Abstract
Although Parkinson's disease (PD) is characterized primarily by loss of nigrostriatal dopaminergic neurons, there is a concomitant loss of norepinephrine (NE) neurons in the locus coeruleus. Dopaminergic lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) are commonly used to model PD, and although MPTP effectively mimics the dopaminergic neuropathology of PD in mice, it fails to produce PD-like motor deficits. We hypothesized that MPTP is unable to recapitulate the motor abnormalities of PD either because the behavioral paradigms used to measure coordinated behavior in mice are not sensitive enough or because MPTP in the absence of NE loss is insufficient to impair motor control. We tested both possibilities by developing a battery of coordinated movement tests and examining motor deficits in dopamine β-hydroxylase knockout (Dbh−/−) mice that lack NE altogether. We detected no motor abnormalities in MPTP-treated control mice, despite an 80% loss of striatal dopamine (DA) terminals. Dbh−/− mice, on the other hand, were impaired in most tests and also displayed spontaneous dyskinesias, despite their normal striatal DA content. A subset of these impairments was recapitulated in control mice with 80% NE lesions and reversed in Dbh−/− mice, either by restoration of NE or treatment with a DA agonist. MPTP did not exacerbate baseline motor deficits in Dbh−/− mice. Finally, striatal levels of phospho-ERK-1/2 and ΔFosB/FosB, proteins which are associated with PD and dyskinesias, were elevated in Dbh−/− mice. These results suggest that loss of locus coeruleus neurons contributes to motor dysfunction in PD.
Keywords: dopamine, Parkinson's disease, dyskinesias, dopamine β-hydroxylase
Parkinson's disease (PD) affects ≈1% of the world's aging population (1). Despite this high prevalence and intensive research into its origins, the etiology of PD remains largely unknown. The disease is characterized by degeneration of dopamine (DA) neurons in the substantia nigra pars compacta (SN), and symptoms, which tend to manifest when ≈80% of striatal DA is lost, include bradykinesia, postural instability, rigidity, and resting tremor. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin, is known to produce parkinsonism in humans and causes severe DA loss in animals (2). Because of its ability to recapitulate the neuropathology of PD, MPTP is used widely in PD research. However, MPTP has been unable to reliably reproduce the motor symptoms of PD in mice, which limits the utility of MPTP-treated mice as an animal model of the disease (3). Differences in mouse strain and experimental paradigms may at least partially account for these inconsistencies.
Despite the focus on DA, PD is more accurately described as a multisystem disorder that features a profound albeit underappreciated loss of locus coeruleus (LC) neurons, as well as variable damage to other brain regions (4–6). Postmortem studies indicate that neuronal degeneration in the LC is comparable to that in the substantia nigra pars compacta, and that it may actually precede DA degeneration in PD (5, 7, 8). LC lesions exacerbate PD neuropathology and behavioral symptoms in animal models, suggesting that the noradrenergic system may partially compensate for DA loss (9–11). Therefore, a model that simultaneously mimics both the NE and DA deficits seen in PD could be of enormous value.
We hypothesized that the lack of reproducible motor deficits in MPTP-treated mice could be attributed to either insufficiently sensitive behavioral tests or the fact that MPTP alone does not faithfully reproduce all of the multisystem features of PD, particularly NE loss. We investigated these alternative explanations in two ways. First, we used a combination of motor-coordination tests, both traditional (pole test and stride length measurement) and newer (challenging beam traversal, grid test, automated gait analysis, and abnormal involuntary movements), in an exhaustive effort to detect abnormalities in MPTP-treated mice. Second, we screened for motor deficits in Dbh−/− mice that lack NE and in Dbh−/− mice treated with MPTP.
Results
An Absence of Motor Impairment in MPTP-Treated Mice.
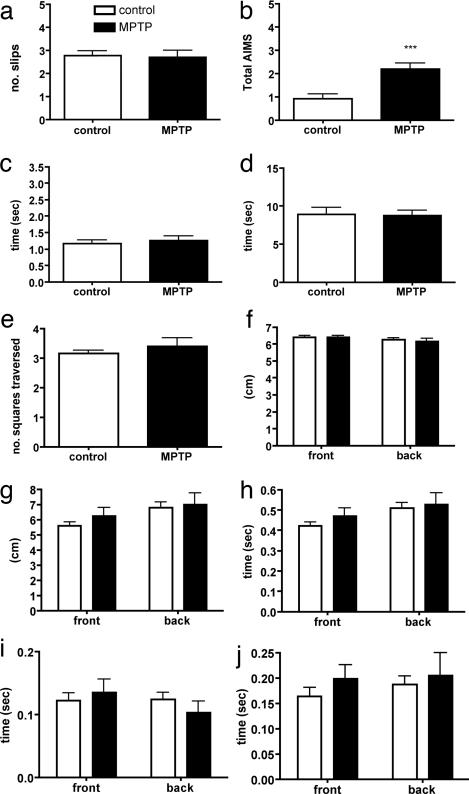
Our efforts to detect motor abnormalities after MPTP treatment entailed testing 10- to 12-month-old mice in six different paradigms designed specifically to assess motor behavior, coordination, and balance. These included the pole test, challenging beam traversal, automated gait analysis, stride length measurement, grid test, and spontaneous dyskinesias [abnormal involuntary movements (AIMs) scoring]. In accord with many previous reports, we were unable to detect impairments on any of these tests in MPTP-treated control mice, despite a dramatic loss of striatal DA, except for an occasional AIMs parameter (P < 0.001, Table 1 and Fig. 1).
Table 1.
Neurochemical analysis of MPTP, DSP4, and 6OHDA lesions
| Treatment | DA in striatum, ng/mg | DOPAC in striatum, ng/mg | DAT in striatum, μg | NE in HC/FC, ng/mg | NE in heart, ng/ml |
|---|---|---|---|---|---|
| Control | 19.82 ± 0.34 | 1.66 ± 0.34 | 15.02 ± 0.72 | HC 1.58 ± 0.33 | 0.14 ± 0.04 |
| FC 0.48 ± 0.03 | |||||
| MPTP | 4.22 ± 0.73*** | 2.17 ± 0.42 | 3.18 ± 0.87*** | FC 0.42 ± 0.06 | N/A |
| DSP4 | N/A | N/A | N/A | HC 0.29 ± 0.09** | 0.082 ± 0.01 |
| 6OHDA | N/A | N/A | N/A | HC 1.52 ± 0.14 | 0.008 ± 0.01** |
Striatal DA and 3,4-dihyroxyphenylacetic acid (DOPAC), hippocampal (HC) NE, and heart NE levels are shown as measured by HPLC and striatal DA transporter (DAT) as measured by Western blot analysis to confirm MPTP, DSP4, and 6-hydroxydopamine (6OHDA) lesions. All values are in ng/mg of wet tissue weight except DAT, for which tubulin-normalized microgram equivalents are shown. N/A, not applicable. **, P = 0.003 compared with control values; ***, P < 0.0001 compared with control values. Control DA and DOPAC, n = 16; control DAT, n = 14; control HC NE, n = 6; control heart NE, n = 6; MPTP DA and DOPAC, n = 8; MPTP DAT, n = 5; DSP4 HC NE, n = 7; 6OHDA HC NE, n = 6; DSP4 heart NE, n = 4; 6OHDA heart NE, n = 6; control frontal cortex (FC) NE n = 3; MPTP n = 5.
Fig. 1.
An absence of motor impairment in MPTP-treated mice. Shown is the effect of MPTP treatment on the performance of control (Dbh+/−) mice on number of slips on challenging beam traversal (a), frequency of abnormal involuntary movements (b), time to orient down in the pole test (c), time to descend in the pole test (d), number of squares traversed on grid (e), stride length distance (f), automated stride length distance (g), stride time (h), brake time (i), and propel time (j). Automated gait analysis results of front and back paws are shown in g–j. Control, n = 24–36; MPTP, n = 16. ***, P < 0.0001 compared with control.
Dbh−/− Mice Have Numerous Abnormalities in Coordinated Movement.
To determine whether concurrent loss of NE is required for MPTP-induced motor deficits, we tested 10- to 12-month-old Dbh−/− mice in the same six behavioral paradigms both at baseline and after MPTP treatment. Surprisingly, untreated Dbh−/− mice were impaired on many tests. Dbh−/− mice slipped more often through the grid than control mice in the challenging beam traversal, took longer to orient down and descend in the pole test, and showed a decreased back-paw brake time according to gait analysis (Fig. 2 and data not shown) (gait F1,70 = 7.976, P = 0.0062). In addition, Dbh−/− mice had higher AIMs scores than saline- or MPTP-treated control mice (Fig. 2b). Specifically, they exhibited greater axial and limb abnormality scores, occasionally displayed a severe resting paw tremor, and demonstrated an abnormal curved posture during sitting and traversal during the challenging beam traversal [compare control mouse in supporting information (SI) Movie 1 to Dbh−/− mouse in SI Movie 2]. MPTP treatment did not exacerbate any phenotypes in Dbh−/− mice (data not shown).
Fig. 2.
Motor impairment in Dbh−/− mice. Shown is the performance of Dbh−/− mice compared with control Dbh+/− mice on challenging beam traversal (a), frequency of abnormal involuntary movements (b), time to orient down in the pole test (c), and time to descend in the pole test (d). Control, n = 36; Dbh−/−, n = 37. **, P < 0.001 compared with control; ***, P < 0.0001 compared with control.
To determine whether the age of the mice had an impact on motor performance, we also tested young (≈3 months old) control and Dbh−/− mice (SI Fig. 6). We found that there was a modest age-related decline in performance in old control mice, particularly for the beam traversal and AIMs scores. Young Dbh−/− mice exhibited a trend toward worse performance compared with young controls, but the differences were not significant. Given the profound impairment in the performance of old Dbh−/− mice compared with controls, these results indicate the existence of an interaction between NE loss and age in the development of deficits in coordinated movement.
LC Lesions in Control Mice Partially Recapitulate Dbh−/− Phenotypes.
One caveat when comparing Dbh−/− mice to MPTP-treated mice is that the former experience a complete and lifelong NE loss, whereas the latter have an 80% adult-onset DA loss. To produce a better comparative group that more closely mimics the LC loss observed in clinical PD, we induced an ≈80% NE lesion in adult control mice by using N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP4; 50 mg/kg, i.p.; Table 1), which selectively lesions LC neurons. Coordinated movement was tested 1 week later (to allow time for the lesions to stabilize). DSP4 lesions in control mice partially recapitulated the Dbh−/− motor phenotypes. DSP4-lesioned mice displayed motor impairments on the challenging beam traversal (F2,60 = 10.18, P = 0.0002, Fig. 3a) and had high AIMs scores (F2,60 = 11.09, P < 0.0001), reminiscent of the Dbh−/− mice (Fig. 3b), but they did not show similar deficits on the pole test (Fig. 3 c and d). The higher AIMs scores in DSP4-treated mice were primarily attributable to axial disturbances and did not include the dramatic resting-paw tremor seen in Dbh−/− mice. Importantly, DSP4 lesioned only central NE. There was a trend toward decreased heart NE, but it was not significant (Table 1). An additional group of mice given a selective peripheral NE lesion with 6-hydroxydopamine (6OHDA; 100 mg/kg, i.p., Table 1) did not demonstrate motor impairment on the challenging beam traversal (Fig. 3e).
Fig. 3.
LC lesions in control mice partially recapitulate Dbh−/− phenotypes. Shown is the performance of DSP4-treated control (Dbh+/−) mice compared with control and Dbh−/− mice on challenging beam traversal (a), frequency of abnormal involuntary movements (b), time to orient down in the pole test (c), and time to descend in the pole test (d). (e) Control mice and mice given peripheral 6OHDA lesions did not differ in number of slips on challenging beam traversal (Student's two-tailed, unpaired t test). Control, n = 36; Dbh−/−, n = 37; DSP4-lesioned control mice, n = 9. For 6OHDA experiment, n = 5 for control and n = 6 for 6OHDA-lesioned mice. *, P < 0.05 compared with untreated control; **, P < 0.01 compared with untreated control; ***, P < 0.001 compared with untreated control; †, P < 0.05 compared with Dbh−/− mice; ††, P < 0.01 compared with Dbh−/− mice.
NE Replacement in Dbh−/− Mice.
We pharmacologically restored central NE to Dbh−/− mice by administering l-3,4-dihydroxyphenylserine (DOPS) + benserazide (see Materials and Methods). Acute NE restoration significantly improved the performance of Dbh−/− mice on the challenging beam traversal (F2,57 = 10.77, P < 0.0001; Fig. 4a) but not on the pole test (Fig. 4 c and d), and actually increased AIMs (F2,57 = 17.61, P < 0.0001) (Fig. 4b).
Fig. 4.
NE replacement in Dbh−/− mice partially restores motor coordination but exacerbates dyskinesias. Shown is the effect of NE replacement with DOPS + benserazide (labeled DOPS) on performance of Dbh−/− mice on challenging beam traversal (a), frequency of abnormal involuntary movements (b), time to orient down in the pole test (c), and time to descend in the pole test (d). Control, n = 36; Dbh−/−, n = 37; Dbh−/− given DOPS + benserazide, n = 7. *, P < 0.05 compared with control; ***, P < 0.001 compared with control; †, P < 0.05 compared with untreated Dbh−/−; ††, P < 0.01 compared with untreated Dbh−/− mice.
Stimulation of DA Receptors Improves Coordinated Movement in Dbh−/− Mice.
Despite increased tissue levels of DA, Dbh−/− mice have decreased basal and evoked extracellular striatal DA (12). We hypothesized that the motor impairments seen in Dbh−/− mice could stem from their hypodopaminergic state. To test this theory, we attempted to restore normal motor function in Dbh−/− mice by administering the D1/D2 agonist apomorphine (0.5 mg/kg). Apomorphine significantly improved performance on the challenging beam traversal (F2,76 = 10.74, P < 0.0001, Fig. 5a) and tended to improve the time to orient down but not the time to descend, in the pole test (Fig. 5 b and c).
Fig. 5.
Stimulation of DA receptors improves motor behavior in Dbh−/− mice. Shown is the effect of the D1/D2 agonist apomorphine (APO) on performance of Dbh−/− mice on challenging beam traversal (a), time to orient down in the pole test (b), and time to descend in the pole test (c). Control, n = 36; Dbh−/−, n = 37; Dbh−/− mice given apomorphine, n = 8. **, P < 0.01 compared with control; ***, P < 0.001 compared with control; †, P < 0.05 compared with untreated Dbh−/− mice.
Blockade of DA Receptors Attenuates Dyskinetic Movements in Dbh−/− Mice.
Dbh−/− mice have an increase in high-affinity state striatal DA receptors and are behaviorally hypersensitive to direct and indirect DA agonists (12). Because some studies have suggested that l-dopa-induced dyskinesias are due, in part, to supersensitive DA receptors (13–15), we hypothesized that the increased AIMs seen in Dbh−/− mice were the result of excessive DA signaling events. In support of this idea, we found that a low dose of the D1/D2 receptor antagonist flupenthixole (0.025 mg/kg) significantly reduced AIMs in Dbh−/− mice (Dbh−/− + saline 4.38 ± 0.44, Dbh−/− + flupenthixole 2.83 ± 0.21; P < 0.05, t test).
Dbh−/− Mice Have Increased Striatal Levels of Phospho-ERK-1/2 and ΔFosB/FosB.
Previous studies have correlated the molecular markers ERK-1/2 and ΔFosB/FosB with DA-associated motor deficits and l-dopa-induced dyskinesias (16–18). Consistent with a molecular PD-like phenotype, Western blot analysis revealed that Dbh−/− mice had significantly more striatal phospho-ERK-1/2 and ΔFosB/FosB than control mice (SI Fig. 7). MPTP treatment did not significantly alter phospho-ERK-1/2 or ΔFosB/FosB protein levels (data not shown).
Discussion
PD is characterized by a loss of nigrostriatal DA neurons. MPTP is commonly used to recapitulate the neuropathology of PD in animal models. Unfortunately, MPTP-treated rodents often do not display the behavioral symptoms of PD. We explored whether this problem could be overcome by using a more sensitive behavioral testing battery and/or by developing a more comprehensive model that includes deficits of the NE system using Dbh−/− mice. We were unable to detect motor impairment in MPTP-treated mice by using our test battery, suggesting that an 80% loss of DA alone is insufficient to produce PD-like behavioral deficits in mice. In contrast, Dbh−/− mice demonstrated robust motor impairments on multiple tests. Dbh−/− mice are bradycardic and hypotensive because of peripheral NE and epinephrine (Epi) deficiency (19). However, peripheral NE/Epi depletion and cardiovascular dysfunction cannot account for the motor phenotypes observed in Dbh−/− mice. A subset of these impairments, particularly the challenging beam, were recapitulated in control mice with LC lesions but not peripheral NE/Epi lesions and were reversed by central NE replacement in adult Dbh−/− mice, indicating that a subset of impairments were specifically related to NE deficiency in the brain and not peripheral NE depletion or developmental abnormalities from gene knockout. Some motor deficits were rescued by DA agonists or antagonists, suggesting that dysregulated DA signaling contributed to these phenotypes.
NE Loss Causes Motor Impairment.
We suggest two possible mechanisms for the motor impairments of Dbh−/− and LC-lesioned mice. First, NE may be directly necessary for normal motor control. The cerebellum, which is important for coordinated movement and balance, receives direct noradrenergic input (20). Second, NE is required for the normal firing patterns of DA neurons and striatal DA release. For example, Dbh−/− mice and DSP4-lesioned animals have low basal and evoked striatal DA release (12, 21). Our results suggest that the motor deficits observed on the challenging beam traversal (and aspects of the pole test) are due to a hypodopaminergic state rather than cerebellar abnormalities. These same motor control tests have been used to detect dopaminergic deficits (22, 23), and the impairments in Dbh−/− mice were reversed by apomorphine, a DA agonist. In addition, the DOPS paradigm we used rescues the motor deficits but does not restore cerebellar NE to Dbh−/− mice (24), and selective cerebellar NE depletion in rats does not worsen baseline performance on a motor test nearly identical to the challenging beam traversal (25). These combined data suggest that at least some of the motor impairments observed in Dbh−/− mice stem from a loss of dopaminergic tone, not cerebellar dysfunction. Some motor abnormalities in Dbh−/− mice (e.g., slowness in orienting down the pole) were not recapitulated by DSP4 lesions in control mice, suggesting that NE systems other than the LC (e.g., the ventral noradrenergic bundle) are important in motor control. Nor were all abnormalities rescued by apomorphine, suggesting that noradrenergic regulation of nondopaminergic motor areas is also important. Interestingly, the motor deficits in Dbh−/− mice were apparent only in older mice, indicating that, at least in this model, an interaction between NE loss and age determines behavioral outcome (see SI Fig. 6).
Why, then, does NE depletion cause these phenotypes, whereas an 80% loss of DA does not? When the DA system is lesioned with MPTP, the small number of DA neurons that are spared have a compensatory increase in activity, resulting in normal or even elevated DA turnover [as measured by dihydroxyphenylacetic acid (DOPAC) levels and DA/DOPAC ratio] despite fewer terminals (Table 1) (26, 27). By contrast, because NE is required for DA-neuron firing and DA release, NE-deficient animals (Dbh−/− mice or DSP4-lesioned animals) have very low extracellular DA levels, despite normal tissue DA content in the striatum (12, 24). Thus, the LC neuron loss in PD may exacerbate the motor symptoms associated with DA-neuron loss by disabling the firing capabilities of surviving DA neurons.
An important remaining question is why does MPTP treatment in the absence of LC lesion cause PD-like motor deficits in primates but not mice? One possibility is that basal ganglia function differs between species. However, although it was initially reported that MPTP did not cause LC cell death in nonhuman primates, it has now become clear that typical nonhuman primate MPTP regimens nearly always result in LC cell death and/or loss of NE from terminal fields (28–34). In contrast, typical MPTP dosing regimens cause profound DA depletion with little effect on LC neurons or NE content in mice (10, 35–38). Furthermore, primate models that attempt to create a “pure” dopaminergic loss with intrastriatal/intranigral toxin injections typically produce only sensorimotor deficits and do not recapitulate the cardinal symptoms of PD (e.g., resting tremor and akinesia) (39, 40). The integrity of the noradrenergic system is not commonly addressed in mouse MPTP studies, and we suspect that differences in noradrenergic damage across strains and dosing regimens contribute to the variability in reported behavioral results in MPTP-treated mice. Thus, it appears that the species difference is a result of differential effects of MPTP, not basal ganglia function, and suggests that NE loss is a critical component of PD-like motor deficits in both mice and nonhuman primate MPTP models. One important consideration with most lesion models (e.g., 6OHDA or MPTP) is that because the substantia nigra pars compacta and striatum both receive direct noradrenergic innervation (41, 42), direct infusion of these toxins damages both DA and NE neurons. Therefore, the only way to create a pure DA lesion would be to pretreat animals with a selective NE transporter inhibitor before toxin administration. A recent study in rats has shown that unilateral infusion of 6-OHDA into the medial forebrain bundle (NE and DA loss) produced dyskinesias of earlier onset and greater severity than in rats given a NE transporter inhibitor + 6-OHDA (DA loss alone) (43). It is not our opinion that a noradrenergic loss rather than a DA loss is solely responsible for the behavioral manifestations of PD. A pure DA loss, if extreme enough, can produce motor impairment in mice (44, 45). Rather, our data imply that the loss of NE and DA has a synergistic effect on the emergence of the parkinsonian phenotype, particularly in the early-to-mid stages of the disease when sufficient numbers of DA neurons are intact and attempting to compensate.
Dbh−/− Mice Exhibit Dyskinetic Behavior.
In addition to the deficits in coordinated movement, Dbh−/− mice also demonstrated dyskinetic behavior on the AIMs scale. Dyskinesias are common side effects of long-term l-dopa therapy and may involve changes in DA-receptor function and signaling (14, 46, 47). Although Dbh−/− mice are typically hypodopaminergic, due to decreased DA release, they have more high-affinity state striatal DA receptors and are behaviorally hypersensitive to direct and indirect DA agonists (12, 48). We propose that under “basal” conditions, Dbh−/− mice have very little DA signaling, but on those rare occasions that a burst of DA is released in the striatum, the hypersensitive DA receptors cause a hyperdopaminergic response manifesting as a dyskinetic-like movement. In support of this hypothesis, DSP4 lesions produce both DA-receptor hypersensitivity (49) and dyskinesias (this study), and the D1/D2 antagonist flupenthixole partially attenuates dyskinesias in Dbh−/− mice. NE replacement with DOPS in Dbh−/− mice worsened dyskinesias, probably by acutely restoring normal DA release without normalizing DA-receptor sensitivity. Interestingly, α2-adrenergic-receptor antagonists are moderately effective in decreasing l-dopa-induced dyskinesias, although the mechanism is not understood (50–52).
Molecular Markers of DA Motor Impairment.
There are molecular markers, such as the immediate early genes, ERK-1/2, and ΔFosB/FosB, that correlate with DA lesions and dyskinetic behavior in animal models and postmortem PD brains (17, 53, 54). Because Dbh−/− and DSP4-lesioned mice have abnormalities in DA transmission and display spontaneous dyskinetic-like movements, we examined striatal levels of phospho-ERK-1/2 and ΔFosB/FosB protein. We found that both ERK-1/2 and ΔFosB/FosB were significantly increased in the striatum of Dbh−/− mice. This finding indicates that NE loss promotes not just motor impairment, but also PD-like changes in striatal gene expression, further confirming the importance of an interaction between the NE and DA systems.
Implications for Preclinical and Clinical PD.
Many animal models have failed to simultaneously recapitulate both the neuropathological and behavioral symptoms of PD, perhaps because they model PD as a pure DA deficit, rather than as a multisystem disorder that includes the NE and DA systems. Although PD is characterized by a loss of DA nigrostriatal neurons, there is also a profound loss of noradrenergic LC neurons that rivals the loss of nigral neurons (4, 5, 7, 8). Therefore, the Dbh−/− mouse is a valuable complement to the current mouse PD models for three reasons: it has a dual impairment of NE and DA transmission that more accurately reflects clinical PD, the neurochemical impairments manifest as disturbances in coordination and balance, and the mice have spontaneous dyskinesias and changes in striatal gene expression reminiscent of those produced by chronic l-dopa treatment.
Early detection and treatment of PD is difficult because the motor symptomology does not become evident until 80% of DA neurons are lost, suggesting the existence of an impressive compensatory system. Our results indicate that this system may involve a significant noradrenergic component. Because LC loss coincides with nigral loss during PD progression, the behavioral manifestation of PD symptoms may involve a threshold loss of both DA and NE. Intriguingly, dopamine β-hydroxylase activity in humans is genetically controlled (55). On the basis of our results, we predict that PD patients with genetically low dopamine β-hydroxylase activity may have earlier onset or more severe motor symptoms and may be more prone to developing dyskinesias. In addition, we have shown that increasing extracellular NE protects DA neurons from MPTP toxicity (56), which implies that noradrenergic drugs could have dual therapeutic functions, by both preventing the progression of DA-neuron loss in PD and, when combined with l-dopa therapy, alleviating PD-associated motor deficits.
Materials and Methods
Animals.
We used 3-month-old and 10- to 12-month-old knockout (Dbh−/−) and control (Dbh+/−) mice that were kept under conditions of a 12-hour light/dark cycle, with the light on between 0700 and 1900 hours at a room temperature of 21°C, and food and water available ad libitum. Animals were treated in accordance with National Institutes of Health Intramural Animal Care and Use Program guidelines. The experiments described in this article followed the Emory University Division of Animal Resources' Care and Use of Laboratory Animals guidelines and were approved by the Emory Institutional Animal Care and Use Committee. Data from male and female mice were combined given that there were no detectable sex differences. Because of breeding technicalities, Dbh+/− mice were used as controls, because they have normal brain NE levels and are behaviorally identical to wild-type (Dbh+/+) mice (57, 58). Mice were maintained on a mixed C57BL/6J and 129SvEv background.
Behavioral Measures.
All mice were handled 1 week before testing. Behavioral tests included challenging beam traversal, pole test, stride length, automated gait analysis, grid test, and AIMs. Detailed methods for behavioral tests are available in SI Methods.
Nigrostriatal DA System Lesions.
MPTP (30 mg/kg; Sigma–Aldrich, St. Louis, MO) was administered s.c. twice, with a 12-h interval between injections (60 mg/kg cumulative dose). Saline was used as the vehicle for MPTP and was injected into both control and Dbh−/− mice. This MPTP dosing regimen in these mice resulted in ≈80% loss of striatal DA in HPLC measurements (Table 1). The lesions were allowed to stabilize for 1 week before mice were subjected to behavioral testing.
LC Lesions.
DSP4, which selectively lesions LC neurons, was administered i.p. to mice at doses of 50 mg/kg. DSP4 was dissolved in saline immediately before injection and used within 2 min to avoid degradation. The ensuing lesions were allowed to stabilize for 1 week before behavioral testing. A dose–response measurement was performed by using 30, 40, and 50 mg/kg DSP4 to find a dose that would create a degree of loss to NE terminals that would parallel the loss of DA terminals with our MPTP paradigm (data not shown). According to neurochemical analysis with HPLC, the 50 mg/kg dose of DSP4 typically results in an ≈80% noradrenergic terminal loss (hippocampus), a finding that is in accord with previous groups (59) and was confirmed in our own studies with HPLC analysis (Table 1). This dose did not result in 5-hydroxytryptamine loss or DA loss and was specific to the noradrenergic system, also in agreement with Fornai et al. (9). Furthermore, this dose of DSP4 did not significantly reduce peripheral (heart tissue) NE (Table 1).
Chemical Sympathectomy.
Control mice (Dbh+/−) 15 months old were injected with 100 mg/kg 6OHDA (coinjected i.p. with 0.01% vitamin C to prevent oxidation) to induce a chemical sympathectomy, and mice were tested on the challenging beam traversal 7 days after 6OHDA administration. 6OHDA is unable to cross the blood–brain barrier and, therefore, specifically lesions the peripheral noradrenergic system without affecting brain catecholamines. Lesions were confirmed with HPLC electrochemical detection by analyzing heart NE content as positive control and brain hippocampal NE as a negative control. This regimen resulted in ≈90% loss of peripheral but not central NE/Epi (60, 61) (Table 1).
NE Replacement in Dbh−/− Mice.
Central NE was acutely restored in Dbh−/− mice with DOPS and benserazide administration (24). DOPS can be converted to NE by aromatic acid decarboxylase, and benserazide is a peripheral aromatic acid decarboxylase inhibitor that restricts NE production to the brain. Mice were injected s.c. with DOPS (0.5 mg/g; Dainappon–Sumitomo Pharmaceuticals, Osaka, Japan) and benserazide (0.125 mg/g; Sigma–Aldrich), then behaviorally tested 5 h later, once maximal NE levels were attained. This dose of DOPS has previously been shown to restore NE in the brain to 11–26% of control levels in the frontal cortex, occipital cortex, brainstem, hippocampus, olfactory bulb, hypothalamus, thalamus, colliculi, amygdala, striatum, and midbrain. However, NE is not restored to the cerebellum at this dosage. In addition, Epi is also not restored in most brain regions, including the striatum and brainstem.
Administration of DA-Receptor Agonists/Antagonists.
Vehicle (0.9% NaCl) and either the mixed D1/D2 agonist apomorphine (0.5 mg/kg; Sigma–Aldrich) or the D1/D2 antagonist flupenthixole (0.025 mg/kg; Sigma–Aldrich) was administered i.p. to Dbh−/− mice 30 min before behavioral testing. Dose–response experiments were performed to select doses that minimized locomotor effects in control mice (data not shown).
Neurochemical Analysis.
HPLC with electrochemical detection was used to determine striatal DA, DOPAC, and frontal cortical, hippocampal, and cerebellar and heart NE. Details are available in SI Methods.
Data Analysis.
All data were analyzed by using Prism 3.0 software (GraphPad, San Diego, CA). Control vs. Dbh−/− mice and control vs. MPTP-treated mice were compared with two-tailed Student's t tests for behavior and Western blot analysis. One-way ANOVA with Bonferroni posthoc analyses was used to compare control and Dbh−/− mice with DSP4, DOPS, 6OHDA, apomorphine, or flupenthixole treatment. Two-way ANOVA with Bonferroni posthoc analysis was used to compare genotype and paws (front and back) for gait analysis parameters. The same untreated controls and Dbh−/− mice were used for comparisons with each treatment.
Supplementary Material
Acknowledgments
We thank T. Guillot for technical assistance with the gait analysis, C. Strauss for critical reading of the manuscript and helpful suggestions, and Dainappon–Sumitomo Pharmaceuticals for their generous donation of DOPS. This work was funded in part by Public Health Service National Institutes of Health Grant U54 ES012068.
Abbreviations
- PD
Parkinson's disease
- NE
norepinephrine
- LC
locus coeruleus
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- DA
dopamine
- AIMs
abnormal involuntary movements
- Epi
epinephrine
- DOPS
l-3,4-dihydroxyphenylserine
- 6OHDA
6-hydroxydopamine
- DSP4
N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702753104/DC1.
References
- 1.de Lau LM, Breteler MM. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Langston JW. Life Sci. 1985;36:201–206. doi: 10.1016/0024-3205(85)90059-1. [DOI] [PubMed] [Google Scholar]
- 3.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exp Neurol. 2002;178:80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- 4.Marien MR, Colpaert FC, Rosenquist AC. Brain Res Brain Res Rev. 2004;45:38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 6.Cash R, Dennis T, L'Heureux R, Raisman R, Javoy-Agid F, Scatton B. Neurology. 1987;37:42–46. doi: 10.1212/wnl.37.1.42. [DOI] [PubMed] [Google Scholar]
- 7.Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 8.Zarow C, Lyness SA, Mortimer JA, Chui HC. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 9.Fornai F, Bassi L, Bonaccorsi I, Giorgi F, Corsini GU. Funct Neurol. 1997;12:193–198. [PubMed] [Google Scholar]
- 10.Marien M, Briley M, Colpaert F. Eur J Pharmacol. 1993;236:487–489. doi: 10.1016/0014-2999(93)90489-5. [DOI] [PubMed] [Google Scholar]
- 11.Mavridis M, Colpaert FC, Millan MJ. Brain Res. 1991;562:216–224. doi: 10.1016/0006-8993(91)90624-5. [DOI] [PubMed] [Google Scholar]
- 12.Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D. Neuropsychopharmacology. 2006;31:2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- 13.de la Fuente-Fernandez R, Schulzer M, Mak E, Calne DB, Stoessl AJ. Brain. 2004;127:888–899. doi: 10.1093/brain/awh102. [DOI] [PubMed] [Google Scholar]
- 14.Gerfen CR. Neuroscientist. 2003;9:455–462. doi: 10.1177/1073858403255839. [DOI] [PubMed] [Google Scholar]
- 15.Nutt JG. Exp Neurol. 2003;184:9–13. doi: 10.1016/s0014-4886(03)00304-2. [DOI] [PubMed] [Google Scholar]
- 16.Gerfen CR, Miyachi S, Paletzki R, Brown P. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavon N, Martin AB, Mendialdua A, Moratalla R. Biol Psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Otano I, Mandelzys A, Morgan JI. Brain Res Mol Brain Res. 1998;53:41–52. doi: 10.1016/s0169-328x(97)00269-6. [DOI] [PubMed] [Google Scholar]
- 19.Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Am J Physiol. 2004;286:R108–R113. doi: 10.1152/ajpregu.00405.2003. [DOI] [PubMed] [Google Scholar]
- 20.Moises HC, Waterhouse BD, Woodward DJ. Brain Res Bull. 1983;10:795–804. doi: 10.1016/0361-9230(83)90211-3. [DOI] [PubMed] [Google Scholar]
- 21.Lategan AJ, Marien MR, Colpaert FC. Life Sci. 1992;50:995–999. doi: 10.1016/0024-3205(92)90093-5. [DOI] [PubMed] [Google Scholar]
- 22.Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa N, Hirose Y, Ohara S, Ono T, Watanabe Y. Res Commun Chem Pathol Pharmacol. 1985;50:435–441. [PubMed] [Google Scholar]
- 24.Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- 25.Watson M, McElligott JG. Brain Res. 1984;296:129–138. doi: 10.1016/0006-8993(84)90518-3. [DOI] [PubMed] [Google Scholar]
- 26.Rousselet E, Joubert C, Callebert J, Parain K, Tremblay L, Orieux G, Launay JM, Cohen-Salmon C, Hirsch EC. Neurobiol Dis. 2003;14:218–228. doi: 10.1016/s0969-9961(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 27.Spooren WP, Vassout A, Waldmeier P, Gentsch C. Eur J Pharmacol. 1998;353:1–4. doi: 10.1016/s0014-2999(98)00382-3. [DOI] [PubMed] [Google Scholar]
- 28.Forno LS, Langston JW, DeLanney LE, Irwin I, Ricaurte GA. Ann Neurol. 1986;20:449–455. doi: 10.1002/ana.410200403. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell IJ, Cross AJ, Sambrook MA, Crossman AR. Neurosci Lett. 1985;61:195–200. doi: 10.1016/0304-3940(85)90424-0. [DOI] [PubMed] [Google Scholar]
- 30.Miyoshi R, Kito S, Ishida H, Katayama S. Res Commun Chem Pathol Pharmacol. 1988;62:93–102. [PubMed] [Google Scholar]
- 31.Mavridis M, Degryse AD, Lategan AJ, Marien MR, Colpaert FC. Neuroscience. 1991;41:507–523. doi: 10.1016/0306-4522(91)90345-o. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka J, Nakamura H, Honda S, Takada K, Kato S. Acta Neuropathol (Berlin) 1988;75:370–376. doi: 10.1007/BF00687790. [DOI] [PubMed] [Google Scholar]
- 33.Herrero MT, Hirsch EC, Kastner A, Ruberg M, Luquin MR, Laguna J, Javoy-Agid F, Obeso JA, Agid Y. Neuroscience. 1993;56:499–511. doi: 10.1016/0306-4522(93)90349-k. [DOI] [PubMed] [Google Scholar]
- 34.Forno LS, DeLanney LE, Irwin I, Langston JW. Adv Neurol. 1993;60:600–608. [PubMed] [Google Scholar]
- 35.Fornai F, Alessandri MG, Torracca MT, Bassi L, Corsini GU. J Pharmacol Exp Ther. 1997;283:100–107. [PubMed] [Google Scholar]
- 36.Archer T, Fredriksson A. J Neural Transm. 2006;113:1119–1129. doi: 10.1007/s00702-005-0402-5. [DOI] [PubMed] [Google Scholar]
- 37.German DC, Liang CL, Manaye KF, Lane K, Sonsalla PK. Neuroscience. 2000;101:1063–1069. doi: 10.1016/s0306-4522(00)00385-7. [DOI] [PubMed] [Google Scholar]
- 38.Gupta M, Felten DL, Gash DM. Brain Res Bull. 1984;13:737–742. doi: 10.1016/0361-9230(84)90234-x. [DOI] [PubMed] [Google Scholar]
- 39.Eslamboli A, Baker HF, Ridley RM, Annett LE. Exp Neurol. 2003;183:418–429. doi: 10.1016/s0014-4886(03)00139-0. [DOI] [PubMed] [Google Scholar]
- 40.Pechadre JC, Larochelle L, Poirier LJ. J Neurol Sci. 1976;28:147–157. doi: 10.1016/0022-510x(76)90100-3. [DOI] [PubMed] [Google Scholar]
- 41.Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR. Synapse. 2004;52:233–244. doi: 10.1002/syn.20023. [DOI] [PubMed] [Google Scholar]
- 42.Udenfriend S, Creveling CR. J Neurochem. 1959;4:350–352. doi: 10.1111/j.1471-4159.1959.tb13216.x. [DOI] [PubMed] [Google Scholar]
- 43.Fulceri F, Biagioni F, Ferrucci M, Lazzeri G, Bartalucci A, Galli V, Ruggieri S, Paparelli A, Fornai F. Brain Res. 2007;1135:219–229. doi: 10.1016/j.brainres.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 44.Hwang DY, Fleming SM, Ardayfio P, Moran-Gates T, Kim H, Tarazi FI, Chesselet MF, Kim KS. J Neurosci. 2005;25:2132–2137. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou QY, Palmiter RD. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 46.Kovoor A, Seyffarth P, Ebert J, Barghshoon S, Chen CK, Schwarz S, Axelrod JD, Cheyette BN, Simon MI, Lester HA, Schwarz J. J Neurosci. 2005;25:2157–2165. doi: 10.1523/JNEUROSCI.2840-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Opacka-Juffry J, Ashworth S, Ahier RG, Hume SP. J Neural Transm. 1998;105:349–364. doi: 10.1007/s007020050063. [DOI] [PubMed] [Google Scholar]
- 48.Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD. Proc Natl Acad Sci USA. 2002;99:13873–13877. doi: 10.1073/pnas.212519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harro J, Merikula A, Lepiku M, Modiri AR, Rinken A, Oreland L. Pharmacol Toxicol. 2000;86:197–202. doi: 10.1034/j.1600-0773.2000.d01-35.x. [DOI] [PubMed] [Google Scholar]
- 50.Rascol O, Arnulf I, Peyro-Saint Paul H, Brefel-Courbon C, Vidailhet M, Thalamas C, Bonnet AM, Descombes S, Bejjani B, Fabre N, et al. Mov Disord. 2001;16:708–713. doi: 10.1002/mds.1143. [DOI] [PubMed] [Google Scholar]
- 51.Archer T, Fredriksson A. J Neural Transm. 2003;110:183–200. doi: 10.1007/s00702-002-0777-5. [DOI] [PubMed] [Google Scholar]
- 52.Domino EF, Ni L, Colpaert F, Marien M. Recept Channels. 2003;9:335–338. doi: 10.3109/713745180. [DOI] [PubMed] [Google Scholar]
- 53.Lundblad M, Picconi B, Lindgren H, Cenci MA. Neurobiol Dis. 2004;16:110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Lundblad M, Usiello A, Carta M, Hakansson K, Fisone G, Cenci MA. Exp Neurol. 2005;194:66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, Kim KS, Kim CH, Malison RT, Gelernter J, Cubells JF. Am J Hum Genet. 2001;68:515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rommelfanger KS, Weinshenker D, Miller GW. J Neurochem. 2004;91:1116–1124. doi: 10.1111/j.1471-4159.2004.02785.x. [DOI] [PubMed] [Google Scholar]
- 57.Thomas SA, Matsumoto AM, Palmiter RD. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 58.Bourdelat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, Liles LC, Weinshenker D. Psychopharmacology (Berlin) 2005;183:72–80. doi: 10.1007/s00213-005-0139-8. [DOI] [PubMed] [Google Scholar]
- 59.Fornai F, Giorgi FS, Gesi M, Chen K, Alessri MG, Shih JC. Synapse. 2001;39:213–221. doi: 10.1002/1098-2396(20010301)39:3<213::AID-SYN1002>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 60.Kruszewska B, Felten SY, Moynihan JA. J Immunol. 1995;155:4613–4620. [PubMed] [Google Scholar]
- 61.Song DK, Im YB, Jung JS, Suh HW, Huh SO, Park SW, Wie MB, Kim YH. J Neurochem. 1999;72:1625–1633. doi: 10.1046/j.1471-4159.1999.721625.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.