Abstract
Estrogen is known to influence pain, but the specific roles of the two estrogen receptors (ERs) in the spinal cord are unknown. In the present study, we have examined the expression of ERα and ERβ in the spinal cord and have looked for defects in pain pathways in ERβ knockout (ERβ−/−) mice. In the spinal cords of 10-month-old WT mice, ERβ-positive cells were localized in lamina II, whereas ERα-positive cells were mainly localized in lamina I. In ERβ−/− mice, there were higher levels of calcitonin gene-regulated peptide and substance P in spinal cord dorsal horn and isolectin B4 in the dorsal root ganglion. In the superficial layers of the spinal cord, there was a decrease in the number of calretinin (CR)-positive neurons, and in the outer layer II, there was a loss of calbindin-positive interneurons. During embryogenesis, ERβ was first detectable in the spinal cord at embryonic day 13.5 (E13.5), and ERα was first detectable at E15.5. During middle and later embryonic stages, ERβ was abundantly expressed in the superficial layers of the dorsal horn. ERα was also expressed in the dorsal horn but was limited to fewer neurons. Double staining for ERβ and CR showed that, in the superficial dorsal horn of WT neonates [postnatal day 0 (P0)], most CR neurons also expressed ERβ. At this stage, few CR-positive cells were detected in the dorsal horn of ERβ−/− mice. Taken together, these findings suggest that, early in embryogenesis, ERβ is involved in dorsal horn morphogenesis and in sensory afferent fiber projections to the dorsal horn and that ERβ is essential for survival of dorsal horn interneurons throughout life.
Keywords: spinal cord, embryo, development, calretinin, pain
Estrogen is known to influence multiple functions in brain tissue, including neuronal development, plasticity and survival, neurotransmitter and neuropeptide synthesis, and neurotransmitter receptors (1–4). There are recent studies on the effects of estradiol on the spinal cord and on the peripheral nervous system (5, 6). Animal experiments as well as observations in humans have shown that somatosensory perception and pain sensitivity are influenced by estrogen (7–10), but little is known about the underlying mechanisms. In the rat and mouse, both estrogen receptor α (ERα) and ERβ have been shown to be expressed in the dorsal horn of adult spinal cords, in laminae I and II, an area involved in receiving and processing nociceptive information (11–14). In an animal model of inflammatory pain, it has been demonstrated that estradiol-induced analgesia can be reversed by tamoxifen (a selective ER modulator) (15).
To better understand the ontogenic role of ERα and ERβ in the spinal cord, it is necessary to know the evolution of the population of ERα- and ERβ-positive neurons during the course of development. Both ERα and ERβ are expressed in dorsal root ganglion (DRG) neurons during the early postnatal period, and both contribute to development and survival of these neurons (16). Developmental changes in the distribution of ERα immunoreactivity have been reported in neurons and fibers of rat prenatal and postnatal spinal cord (17), but knowledge of the distribution and function of ERβ in the spinal cord during embryogenesis is lacking. Our recent study (18) demonstrated that ERβ is the predominant estrogen nuclear receptor in the brain during embryogenesis. At the protein level, ERβ is strongly expressed within laminated CNS structures, including the cerebral cortex, cerebellum, hippocampus, and olfactory bulb, and contributes to neuronal development in these areas (18).
The dorsal horn is also a cytoarchitecturally laminated region. The superficial laminae of the spinal cord (laminae I and II) receive primary afferent fibers that mostly convey nociceptive and thermoceptive inputs to associative and second-order neurons. Proper development of the superficial laminae of the dorsal horn is required for the function of sensory pathways (19–23). In mammals and birds, calbindin-D28K (CB)-positive and calretinin (CR)-positive neurons are mainly localized in the superficial areas of the dorsal horn, which are the sites for terminals of type A collaterals of primary afferents (24–29). Therefore, CB- and CR-positive neurons might be involved in spinal nociceptive processing, visceral regulation, and dorsal column sensory pathways.
In the present study, we demonstrate ERβ expression in the spinal cord during embryogenesis and use ERβ knockout (ERβ−/−) mouse embryos to explore a role of ERβ in dorsal horn morphogenesis, especially CB- and CR-positive interneurons located in the superficial layers. Given that this region of the dorsal horn processes nociceptive information, we extended our analysis of the targets of afferent projections to specific laminae in the dorsal spinal cord and expression of nociceptive receptors in DRG. These findings suggest that ERβ plays an important role in the dorsal horn development and thus affects sensory function and pain sensitivity.
Results
Pattern of ERβ and ERα Expression in the Developing Spinal Cord.
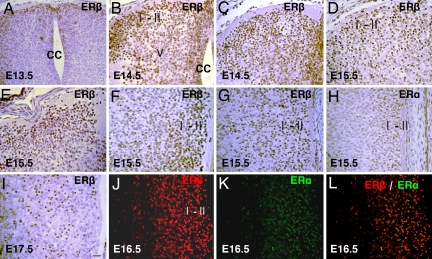
ERβ expression appeared in the embryonic spinal cord as early as embryonic day 13.5 (E13.5), and, at this time, most of the ERβ-positive cells were specifically localized in the superficial layers of the dorsal horn, with a few cells in the lateral and anterior part of mantle layer (Fig. 1A). At E14.5, ERβ expression in the spinal cord increased significantly. ERβ-positive neurons occupied laminae I–II. Distinct immunoreactive cells were also present in the lateral region of lamina V and near the central canal (Fig. 1 B and C). At E15.5 and E16.5, strong ERβ expression was mainly seen in laminae I–II of the dorsal horn (Fig. 1 D and E). Sagittal sections showed ERβ widely expressed in laminae I and II throughout the rostrocaudal part of the spinal cord, with the strongest signal localized in the lumbar sacral region (Fig. 1 F and G). ERα expression was first detectable in the spinal cord at E 15.5. At this stage, some ERα-positive cells appeared in the dorsal horn, most of them in laminae I and II (Fig. 1H). By E17.5, ERβ-positive cells were mainly localized in lamina II, and fewer cells were found in lamina I (Fig. 1I). At E15.5 and E 16.5, double staining showed that, in the dorsal horn, most of the ERα-positive cells also expressed ERβ (Fig. 1 J–L).
Fig. 1.
ERβ and ERα expression in the spinal cord during embryogenesis. (A) At E13.5, ERβ is mainly localized in the superficial layers of the dorsal horn in the lumbar region of the spinal cord. (B and C) In E14.5 lumbar (B) and thoracic (C) spinal cords, ERβ is strongly expressed in the laminae I-II. There is also distinct nuclear staining in the lateral region of lamina V and near the central canal. (D and E) In E15.5 thoracic (D) and cervical (E) spinal cords, ERβ is found in laminae I–II; some ERβ-positive cells are also localized in laminae III–V. (F) In the sagittal section of lumbo–sacral spinal cords at E15.5, dense and deep ERβ staining is seen in the superficial layers. (G and H) In sagittal sections of thoracic spinal cords at E15.5, there are some ERα-positive cells in the dorsal horn (H), and the number of ERβ-positive cells far exceeds that of ERα-positive cells (G). (I) At E17.5 in the lumbar spinal cord, more ERβ-labeled cells appear in lamina II. (J–L) At E16.5 in the dorsal horn of the lumbar spinal cord, most of the ERα-positive cells also express ERβ. CC, central canal. (Scale bar: 20 μm.)
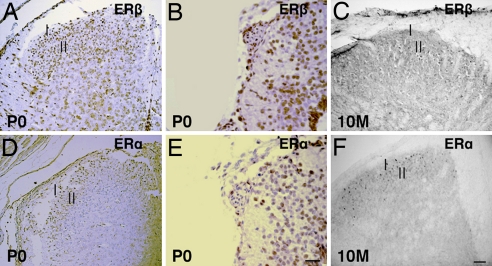
On the first day of postnatal life [postnatal day 0 (P0)], both ERβ (Fig. 2 A and B) and ERα (Fig. 2 D and E) were mainly localized in the superficial layers of the dorsal horn. ERβ-positive neurons were less numerous than ERα-containing neurons in the outer layer but were more abundant in the deeper layers of the spinal cord. In the adult spinal cord, the expression patterns of ERβ (Fig. 2C) and ERα (Fig. 2F) were similar to those seen at P0, but expression of ERβ was lower than that of ERα. There was no observable ERβ staining in either the negative controls or in ERβ−/− mice.
Fig. 2.
ERβ and ERα expression in the postnatal spinal cord. (A, B, D, and E) At P0 in lumbar (A and D) and sacral (B and E) regions of the spinal cord, ERβ-labeled neurons (A and B) appear less numerous in the outer dorsal horn but more numerous in the deeper layers of the spinal cord than ERα-labeled neurons (D and E). (C and F) In 10-month-old mouse spinal cords, more ERβ-stained cells (C) are localized in lamina II, whereas ERα-stained cells (F) are mainly localized in lamina I. (Scale bars: A, D, C, and F, 50 μm; B and E, 20 μm).
ERβ Contributes to Dorsal Horn Morphogenesis Through Modulation of Interneuron Development.
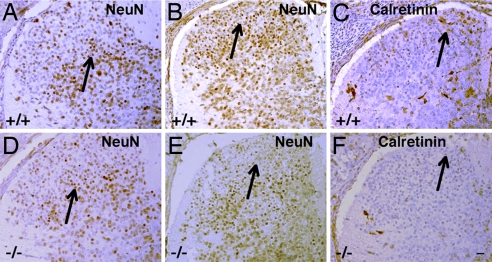
At E17.5 in WT mice, we found that neuron-specific nuclear protein (NeuN)-labeled neurons were evenly distributed at all levels of the spinal cord (Fig. 3 A and B). In contrast, in ERβ−/− mouse dorsal horns, there was a retardation in the neuronal development, with fewer NeuN-labeled neurons in the dorsal horn (Fig. 3 D and E). The number of CR-positive neurons in the spinal cord, particularly in the superficial layer, of ERβ−/− (Fig. 3F) mice was lower than in WT littermates (Fig. 3C).
Fig. 3.
Expression of NeuN and calretinin in the dorsal horn of WT and ERβ−/− female mice at E17.5. (A, B, D, and E) At E17.5, in thoracic (A and D) and lumbar (B and E) dorsal horn, there are fewer NeuN-labeled neurons in ERβ−/− mice (D and E) than in WT littermates (A and B). (C and F) Calretinin expression in the lumbar dorsal horn of ERβ−/− mice (F) is markedly lower than in WT mice (C); the reduction is especially noticeable in the superficial layer. Arrows in A and D indicate laminae III–V of thoracic dorsal horn, and arrows in B, C, E, and F indicate laminae I–II of lumbar dorsal horn. (Scale bar: 20 μm.)
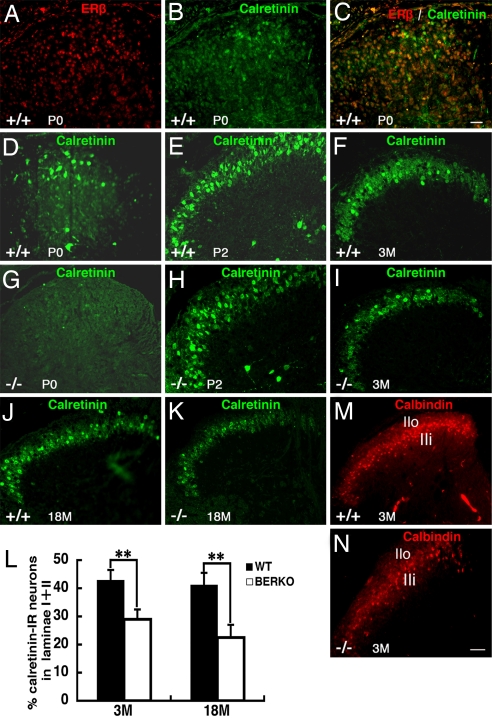
After birth, CR expression was clearly localized in the lamina II of the spinal cord. Double staining for ERβ and CR showed that, in the dorsal horn of neonates (P0), most of the CR neurons in the superficial dorsal horn also expressed ERβ (Fig. 4 A–C). At this stage, few CR-positive cells were detected in the dorsal horn of ERβ−/− mice (Fig. 4G). At P2, most of the CR-labeled cells were localized in the lamina II, with some positive cells in lamina I (Fig. 4E); in ERβ−/− mice, there were fewer CR-positive cells in the medial part of lamina II (Fig. 4H). In the adult spinal cord, both CR and CB were strongly expressed in the lamina II; CR-positive cells mainly occupied the lateral part, whereas CB-positive cells mainly localized in the medial part (Fig. 4F, J, and M). In ERβ−/− mice, the number of CR-positive neurons in lamina II in mice at 3 and 18 months of age (Fig. 4I, K, and L) was much lower than that in age-matched WT mice. In ERβ−/− mice, a decrease in the number of CB-positive cells was also seen in the outer layer II (IIo) at 3 months of age (Fig. 4N).
Fig. 4.
Expression of calretinin and calbindin in the superficial layers of the dorsal horn in the lumbar region in postnatal WT and ERβ−/− female mice. (A–C) At P0, double staining for ERβ and calretinin shows that, in the dorsal horn, most of the calretinin-positive neurons also express ERβ. (D and G) There are fewer calretinin-positive cells in ERβ−/− mice (G) than in WT mice (D). (E and H) At P2, in ERβ−/− mice (H), there are fewer calretinin-positive cells in the medial part of lamina II compared with WT mice (E). (F and I–K) Expression of calretinin in lamina II is significantly lower in ERβ−/− mice (I and K) than WT mice (F and J) at 3 (F and I) and 18 (J and K) months of age. (L) The average percentage of calretinin-labeled cells in laminae I and II of spinal cord dorsal horn at 3 and 18 months of age is shown (n = 3; error bar, SD; **, P < 0.01, Student's t test). BERKO, ERβ−/−. (M) In 3-month-old WT mice, calbindin is mainly localized in laminae I–II of the dorsal horn. (N) Decreased calbindin-labeled cells are seen in the outer layer II (IIo) and lamina I of ERβ −/− mouse dorsal horn. (Scale bars: A–E, G, and H, 20 μm; F, I–K, M, and N, 50 μm).
Central Afferent Targeting Is Impaired in ERβ Mutants.
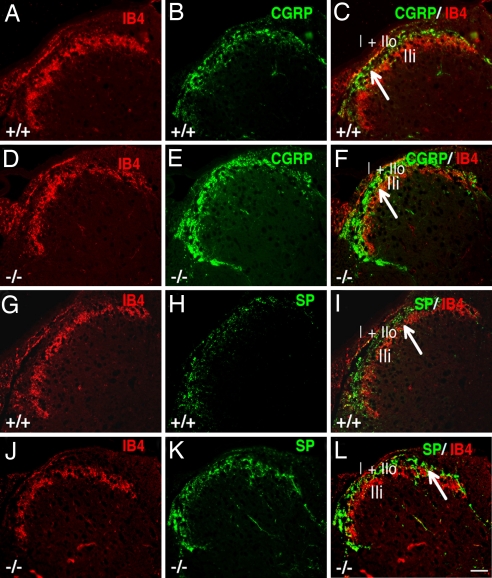
The distribution of peptide-containing [substance P (SP)/calcitonin gene-regulated peptide (CGRP)] and nonpeptide-containing [isolectin B4(IB4)] thin primary afferents (C fibers) that terminate in laminae I–II was examined in female mice at 3 months of age. In WT mice, IB4+ afferents project predominantly to inner lamina II (IIi) (Fig. 5 A and G), whereas CGRP+ and SP+ peptidergic afferents predominantly project to lamina I and outer layer II (IIo) and, to a lesser extent, to lamina IIi (Fig. 5 B and H). Although the expression pattern of IB4 appeared similar in ERβ−/− (Fig. 5 D and J) and WT mice (Fig. 5 A and G), expressions of CGRP and SP in ERβ−/− mouse primary afferents (Fig. 5 E and K) were higher than those in WT mice (Fig. 5 B and H). Double labeling of IB4 and CGRP or SP clearly showed more CGRP and SP afferent innervation in lamina IIi in ERβ−/− (Fig. 5 F and L) than in WT mice (Fig. 5 C and I).
Fig. 5.
Distribution of CGRP, SP, and IB4 in lumbar superficial dorsal horn of 3-month-old, female WT and ERβ−/−mice. (A, D, G, and J) IB4-labeled afferents terminate in inner lamina II and show similar expression pattern in ERβ−/− (D and J) and WT mice (A and G). (B, E, H, and K) Expression of CGRP and SP in superficial dorsal horn is higher in ERβ−/− (E and K) than in WT mice (B and H). (C, F, I, and L) Double labeling of IB4 and CGRP or SP clearly shows more CGRP or SP afferent innervation detected in lamina IIi in ERβ−/− mice (F and L) compared with WT mice (C and I). Arrows in C, F, I, and L indicate inner lamina II. (Scale bar: 20 μm.)
Expression of Nociceptive Receptors in the DRG of ERβ−/− Female Mice at 3 Months of Age.
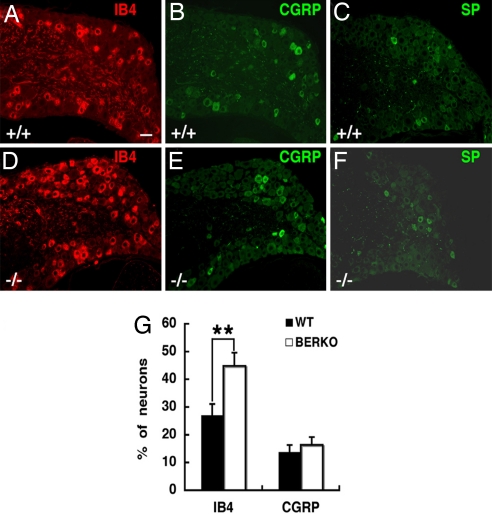
In addition to evaluating distribution of primary afferents in the dorsal horn of ERβ−/− mice, we also examined the CGRP-, IB4-, and SP-labeled cell bodies in lumbar (L4–5) DRG at 3 months of age. Expressions of CGRP and SP were similar in ERβ−/− (Fig. 6 E and F) and WT mice (Fig. 6 B and C), but the percentage of IB4-positive cells in DRG was significantly higher in ERβ−/− (Fig. 6D) than in WT mice (Fig. 6 A and G).
Fig. 6.
Expression of IB4, CGRP, and SP in the DRG of female WT and ERβ−/−mice at 3 months of age. (A and D) The number of IB4-labeled cells in DRG is higher in ERβ−/− mice (D) than in WT littermates (A). (B, C, E, and F) Expression of CGRP (B and E), and SP (C and F) is similar in ERβ−/− (E and F) and WT mice (B and C). (G) The average percentages of DRG neurons expressing IB4 or CGRP in WT and ERβ−/− female mice at 3 months of age are shown (n = 3; error bar, SD; **, P < 0.01, Student's t test). BERKO, ERβ−/−. (Scale bar: 20 μm.)
Discussion
In this study, we demonstrate that ERβ is expressed in the spinal cord as early as E13.5. During middle and later embryonic ages, ERβ was strongly expressed in the dorsal horn, mainly in the laminae I and II. In contrast, ERα-positive neurons were not detected before E15.5, and the level of expression of ERα was lower than that of ERβ. Thus, ERβ is the predominant estrogen nuclear receptor involved in the development of the dorsal horn. At E15.5 and E16.5, in the dorsal horn, most of the ERα-positive cells also expressed ERβ. In vitro studies have reported that ERα and ERβ have the ability to heterodimerize when they coexist in the same nucleus and that hetero- and homodimers display different transcriptional activities (30–33). Colocalization of ERα and ERβ provides evidence that ERα and ERβ have the opportunity to interact in vivo within the spinal cord.
Our previous studies have demonstrated that ERβ is the predominant estrogen nuclear receptor in the brain during embryogenesis and that it contributes to embryonic and postnatal cortical development through influencing neuronal differentiation and migration (34, 35). In the cerebellum, ERβ expression occurs in each neuronal type at different postnatal days and is involved in the regulation of differentiation and maintenance of various types of neurons (36). In the present study, we demonstrated that there is retarded neuronal development in the dorsal horns of ERβ−/− mice at E17.5. This was evident in the paucity of NeuN-labeled neurons in the dorsal horn and the loss of CR-positive neurons in the superficial layers. Double staining for ERβ and CR showed that, in the dorsal horn of WT neonates (P0), most of the CR-positive neurons in the superficial dorsal horn also expressed ERβ. In ERβ−/−mice at P0, there were few CR-positive cells, indicating that the developmental neuronal deficit remained. Furthermore, ERβ is essential for interneuron survival throughout life because, in ERβ−/−mice, there were abnormalities in distribution and number of interneurons in the adult spinal cord. The number of CR-labeled cells in lamina II in 3- and 18-month-old mice was much lower in ERβ−/− than in WT mice. There was also a decrease in CB-positive cells in the outer layer II (IIo) in ERβ−/− mice at 3 months of age. The worsening of the neuronal losses with age suggests that ERβ is essential throughout life for maintenance of the integrity of sensory pathways in the spinal cord.
Our recent study (18) also demonstrated that ERβ is necessary for the development of CR-positive neurons in the embryonic mouse brain. All of these data suggest that ERβ can affect dorsal horn morphogenesis through modulating interneuron development of the superficial laminae of the dorsal horn.
To date, the exact function of CR and CB in the nervous system remains to be discovered. However, it has been proposed that the interneurons in the spinal cord that express these proteins may relate to sensory pathways (37–39). In the dorsal horn, DRG afferents innervate not only the secondary sensory neurons but also the lamina II interneurons, which can have either excitatory or inhibitory effects on the secondary sensory neurons such that pain signals can be modulated (40–42). Nociceptive neurons are specified early during development and precede the formation of synaptic contacts with their future peripheral or central targets (41). The establishment of laminae I and II is essential in the development and functional maturation of nociceptive circuits and subsequent processing of noxious and thermal sensitivity in mammals (43, 44). The level of ERβ has been implicated in altered stability of synaptic connections in the hippocampus (45). Therefore, CR- and CB-positive interneurons may contribute to establishing proper connections with the corresponding primary afferents.
We investigated the distribution of the primary afferents that target dorsal horn neurons. In 3-month-old WT mice, there were more CGRP- and SP-positive fibers in the dorsal horn of ERβ−/− than in that of WT mice. In contrast, there was no difference in IB4-positive fibers between ERβ−/− and WT mice. Peptidergic fiber terminals expressing SP and/or CGRP have been observed in laminae I–II at E18–19, whereas the IB4+ subset of C fiber synaptic terminals appeared at P5 (46–49). Loss of ERβ had little effect on CGRP or SP in DRG neurons. We found that expression of CGRP and SP in lumbar (L4–5) DRG was similar in ERβ−/− and WT mice but that there was higher IB4 expression in ERβ−/− mice. On the basis of these data, we can infer that peptidergic fibers do carry peptides from the DRG to the dorsal horn but that, in ERβ−/− mice, they have fewer interneurons in the dorsal horn with which to interact. This leads to accumulation of SP and CGRP in the afferent fibers.
A recent study has reported that ERβ 401, a selective ERβ agonist, is antihyperalgesic in preclinical models of chemical-induced and acute inflammatory pain (50). Combining this information with our results, we can infer that endogenous ERβ receptor activation in the spinal cord in CR and CB neurons may be the site at which ERβ agonists can modulate pain sensitivity.
Materials and Methods
Animals and Tissue Preparation.
ERβ−/− mice were generated as described in ref. 34. Heterozygous mice were used for breeding. ERβ+/− female mice were mated overnight with ERβ+/− males and inspected at 9:00 a.m. on the following day for the presence of vaginal plugs. Noon of this day was assumed to correspond to E0.5. All animals were housed in the Karolinska University Hospital Animal Facility (Huddinge, Sweden) in a controlled environment on a 12-h light/12-h dark illumination schedule and were fed a standard pellet diet with water provided ad libitum. To obtain embryos, pregnant mice were anesthetized deeply with CO2 and were perfused with PBS followed by 4% paraformaldehyde (in 0.1 M PBS, pH 7.4). Embryos were taken out and put on ice, and spinal cords were dissected and postfixed in the same fixative overnight at 4°C. For the P0 and P2 pups, spinal cords were dissected and postfixed in 4% paraformaldehyde overnight at 4°C. Three- and 10-month-old mice were perfused individually with PBS followed by 4% paraformaldehyde, and spinal cords and DRG were then removed and postfixed overnight. Sex was determined after direct visual inspection of the gonads with a dissecting microscope, and the tail and limbs were removed from each embryo for genotyping. Both male and female embryos were used to study ERβ expression, and there were no observable differences between them in our experiments. To explore ERβ function in the spinal cord, only female embryos and adult spinal cords were used in this study. After fixation, spinal cords and DRG were processed for either paraffin (6-μm) or frozen (30-μm) sections.
Immunohistochemistry.
Paraffin sections were deparaffinized in xylene, rehydrated through graded alcohol, and processed for antigen retrieval by boiling in 10 mM citrate buffer (pH 6.0) for 2 min. The sections were incubated in 0.5% H2O2 in PBS for 30 min at room temperature to quench endogenous peroxidase and then were incubated in 0.5% Triton X-100 in PBS for 30 min. To block nonspecific binding, sections were incubated in 3% BSA for 1 h at 4°C. For ERβ staining, retrieval was improved by incubating the sections with 0.15 units/ml β-galactosidase for 2 h. Sections were then incubated with anti-ERβ 1:200, anti-calretinin 1:2,000, anti-NeuN 1:200, anti-ERα 1:200, anti-CGRP 1:500, anti-IB4 1:200, or anti-SP 1:100 in 1% BSA and 0.1% Triton X-100 overnight at room temperature. BSA replaced primary antibodies in negative controls. After washing, sections were incubated with the corresponding secondary antibodies in 1:200 dilutions for 2 h at room temperature. The Vectastain ABC kit (Vector Laboratories, Burlingame, CA) was used for the avidin–biotin complex (ABC) method according to the manufacturer's instructions. Peroxidase activity was visualized with 3,3-diaminobenzidine (DAKO, Carpenteria, CA). The sections were lightly counterstained with hematoxylin, dehydrated through an ethanol series to xylene, and mounted. For immunofluorescence, slides were directly mounted in Vectashield antifading medium (Vector Laboratories). The sections were examined under a Zeiss (Göttingen, Germany) fluorescence microscope with filters suitable for selectively detecting the fluorescence of FITC (green) and Cy3 (red) or were examined under a light microscope. For colocalization, images from the same section but showing different antigen signals were overlaid. Frozen sections were processed for detecting ERβ and ERα expression in 10-month-old spinal cords and for detecting calbindin in 3-month-old spinal cord. Sections were blocked for 30 min with 1% H2O2 and followed by 10% normal serum, were rinsed three times with PBS, and were incubated overnight with the antibodies ERβ 1:500, ERα1:500, and calbindin 1:1,000. These sections were processed further with biotinylated secondary antibodies for ERβ and ERα and with Cy3-labeled antimouse IgG for calbindin. Visualization was done with 3,3-diaminobenzidine or with a fluorescence microscope.
Data Analysis.
Stained spinal cord and DRG sections (10–12 sections for each mouse) were examined under a fluorescence microscope, and images were captured under ×20 magnification. Percentage of CR-immunopositive cells in laminae I–II and percentages of IB4 and CGRP in DRG were calculated. Estimates of the number of CR-immunoreactive cells in laminae I–II of lumbar spinal cords and IB4- and CGRP-labeled cells in L4–5 DRG were made based on the counts of the 10 images showing the highest number of labeled neurons. Statistical analysis was performed using Student's t test.
Chemicals and Antibodies.
We purchased β-galactosidase and biotin-conjugated IB4 from Sigma–Aldrich (St. Louis, MO). The following antibodies were used: rabbit polyclonal anti-calretinin from Swant, (Bellinzona, Switzerland), mouse anti-calbindin and rabbit anti-CGRP from Sigma-Aldrich, rabbit polyclonal anti-ERα from Santa Cruz Biotechnology (Santa Cruz, CA), and mouse anti-NeuN from Chemicon (Temecula, CA). The chicken polyclonal anti-ERβ 503 was produced in our laboratory (18), Cy3 anti-SABC, Cy3 anti-mouse, and FITC anti-rabbit antibodies were from Jackson ImmunoResearch (West Grove, PA), and biotinylated goat anti-rabbit IgG and rabbit anti-chicken/turkey IgG were from Zymed (South San Francisco, CA).
Acknowledgments
This study was supported by the Swedish Cancer Society, Karo Bio AB, Konung Gustaf V:s och Drottning Victorias Stiftelse, the European Commission-funded Network of Excellence CASCADE (FOOD-CT-2004-506319), and the European Commission-funded Integrated Project CRESCENDO (LSHM-CT-2005-018652).
Abbreviations
- ER
estrogen receptor
- DRG
dorsal root ganglion
- CB
calbindin-D28K
- CR
calretinin
- Pn
postnatal day n
- En
embryonic day n
- NeuN
neuron-specific nuclear protein
- SP
substance P
- CGRP
calcitonin gene-regulated peptide
- IB4
isolectin B4.
Footnotes
Conflict of interest statement: J.-Å.G. is a shareholder and consultant of Karo Bio AB.
References
- 1.McEwen BS, Alves SE. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 3.Behl C, Manthey D. J Neurocytol. 2000;29:351–358. doi: 10.1023/a:1007109222673. [DOI] [PubMed] [Google Scholar]
- 4.Beyer C. Anat Embryol. 1999;199:379–390. doi: 10.1007/s004290050236. [DOI] [PubMed] [Google Scholar]
- 5.Chaovipoch P, Jelks KA, Gerhold LM, West EJ, Chongthammakun S, Floyd CL. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- 6.Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. J Neurosci Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- 7.Amandusson A, Hallbeck M, Hallbeck AL, Hermanson O, Blomqvist A. Pain. 1999;83:243–248. doi: 10.1016/s0304-3959(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 8.Liu NJ, Gintzler AR. Pain. 2000;85:273–281. doi: 10.1016/s0304-3959(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 9.Dao TT, LeResche L. J Orofac Pain. 2000;14:169–184. [PubMed] [Google Scholar]
- 10.Ji Y, Murphy AZ, Traub RJ. J Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanderhorst VG, Gustafsson JA, Ulfhake B. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- 12.Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Cell Tissue Res. 2001;304:193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- 13.Shughrue PJ, Lane MV, Merchenthaler I. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Papka RE, Mowa CN. Int Rev Cytol. 2003;231:91–127. doi: 10.1016/s0074-7696(03)31003-4. [DOI] [PubMed] [Google Scholar]
- 15.Kuba T, Wu HB, Nazarian A, Festa ED, Barr GA, Jenab S, Inturrisi CE, Quinones-Jenab V. Horm Behav. 2006;49:441–449. doi: 10.1016/j.yhbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Patrone C, Andersson S, Korhonen L, Lindholm D. Proc Natl Acad Sci USA. 1999;96:10905–10910. doi: 10.1073/pnas.96.19.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke KA, Schroeder DM, Abel RA, Richardson SC, Bigsby RM, Nephew KP. J Neurosci Res. 2000;61:329–337. doi: 10.1002/1097-4547(20000801)61:3<329::AID-JNR11>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Fan X, Warner M, Gustafsson JA. Proc Natl Acad Sci USA. 2006;103:19338–19343. doi: 10.1073/pnas.0609663103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cervero F, Iggo A. Brain. 1980;103:717–772. doi: 10.1093/brain/103.4.717. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura M, Jessell TM. J Neurophysiol. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- 21.Pan YZ, Pan HL. J Neurophysiol. 2004;91:2413–2421. doi: 10.1152/jn.01242.2003. [DOI] [PubMed] [Google Scholar]
- 22.Light AR, Perl ER. J Comp Neurol. 1979;186:117–131. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- 23.Woolf CJ, Fitzgerald M. J Comp Neurol. 1983;221:313–328. doi: 10.1002/cne.902210307. [DOI] [PubMed] [Google Scholar]
- 24.Ren K, Ruda MA. Brain Res Brain Res Rev. 1994;19:163–179. doi: 10.1016/0165-0173(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 25.Papka RE, Collins J, Copelin T, Wilson K. Cell Tissue Res. 1999;298:63–74. doi: 10.1007/s004419900071. [DOI] [PubMed] [Google Scholar]
- 26.Lunam CA. Cell Tissue Res. 1989;257:149–153. doi: 10.1007/BF00221645. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Carr PA, Baimbridge KG, Nagy JI. Brain Res Bull. 1989;23:493–508. doi: 10.1016/0361-9230(89)90195-0. [DOI] [PubMed] [Google Scholar]
- 28.Antal M, Polgar E. Eur J Neurosci. 1993;5:782–794. doi: 10.1111/j.1460-9568.1993.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 29.Resibois A, Rogers JH. Neuroscience. 1992;46:101–134. doi: 10.1016/0306-4522(92)90012-q. [DOI] [PubMed] [Google Scholar]
- 30.Cowley SM, Hoare S, Mosselman S, Parker MG. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 31.Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M. Mol Cell Biol. 2004;24:7681–7694. doi: 10.1128/MCB.24.17.7681-7694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews J, Gustafsson JA. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Andersson S, Warner M, Gustafsson JA. Proc Natl Acad Sci USA. 2003;100:703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Andersson S, Warner M, Gustafsson JA. Proc Natl Acad Sci USA. 2001;98:2792–2796. doi: 10.1073/pnas.041617498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikeda Y, Nagai A. Brain Res. 2006;1083:39–49. doi: 10.1016/j.brainres.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 37.Huang HY, Cheng JK, Shih YH, Chen PH, Wang CL, Tsaur ML. Eur J Neurosci. 2006;22:1149–1157. doi: 10.1111/j.1460-9568.2005.04283.x. [DOI] [PubMed] [Google Scholar]
- 38.Ren K, Ruda MA, Jacobowitz DM. Brain Res Bull. 1993;31:13–22. doi: 10.1016/0361-9230(93)90004-u. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida S, Senba E, Kubota Y, Hagihira S, Yoshiya I, Emson PC, Tohyama M. Neuroscience. 1990;37:839–848. doi: 10.1016/0306-4522(90)90113-i. [DOI] [PubMed] [Google Scholar]
- 40.Jackman A, Fitzgerald M. J Comp Neurol. 2000;418:281–298. [PubMed] [Google Scholar]
- 41.Fitzgerald M. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Bao L. Curr Opin Neurobiol. 2006;16:460–466. doi: 10.1016/j.conb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Burstein R, Dado RJ, Giesler GJ., Jr Brain Res. 1990;511:329–337. doi: 10.1016/0006-8993(90)90179-f. [DOI] [PubMed] [Google Scholar]
- 44.Olave MJ, Maxwell DJ. Neuroscience. 2004;126:391–403. doi: 10.1016/j.neuroscience.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 45.Szymczak S, Kalita K, Jaworski J, Mioduszewska B, Savonenko A, Markowska A, Merchenthaler I, Kaczmarek L. Hippocampus. 2006;16:453–463. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald M, Swett J. Neurosci Lett. 1983;43:149–154. doi: 10.1016/0304-3940(83)90179-9. [DOI] [PubMed] [Google Scholar]
- 47.Marti E, Gibson SJ, Polak JM, Facer P, Springall DR, Van Aswegen G, Aitchison M, Koltzenburg M. J Comp Neurol. 1987;266:332–359. doi: 10.1002/cne.902660304. [DOI] [PubMed] [Google Scholar]
- 48.Benn SC, Costigan M, Tate S, Fitzgerald M, Woolf CJ. J Neurosci. 2001;21:6077–6085. doi: 10.1523/JNEUROSCI.21-16-06077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pignatelli D, Ribeiro-da-Silva A, Coimbra A. Brain Res. 1989;491:33–44. doi: 10.1016/0006-8993(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 50.Leventhal L, Brandt MR, Cummons TA, Piesla MJ, Rogers KE, Harris HA. Eur J Pharmacol. 2006;553:146–148. doi: 10.1016/j.ejphar.2006.09.033. [DOI] [PubMed] [Google Scholar]