Abstract
There is evidence that the primate prefrontal cortex is involved in the monitoring of the order in which stimuli occur. The prefrontal cortical areas, however, involved in the capacity of the human brain to encode and hold “in mind” the precise order of occurrence of a limited number of visual stimuli after a single exposure are not known. Changes in regional cerebral activity were measured with functional magnetic resonance imaging while subjects were coding the precise order of a short sequence of abstract visual stimuli. The results demonstrate the involvement of areas 46 and 9/46, within the mid-dorsolateral subdivision of the prefrontal cortex, in the coding of the precise order of a short sequence of visual stimuli in working memory, consistent with earlier results from monkey lesion studies. The availability of such detailed serial-order information in working memory allows high-level cognitive planning and mental manipulation, functions that depend on prefrontal cortex.
Keywords: functional magnetic resonance imaging, human, serial-order memory
Only a few items can occupy our working memory (1, 2). The capacity to monitor the precise order of a short sequence of visual stimuli/events after a single exposure and to hold their order “in mind” is fundamental to our ability for efficient “on-line” high-level planning and mental manipulation when precise order of what has just happened and what is about to happen is of the essence. It has been shown that, in the monkey, the capacity to monitor the precise order of a short sequence of visual stimuli after a single exposure depends critically on the mid-dorsolateral prefrontal cortex (DLPFC), which includes architectonic areas 46 and 9/46 (3). Lesions restricted to mid-DLPFC abolish the ability to monitor the precise order of short sequences of stimuli (4–5 stimuli), although they leave intact memory for these stimuli (3). The key region in the human prefrontal cortex for the encoding and retrieval of the precise order of a short sequence of stimuli in working memory is not known.
Although there is evidence that lesions of the prefrontal cortex in the human brain can impair the ability to discriminate between more-recent and less-recent stimuli in long sequences (4–7), these studies addressed a different question because of the use of long lists of stimuli in which the precise encoding of serial order after a single exposure is not possible. Some of these earlier studies (4, 7) examined organization strategies during the encoding of long lists in long-term episodic memory and retrieval from long-term memory based, partly, on the quality of organizational strategies. Furthermore, the large size of the lesions studied, which included damage to several prefrontal areas, precluded any conclusions about the key architectonic areas of the anatomically heterogeneous prefrontal cortex that might be involved in serial-order short-term memory (4–7), except for one study which indicated that the most common overlap in lesions giving rise to problems in recency discrimination was the mid-DLPFC (6). Similarly, the few functional neuroimaging studies that examined temporal order memory used long lists of stimuli and experimental designs in which the precise encoding of order was not possible and, therefore, like the lesion studies, examined activity related to the effects of organization on controlled retrieval from long-term episodic memory (8, 9). Thus, there is no answer to the question posed here: Are areas 46 and 9/46 of the human mid-DLPFC involved in the monitoring of the precise order of a short sequence of visual stimuli within working memory? The present functional magnetic resonance imaging (fMRI) study was designed to address this question by using an experimental design that could separate the encoding of the precise order of a short sequence of visual stimuli in working memory from the simple exposure and entry of those stimuli in memory.
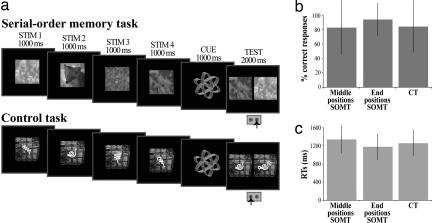
Seventeen normal human subjects were scanned while performing a serial-order memory task (SOMT) and a control task (CT). The order of presentation of these two tasks was counterbalanced across subjects. On each trial of the SOMT, the subject observed a sequence of four new abstract visual stimuli. The sequence was followed by a test display during which two of these stimuli were presented simultaneously (Fig. 1a) and the subject had to indicate, by pressing the appropriate response key, which one of the two stimuli occurred earlier in the sequence. In the CT, again a sequence of four new abstract visual stimuli was presented on each trial, but in the test display one of these stimuli was shown together with a new stimulus and the subject had to indicate which one was the stimulus in the sequence by pressing the appropriate response key (Fig. 1a). The subjects had been instructed to encode the order of the four new stimuli on each trial of the SOMT, but to encode only their physical characteristics in the CT (see Methods). Thus, the SOMT required encoding of the precise order of the stimuli in the sequence, and the CT required encoding of their physical characteristics. Note that the difficulty of recognition memory tasks (like the CT) increases as the similarity among the presented items increases, because a more precise memory of the encoded stimuli is required. In this study, the difficulty of the CT was matched to the difficult part of the SOMT, i.e., the serial order of the stimuli occurring in the middle positions (see Fig. 1 b and c and Results). In addition, in both tasks, the four stimuli were presented successively for 1,000 ms each interspersed with a delay varying from 2.5 to 7.5 s (average = 3.5 s). This delay enabled the signal for each stimulus presentation to be measured separately. This design allowed us to examine activity related to the encoding of the serial order but not to the maintenance of this information within working memory.
Fig. 1.
Behavioral tasks and performance. (a) In both the SOMT and the CT, four new abstract nonverbal black and white stimuli were presented successively for 1,000 ms each. The presentation of the sequence was followed by a cue (1,000 ms) and then the test period (2,000 ms). A random delay varying from 2.5 to 7.5 s (average = 3.5 s) was interspersed between the presentation of each one of the four stimuli, the cue, and the test period to enable the signal for each of these events to be measured. In the test period of the SOMT, two of the four stimuli from the preceding sequence were shown, and the subject had to indicate which one occurred earlier in the sequence. In the test period of the CT, one of the four stimuli from the preceding sequence and a new stimulus were shown, and the subject had to indicate the stimulus presented in the preceding sequence. Note that four new stimuli were used during the sequence presentation in all trials. (b) The subjects' mean (±SD) percent correct responses during the test period for judgments involving stimuli presented in the middle and end positions in the SOMT and all positions in the CT. Performance was significantly higher for the end positions in SOMT than for judgments involving the middle positions in SOMT and all positions in the CT (see Behavioral Performance During Scanning). (c) The subjects' mean (±SD) reaction times in the correct trials for judgments involving stimuli in the middle and end positions in SOMT and all positions in CT. Note that the mean reaction time for judgments involving the end positions in SOMT was significantly lower than for judgments involving the middle positions in SOMT and the CT (see Behavioral Performance During Scanning).
Results
Behavioral Performance During Scanning.
In the test period of the CT, there was no significant difference in behavioral performance for stimuli occurring at the end positions (i.e., positions 1 and 4) during the sequence versus stimuli occurring at the middle positions (i.e., positions 2 and 3) (mean percent correct performance for the middle positions, 84.7 ± 36.0% and for the end positions, 84.8 ± 35.9%, t = 0.03, P < 0.97; mean reaction time for the middle positions, 1,268.7 ± 288.5 ms and for the end positions, 1,251.3 ± 281.9 ms, t = −0.96, P < 0.34). Thus, performance for the test period of the CT is presented for all of the stimuli together (Fig. 1 b and c).
As can be seen in Fig. 1b, there were differences in percent correct performance between judgments of the order of presentation of stimuli that occupied the middle positions in the SOMT, judgments of the order of stimuli in the end positions in the SOMT, and recognition judgments in the CT (F(2,2320) = 21.1, P < 0.00001, one-way ANOVA). Post hoc comparisons (at P < 0.000001, Fisher's test) showed that the percent correct performance for the end positions in the SOMT (94.5 ± 22.7%) was significantly higher than performance for both the middle positions in the SOMT (83.3 ± 37.4%) and the CT (84.7 ± 35.9%). However, the percent correct performance for the middle positions in the SOMT was not significantly different from correct performance in the CT (P < 0.39, Fisher's test). These data show that the difficulty of the CT was well matched with that of the middle positions of the SOMT, but that the CT was more difficult than the easier end positions in the SOMT. Furthermore, as can be seen in Fig. 1c, the mean reaction time for judgments involving the end positions in the SOMT (1,179.8 ± 282.8 SD) was significantly lower than for judgments involving the middle positions in the SOMT (1,344.3 ± 304.2 SD) and the CT (1,260.1 ± 285.3 SD) (F(2,2015) = 41.2, P < 0.0001, one-way ANOVA), again showing that the end positions in the SOMT were easier than both the CT and the middle positions in the SOMT.
fMRI Blood Oxygenation Level Dependent (BOLD) Response Analyses.
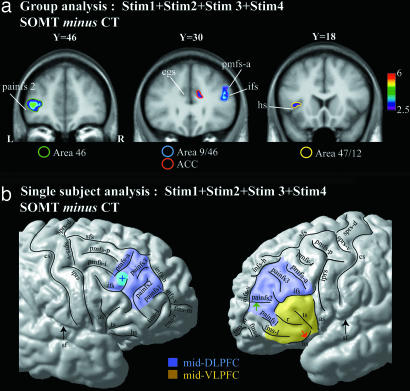
Note that the analysis of the BOLD signal was performed on the correct trials. To assess which ones of the many prefrontal cortical areas were involved in the encoding of the serial order of the visual stimuli, we first compared the BOLD signal obtained during the presentation of all of the four stimuli (i.e., STIM1 + STIM2 + STIM3 + STIM4, Fig. 1a) in the SOMT with the corresponding signal during the presentation of all of the four stimuli in the CT (i.e., SOMT minus CT). It is important to note that we examined the BOLD signal only in the 1,000-ms periods during which the four stimuli on each trial were presented, because the only difference between the SOMT and the CT during the presentation of the four stimuli is that, in the SOMT, the subject has to encode the order of the stimuli. The comparison SOMT minus CT demonstrated two peaks of increased activity within the mid-DLPFC during the encoding of the serial order of the stimuli. The coordinates of the activity peaks are provided in the Montreal Neurological Institute (MNI) standard stereotaxic space. There was a peak of increased activity in the right mid-DLPFC area 9/46 (x = 49, y = 34, z = 23, t = 3.74) and a peak in the left mid-DLPFC area 46 (x = −40, y = 46, z = 4, t = 4.64) (Fig. 2a). In addition, there was increased activity in the right anterior cingulate cortex (ACC) (x = 12, y = 30, z = 23, t = 3.03).
Fig. 2.
Peaks of increased activity during the presentation of all four stimuli from the comparison SOMT minus the CT. (a) Group analysis, i.e., average activity increases from all 17 subjects. The t statistical map of activity has been superimposed on the average T1 anatomical acquisition of the 17 subjects transformed into the MNI standard stereotaxic space. The areas surrounded by a blue, green, yellow, and red circle indicate, respectively, the location of the activity increase within areas 9/46, 46, 47/12, and the ACC. The y value indicates the anteroposterior level in millimeters within the MNI stereotaxic space. The color scale indicates the t value range. (b) Schematic representation of the activity increases on the cortical surface rendering in standard stereotaxic space of a single right and left hemisphere. The activity increase observed within areas 9/46 during the presentation of the four stimuli in the comparison SOMT minus CT is represented by a blue cross in a blue circle. The green arrow points to the painfs2 in the depth of which the activity increase within area 46 is observed. The red arrow points to the horizontal sulcus in the depth of which the activity increase within the mid-VLPFC is observed. The blue and the yellow areas represent the location of the mid-DLPFC (i.e., areas 9/46 and 46) and the mid-VLPFC, respectively. L and R, left and right hemispheres; as, ascending sulcus; cgs, cingulate sulcus; cs, central sulcus; fms-l, fms-i, and fms-m, lateral, intermediate, and medial frontomarginal sulci; hs, horizontal sulcus; ifs, inferior frontal sulcus; infs-h and infs-v, horizontal and vertical branches of the intermediate frontal sulcus; iprs, inferior precentral sulcus; painfs1, painfs2, and painfs3, paraintermediate frontal sulci 1, 2, and 3; pmfs-p, pmfs-i, and pmfs-a, posterior, intermediate, and anterior posterior middle frontal sulci; r, sulcus radiatus; sf, Sylvian fissure; sfs, superior frontal sulcus; sprs-d and sprs-v, dorsal and ventral branches of the superior precentral sulcus; ts, triangular sulcus.
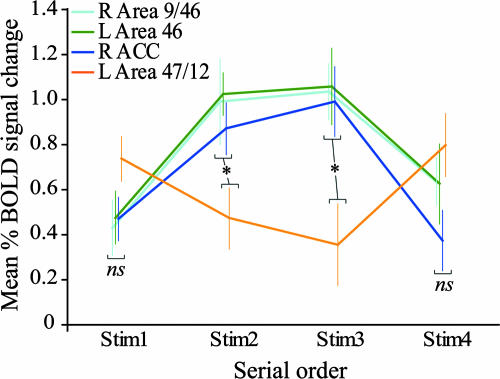
After the overall analysis, we examined the relative involvement of the above areas in the successive encoding of the first, second, third, and fourth stimuli in the SOMT. For this goal, the mean percent BOLD signal change was measured as follows: First, the regions of interest were identified in each single subject on the basis of the overall comparison of the BOLD signal obtained during the presentation of all four stimuli in the SOMT with the corresponding signal during the presentation of all four stimuli in the CT (i.e., SOMT Stim1 + Stim2 + Stim3 + Stim4 minus CT Stim1 + Stim2 + Stim3 + Stim4). Second, the percent BOLD signal change was measured within a gray matter volume of a 5-mm3 radius centered on the peaks observed in each region of interest in each single subject for each one of the following comparisons: Stim1, SOMT minus CT; Stim2, SOMT minus CT; Stim3, SOMT minus CT; and Stim4, SOMT minus CT. Finally, the percent BOLD signal change obtained in the 17 subjects was averaged for each region of interest and for each comparison. As can be seen in Fig. 3, the mean percent BOLD signal change was greater during the encoding of the second and third stimuli than during the encoding of the first and the last stimuli in the right area 9/46, the left area 46, and the ACC. It is interesting to note here that the above overall comparison (i.e., Stim1 + Stim2 + Stim3 + Stim4, SOMT minus CT) revealed also an increase in activity in one location in the mid-ventrolateral prefrontal cortical (VLPFC) region (x = −39, y = 18, z = 6, t = 3.13). This activity increase was situated within the horizontal sulcus, which is occupied by architectonic area 47/12 at the border with area 45. In this region, the mean percent BOLD signal change to the stimuli that occupied the first and the last positions in the sequence was greater than that to the middle positions (Fig. 3) (see below for comment).
Fig. 3.
Mean (±SEM) percent BOLD signal change within areas 9/46, 46, 47/12, and the ACC during the presentation of the first, second, third, and fourth stimuli in the sequence. The signal change was based on the group comparison SOMT minus CT. The percent BOLD signal change varied significantly between the above frontal regions during the successive presentation of stimuli in the SOMT [F(9,159) = 3.35, P < 0.00089, 4 (frontal areas) × 4 (stimulus sequence positions) repeated-measures ANOVA]. Post hoc comparison (Fisher's test, P < 0.05) showed that the mean percent BOLD signal change was significantly greater during the presentation of the second and the third stimuli (i.e., the middle positions in the sequence) compared with the signal observed during the presentation of the first and the fourth stimuli (i.e., the end positions) in the right area 9/46, in the left area 46, and in the right ACC. By contrast, the mean percent BOLD signal change was significantly greater during the presentation of the first and the fourth stimuli compared with the signal during the presentation of the second and the third stimuli in left area 47/12. The mean percent BOLD signal change during the presentation of the first and the last stimuli was not statistically different between the following areas: right 9/46, left 46, right ACC, and left 47/12. By contrast, the mean percent BOLD signal change was significantly greater during the presentation of the second and the third stimuli in the right area 9/46, in the left area 46, and in the right ACC compared with the signal in the left area 47/12. ns, not significant at P < 0.05 (Fisher's test); *, significant at P < 0.05 (Fisher's test).
We also examined, on a subject-by-subject basis, the exact location within the prefrontal cortex of the increases in activity related to the encoding of the order of the stimuli. This examination confirmed that, in each subject, one activity peak was always located in the right hemisphere on the middle frontal gyrus above the rostral part of the inferior frontal sulcus and in front of the anterior segment of the posterior middle frontal sulcus (see Fig. 2b). Architectonic studies in our laboratory have shown that this part of the middle frontal gyrus is occupied by area 9/46 (10). The other increase in activity occurred adjacent and within the intermediate sulcus where our architectonic studies indicate that area 46 is located (i.e., painfs1 and painfs2, Fig. 2b) (10). Thus, the subject-by-subject analysis provided strong evidence that the activity increases were occurring within the mid-DLPFC (i.e., architectonic areas 46 and 9/46).
The difficulty of the middle positions of the SOMT was well matched to the difficulty of the CT, but the end positions of the SOMT were easier than the CT (see Fig. 1b and Behavioral Performance During Scanning). We therefore proceeded to compare separately activity during the encoding of stimuli in the end positions and the middle positions of the SOMT with activity in the CT. Both comparisons yielded significantly increased activity in the mid-DLPFC (end positions SOMT minus end positions CT: area 9/46, x = 51, y = 36, z = 21, t = 3.71; area 46, x = −43, y = 44, z = 4, t = 3.65; middle positions SOMT minus middle positions CT: area 9/46, x = 44, y = 30, z = 26, t = 4.64; area 46, x = −36, y = 46, z = 5, t = 4.33). In other words, regardless of whether we compared the encoding of the middle positions in the SOMT that were equal in difficulty to the CT or the encoding of the end positions in the SOMT that were easier than the CT, there was significantly greater activity in the mid-DLPFC during serial-order encoding. Consequently, the pattern of activity observed in the mid-DLPFC cannot be attributed to the relative difficulty of the tasks being compared. It should also be noted that a recent study has shown greater activity in lateral prefrontal cortex in tasks that are easier to perform because they allow the coding of structure in sequences of spatial and verbal stimuli relative to more difficult unstructured CTs (11).
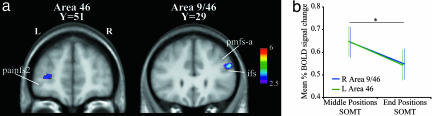
To examine further whether modulation of the BOLD signal to increasing demands in the SOMT would be specific to the mid-DLPFC, we compared the signal obtained during the test period when the subjects were retrieving (i.e., judging) the relative order of stimuli that occupied the more difficult middle positions (i.e., positions 2 and 3) with the signal during judgment of the relative order of stimuli that occupied the easier first and last positions (i.e., middle positions SOMT minus end positions SOMT). The results showed that during judgment of the serial order of stimuli that occupied the more challenging middle positions, there was greater activity in the right mid-DLPFC area 9/46 (x = 49, y = 29, z = 27, t = 3.91) and the left area 46 (x = −30, y = 51, z = 8, t = 3.29) (Fig. 4a). Again, a subject-by-subject analysis showed that the peaks of activity were always in the middle frontal gyrus above the inferior frontal sulcus and anterior to the posterior middle frontal sulcus, i.e., where area 9/46 is located, and within the intermediate frontal sulcus where area 46 is located (10).
Fig. 4.
Activity related to the test period. (a) Peaks of greater activity during the test period of the SOMT when the subjects were judging the relative order of stimuli that had occupied the middle positions during the sequence presentation compared with judgments of the relative order of stimuli that had occupied the end positions (i.e., middle positions SOMT minus end positions SOMT in the whole group of 17 subjects). The t statistical map of activity has been superimposed on the average T1 anatomical acquisition of the 17 subjects transformed into standard stereotaxic space. The y value indicates the anteroposterior level in millimeters within the MNI stereotaxic space. The color scale indicates the t value range. (b) Mean (±SEM) percent BOLD signal change within areas 9/46 and 46 during judgments involving the middle positions and end positions in the test period of the SOMT in comparison with the test period of the CT. *, significant at P < 0.08 (t test).
Finally, we examined the signal in the mid-DLPFC (areas 46 and 9/46) during the test period related to judgments of the order of stimuli in the middle positions and in the end positions of SOMT in comparison with the test period of the CT. For this goal, we first identified the activity peaks in the mid-DLPFC (areas 46 and 9/46) in each individual subject from the comparison “all test periods in SOMT minus all test periods in CT.” The percent BOLD signal change was then measured within a gray matter volume of a 5-mm3 radius centered on these peaks for each one of the following comparisons: “test period of judgments involving the middle positions of SOMT minus judgments involving all positions of CT” and “test period of judgments involving the end positions of SOMT minus judgments involving all positions of CT.” Finally, the percent of the BOLD signal change obtained in the 17 subjects was averaged for each region of interest and for each comparison. The results showed that the mean percent BOLD signal change for the middle positions of SOMT was higher than for the end positions of SOMT in areas 46 and 9/46 combined (t = 2.81, P < 0.008) (see Fig. 4b).
Discussion
The present findings demonstrate unambiguously that activity increases related to the encoding of the serial order of a small number of visual events in working memory occur within the human mid-DLPFC (areas 46 and 9/46) and the anatomically and functionally related ACC (Fig. 2). The greater response in these areas related to the encoding stimuli that occupied the middle positions in the sequence (i.e., the second and third positions in the four stimulus sequence) in comparison with the response to stimuli that occupied the first and last positions. These findings parallel remarkably results of the analysis of the effects of lesions to the mid-DLPFC in the monkey on serial-order short-term memory. In the monkey, absence of this area prevented the precise coding of order: only an approximate order could be inferred, indirectly, on the basis of the greater saliency of the first and last stimuli (i.e., on the basis of the classic primacy and recency effects) relative to the less-salient middle positions in the sequence (3). Interestingly, monkeys with mid-DLPFC lesions that cannot code the serial order of stimuli after a single presentation are still able to learn to select visual stimuli according to a learned fixed order, i.e., they are able to learn a sequence on the basis of repeated trials (12). Thus, mid-DLPFC lesions abolish the neural representation of explicit ordinal position of a short series of stimuli based on a single exposure, leaving intact the learning of fixed chains of associations (i.e., learning a sequence of stimuli) and indirect inference of approximate order based on the greater saliency of the first and last items in a list.
The analysis of the mean percent BOLD response change demonstrated that, in the mid-DLPFC (areas 9/46 and 46) and the ACC, there was increased activity to the coding of all of the positions in the sequence but with greater activity for the coding of the middle positions, which are the most challenging in terms of serial-order coding (Fig. 3). By contrast, in the mid-VLPFC, there was greater activity to the stimuli occupying the first and the last positions in the sequence (Fig. 3). In other words, the mid-VLPFC was responding primarily to the more salient first and last stimuli (i.e., it appeared to be involved in the coding of primacy and recency). The present functional neuroimaging results are consistent with recent single neuron recording studies in the monkey. In one study in which the monkeys had to code only two sequentially presented stimuli, neurons in the mid-VLPFC responded to these two stimuli differentially (13), perhaps coding their relative saliency. By contrast, another single neuron recording study in the monkey with longer sequences (three stimuli) reported neurons in the DLPFC, but not the VLPFC, that coded the order of stimuli (14).
The involvement of the ACC in serial-order memory is consistent with anatomical, electrophysiological, and lesion studies in the monkey. The ACC is strongly connected with areas 9/46 and 46 (15–17) and is also closely related to these areas in evolutionary development (15, 18). Although the ACC works closely with the mid-DLPFC, ACC lesions in the monkey do not yield working memory deficits similar to those obtained after DLPFC lesions (19). Similarly, ACC neurons do not encode information in working memory in the same way as mid-DLPFC neurons (20). Although the exact role of ACC in serial-order memory remains unclear, one hypothesis is that ACC neurons encode the distance of events with respect to the goal (20) and not the order of occurrence of events in relation to the first event. Further work will be necessary to clarify the role of ACC in serial-order memory.
At present, there have been two prominent approaches to the study of the encoding of serial order in working memory. The first approach focused on the study of phonologically coded, stored, rehearsed, and recalled verbal material (21–24). This type of research, which examined the phonological coding of verbal stimuli (e.g., numbers) and the phonological storage and rehearsal of these verbal stimuli, led to the concept of the phonological (articulatory) loop (1) and the demonstration from lesion studies that the left supramarginal gyrus, together with Broca's speech region in the left cerebral hemisphere and the left premotor cortex, constitute an articulatory neural network involved in the processing of various aspects of the phonologically coded stimuli (25, 26). Several neuroimaging studies (27–29) have also shown that the left supramarginal, Broca's speech region, and the left premotor cortical regions play an important role in the phonological coding and rehearsal needed to maintain verbal material in phonological short-term memory. The second research approach examined the coding of the serial order of nonverbal images, i.e., non-phonologically coded material. The neural basis of this type of serial-order coding, which does not critically involve the phonological system (i.e., an aspect of linguistic processing), has been studied in monkeys (3, 13, 14, 30) and in patients with brain lesions (6). The importance of the prefrontal cortex for the coding of the order of stimuli was first suggested by Milner (31) based on impaired performance by patients with frontal lesions on a short-term memory task involving the recurrence of a small number of stimuli. The present functional neuroimaging study provides evidence that the mid-DLPFC (areas 46 and 9/46) constitutes the critical part of the prefrontal cortex for the precise encoding in working memory of the order of a short sequence of visual nonverbal and non-phonologically coded abstract stimuli. The results are also consistent with the finding that frontal lesions in human subjects that encroach upon the mid-DLPFC impair judgments of the relative order of stimuli in a long series of stimuli (6). Note that, in the latter study, verbalization is of limited value in the coding of the relative order of stimuli.
In conclusion, the present fMRI results, together with the anatomical and neurophysiological evidence referred to above, suggest that the mid-DLPFC is necessary for the monitoring of the precise order of a limited number of visual stimuli. The availability of such detailed information in working memory is central to the ability to manipulate “in the mind” the order of stimuli and, therefore, to plan a series of steps of action which can then be translated (if necessary) into movement by the premotor/motor regions of the cortex (32–34). Areas 46 and 9/46, which monitor serial order in current awareness, have strong connections with premotor cortical areas (15, 35) where the sequence of motor action must then be coded. Interestingly, lesions of area 8 and premotor area 6 do not impair the entry into working memory, after a single exposure, of the serial order of visual stimuli (3), a finding that further highlights the specificity of the contribution of the mid-DLPFC in monitoring and manipulating order in the mind versus the motor programs that will ultimately translate high-level cognition into action.
Methods
Subjects.
Seventeen right-handed normal human subjects (8 females and 9 males, mean age = 26.8 ± 2.8 years) participated in the present fMRI study after informed, written consent according to the guidelines established by the Ethics Committee of the Montreal Neurological Hospital and Institute. The subjects were trained on the SOMT and the CT 1 day before the scanning session until they reached at least 85% correct responses in the middle positions and end positions of the SOMT and in the CT.
Stimuli and Testing Procedure During Scanning.
The stimuli were abstract black and white designs to discourage the use of verbal mediation. In addition, the subjects were instructed not to use verbal mediation during the performance of the task. On each trial of the SOMT, four new abstract visual stimuli were presented successively on the screen for 1,000 ms each, followed by a test display during which two of these stimuli were shown simultaneously (Fig. 1) and the subject had to indicate, by pressing the appropriate response key, which one of the two stimuli occurred earlier in the sequence. In a random 50% of the test displays, the first and the last stimuli of the sequence were presented (i.e., end positions in the SOMT), and in the remaining 50%, the second and the third stimuli of the sequence were presented (i.e., middle positions in the SOMT). In the CT, again four new abstract visual stimuli were presented successively, but in the test display one of these stimuli was shown together with a new stimulus and the subject had to indicate which one was the stimulus presented in the sequence by pressing the appropriate response key (Fig. 1). In a random 50% of the test displays, the first or the last stimulus of the sequence was presented together with a new stimulus (i.e., end positions in the CT), and in the remaining 50%, the second or the third stimulus of the sequence was shown together with a new stimulus (i.e., middle positions in the CT). Because of the abstract nonverbal nature of the stimuli, the instruction given to the subjects not to attempt to verbalize the stimuli, and because new stimuli were used in all of the trials of both the SOMT and the CT, the influence of verbalization was minimized in the present experiment. The subjects confirmed during the debriefing after the experiment that they had not attempted to verbalize these constantly changing nonverbal stimuli.
In one condition, the stimuli were abstract designs occupying the whole image and, in the other condition, they were smaller abstract designs embedded in a uniform abstract design background. In the example in Fig. 1a, the embedded abstract figures were abstract line drawings (squiggles). The embedded stimuli were different from trial to trial. The difference in the overall physical features of the stimuli (i.e., whether they occupied the whole image or were embedded in a uniform background) enabled the subjects to know which task they were performing at the beginning of each trial. Note that, for 9 of the 17 subjects, the overall physical features of the stimuli indicating the SOMT and the CT corresponded to the example displayed in Fig. 1a and, for the other eight subjects, the overall physical features of the stimuli indicating the SOMT and the CT were reversed.
FMRI Scanning and Data Analysis.
Each normal volunteer was scanned by using a 1.5 T Siemens Sonata MRI Scanner (Siemens AG, Erlangen, Germany). After a high-resolution T1 anatomical scan (whole head, 1-mm3 isotropic resolution), six functional runs of 248 images each [38 oblique T2* gradient echoplanar images, voxel size = 3.4 × 3.4 × 3.4 mm, repetition time (TR) = 3.5 s, echo time (TE) = 45 ms, flip angle = 90°] sensitive to the BOLD signal were acquired. The repetition time of 3.5 s enabled us to scan the entire brain with the voxel resolution described above. The onset of the first trial in each run was synchronized with the scanner acquisition via a trigger signal generated by the scanner. Behavioral and imaging data were acquired in all trials. In each functional run, the subjects performed 12 SOMT (i.e., six “end positions SOMT” and six “middle positions SOMT”) and 12 CT trials. Stimulus presentation and the recording of motor responses were computer controlled and were programmed with E-prime software, Version 1.1 (Psychology Software Tools, Pittsburgh, PA).
Images from all runs were first realigned with an AFNI image registration software, using the third frame of the first run as a reference (36), and then smoothed with an in-house MINC blurring software (mincblur), using a 6-mm FWHM isotropic Gaussian kernel. Subsequently, all images were nonlinearly transformed into the MNI stereotaxic space, using in-house dedicated software (37). The nonlinear transform was estimated on MRI data blurred with an 8-mm FWHM Gaussian kernel and a 3D lattice grid with 4-mm spacing between nodes. The data analysis was performed by using fmristat (available at www.math.mcgill.ca/keith/fmristat) (38) [see supporting information (SI) Text for additional information on the statistical fMRI data analysis).
Supplementary Material
Acknowledgments
We thank C. Chapados for technical help. This work was supported by Canadian Institutes of Health Research (CIHR) Grant FRN 37753 (to M.P.) and a CIHR fellowship (to C.A.).
Abbreviations
- ACC
anterior cingulate cortex
- BOLD
blood oxygenation level dependent
- CT
control task
- DLPFC
dorsolateral prefrontal cortex
- fMRI
functional magnetic resonance imaging
- SOMT
serial-order memory task
- VLPFC
ventrolateral prefrontal cortex.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706220104/DC1.
References
- 1.Baddeley A. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 2.Cowan N. Psychol Bull. 1988;104:163–191. doi: 10.1037/0033-2909.104.2.163. [DOI] [PubMed] [Google Scholar]
- 3.Petrides M. Proc R Soc London Ser B. 1991;246:299–306. doi: 10.1098/rspb.1991.0158. [DOI] [PubMed] [Google Scholar]
- 4.Butters MA, Kaszniak AW, Glisky EL, Eslinger PJ, Schacter DL. Neuropsychology. 1994;8:343–353. [Google Scholar]
- 5.Kesner RP, Hopkins RO, Fineman B. Neuropsychologia. 1994;32:881–891. doi: 10.1016/0028-3932(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 6.Milner B, Corsi P, Leonard G. Neuropsychologia. 1991;29:601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- 7.Shimamura AP, Janowsky JS, Squire LR. Neuropsychologia. 1990;28:803–814. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- 8.Eyler Zorrilla LT, Aguirre GK, Zarahan E, Cannon TD, D'Esposito M. NeuroReport. 1996;7:2803–2806. doi: 10.1097/00001756-199611040-00079. [DOI] [PubMed] [Google Scholar]
- 9.Konishi S, Uchida I, Okuaki T, Machida T, Shirouzu I, Miyashita Y. J Neurosci. 2002;22:9549–9555. doi: 10.1523/JNEUROSCI.22-21-09549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrides M, Pandya DN. In: The Frontal Cortex. Paxinos G, Mai JK, editors. San Diego: Elsevier Academic; 2004. pp. 950–972. [Google Scholar]
- 11.Bor D, Owen AM. Cereb Cortex. 2007;17:778–786. doi: 10.1093/cercor/bhk035. [DOI] [PubMed] [Google Scholar]
- 12.Petrides M. J Neurosci. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue M, Mikami A. J Neurophysiol. 2006;95:1008–1041. doi: 10.1152/jn.00552.2005. [DOI] [PubMed] [Google Scholar]
- 14.Ninokura Y, Mushiake H, Tanji J. J Neurophysiol. 2004;91:555–560. doi: 10.1152/jn.00694.2003. [DOI] [PubMed] [Google Scholar]
- 15.Barbas H, Pandya DN. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 16.Morris R, Pandya DN, Petrides M. J Comp Neurol. 1999;407:183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Mufson EJ, Pandya DN. J Comp Neurol. 1984;225:31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- 18.Sanides F. Die Architektonik des Menschlichen Stirnhirns. Berlin: Springer; 1962. [Google Scholar]
- 19.Rushworth MF, Hadland KA, Gaffan D, Passingham RE. J Cognit Neurosci. 2003;15:338–353. doi: 10.1162/089892903321593072. [DOI] [PubMed] [Google Scholar]
- 20.Procyk E, Joseph JP. Eur J Neurosci. 2001;14:1041–1046. doi: 10.1046/j.0953-816x.2001.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown GDA, Preece T, Hulme C. Psych Rev. 2000;107:127–181. doi: 10.1037/0033-295x.107.1.127. [DOI] [PubMed] [Google Scholar]
- 22.Burgess N, Hitch GJ. Psych Rev. 1999;106:551–581. [Google Scholar]
- 23.Henson RNA. Cog Psych. 1998;36:73–137. doi: 10.1006/cogp.1998.0685. [DOI] [PubMed] [Google Scholar]
- 24.Howard MW, Kahana MJ. J Exp Psychol Learn Mem Cognit. 1999;25:923–941. doi: 10.1037//0278-7393.25.4.923. [DOI] [PubMed] [Google Scholar]
- 25.Vallar G, Di Betta AM, Silveri MC. Neuropsychologia. 1997;35:795–812. doi: 10.1016/s0028-3932(96)00127-3. [DOI] [PubMed] [Google Scholar]
- 26.Warrington EK, Logue V, Pratt RT. Neuropsychologia. 1971;9:377–387. doi: 10.1016/0028-3932(71)90002-9. [DOI] [PubMed] [Google Scholar]
- 27.Henson RNA, Burgess N, Frith CD. Neuropsychologia. 2000;38:426–440. doi: 10.1016/s0028-3932(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 28.Marshuetz C, Smith EE, Jonides J, DeGutis J, Chenevert TL. J Cognit Neurosci. 2000;12:130–144. doi: 10.1162/08989290051137459. [DOI] [PubMed] [Google Scholar]
- 29.Paulesu R, Frith CD, Frackowiak RSJ. Nature. 1993;362:323–330. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 30.Ninokura Y, Mushiake H, Tanji J. J Neurophysiol. 2003;89:2868–2873. doi: 10.1152/jn.00647.2002. [DOI] [PubMed] [Google Scholar]
- 31.Milner B. In: Analysis of Behavioral Change. Weiskrantz L, editor. New York: Harper & Row; 1968. pp. 328–375. [Google Scholar]
- 32.Carpenter AF, Georgopoulos AP, Pellizzer G. Science. 1999;283:1752–1757. doi: 10.1126/science.283.5408.1752. [DOI] [PubMed] [Google Scholar]
- 33.Ohbayashi M, Ohki K, Miyashita y. Science. 2003;301:233–236. doi: 10.1126/science.1084884. [DOI] [PubMed] [Google Scholar]
- 34.Shima K, Tanji J. J Neurophysiol. 1998;80:3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- 35.Petrides M, Pandya DN. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 36.Cox RW, Jesmanowicz A. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 37.Collins DL, Neelin P, Peters TM, Evans AC. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 38.Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. NeuroImage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.