Abstract
Increased tissue factor (TF)-dependent procoagulant activity in sepsis may be partly due to decreased expression or function of tissue factor pathway inhibitor (TFPI). To test this hypothesis, baboons were infused with live Escherichia coli and sacrificed after 2, 8, or 24 hours. Confocal and electron microscopy revealed increased leukocyte infiltration and fibrin deposition in the intravascular and interstitial compartments. Large amounts of TF were detected by immunostaining in leukocytes and platelet-rich microthrombi. TF induction was documented by quantitative reverse transcriptase-polymerase chain reaction, enzyme-linked immunosorbent assay, and coagulation assays. Lung-associated TFPI antigen and mRNA decreased during sepsis, and TFPI activity diminished abruptly at 2 hours. Blocking antibodies against TFPI increased fibrin deposition in septic baboon lungs, suggesting that TF-dependent coagulation might be aggravated by reduced endothelial TFPI. Decreased TFPI activity coincided with the release of tissue plasminogen activator and the peak of plasmin generation, suggesting that TFPI could undergo proteolytic inactivation by plasmin. Enhanced plasmin produced in septic baboons by infusion of blocking antibodies against plasminogen activator inhibitor-1 led to decreased lung-associated TFPI and unforeseen massive fibrin deposition. We conclude that activation of TF-driven coagulation not adequately countered by TFPI may underlie the widespread thrombotic complications of sepsis.
Sepsis is a serious medical condition caused by a severe infection leading to a systemic response syndrome that includes widespread activation of inflammation and coagulation and may progress to dysfunction of the circulatory system, acute respiratory distress syndrome, and onset of multiple organ dysfunction,1,2 which are leading causes of morbidity and mortality in sepsis.3
Although the pathogenesis of septic acute respiratory distress syndrome is not precisely understood, it is well accepted that inflammation, coagulation, and apoptosis are intimately linked in sepsis.4 Activation of tissue factor (TF)-dependent coagulation leads to formation of thrombin and subsequent deposition of fibrin.5,6
Tissue factor pathway inhibitor (TFPI) is the main inhibitor of the serine proteases involved in the TF-driven pathway in vivo. Two forms of TFPI are produced through alternative mRNA splicing. TFPI-α contains three Kunitz-type domains,7 whereas TFPI-β has the Kunitz-3 domain and C terminus of TFPI-α replaced with an unrelated C-terminal domain directly attached to the membrane via a glycosyl phosphatidylinositol anchor.8,9 In the mouse, TFPI-β mRNA has a similar tissue distribution as TFPI-α mRNA, but the encoded TFPI-β protein has yet to be detected.8 TFPI can be cleaved and inactivated by plasmin10 or by neutrophil elastase.11
Although heightened TF-induced coagulation is a consistent finding in disseminated intravascular coagulation and multiple organ dysfunction associated with sepsis, there are controversial reports on the changes of TFPI in plasma during sepsis, ranging from increased12,13,14 to decreased15,16 or unchanged levels.17 Moreover, despite being widely accepted that the endothelium is the most important source of TFPI in vivo,18,19 the information concerning the role and dynamics of Escherichia coli (EC)-associated TFPI during sepsis is still scant. This renders the pathophysiological role of TFPI in sepsis elusive.
Because endothelial dysfunction plays a key role in the pathogenesis of sepsis20 and because the lung is rich in microvessels and expresses large amounts of TFPI,21 we examined the time course changes of TF and TFPI in the lung and plasma of baboons challenged with E. coli. Our specific objective was to determine the role of TFPI in the pathophysiology of sepsis and the time frame in which the balance between the TF-dependent procoagulant and the TFPI anticoagulant activities are impaired in the baboon lung during sepsis. Our results revealed that i)TFPI immunodepletion leads to increased fibrin deposition in the lung; ii) bacterial infusion leads to a large drop in TFPI activity after 2 hours, which also coincides with the maximum release of tissue plasminogen activator (t-PA) from EC and the peak of plasmin generation; and iii) augmented plasmin generation achieved through inhibition of plasminogen activator inhibitor-1 (PAI-1) associates with decreased TFPI antigen and activity and increased fibrin deposition in the lung. We suggest that plasmin-dependent proteolysis of TFPI may be partly responsible for the detected loss of TFPI function. Our study analyzed, for the first time, the TFPI functional activity, antigen, and mRNA levels during the initial phases of sepsis.
Materials and Methods
Reagents
Antibodies and suppliers used were as follows: monoclonal antibody (mAb) against Kunitz 3 domain of human recombinant TFPI (r-TFPI) and full-length human r-TFPI expressed in Chinese Hamster Ovary cells22 [gifts from Dr. T. Hamuro (KAKETSUKEN, Kumamoto, Japan)]; rabbit anti-human r-TFPI1–249 IgG and mAbs against human full-length r-TFPI (raised and characterized in-house); mAb 10H10 anti-human TF [gift from Dr. J. Morrissey (University of Illinois at Urbana-Champaign, Urbana, IL)]; rabbit anti-human TF IgG [gift from Dr. W. Ruf (Scripps Research Institute, La Jolla, CA)]; mAb anti-human t-PA and rabbit anti-hirudin IgG (American Diagnostica Inc., Greenwich, CT); rabbit anti-human neutrophil elastase (Calbiochem, San Diego, CA); rabbit anti-human plasmin-α2 antiplasmin complex (Boehringer, Mannheim, Germany); mAb anti-CD68, mAb anti-GPIIb-IIIa and rabbit anti-human myeloperoxidase (DakoCytomation, Carpinteria, CA); antibodies anti-human t-PA [gift from R.H. Lijnen (University of Leuven, Leuven, Belgium)]; and peroxidase- or fluorophore-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Human coagulation factors VIIa, X, and Xa were from Enzyme Research Laboratories (South Bend, IN). Protein G-purified mAb anti-human PAI-1 (CLB-2C8) has been described previously.23 Chromogenic substrate S-2765 for factor Xa (FXa) was purchased from DiaPharma (Westchester, OH). Human r-TF (Innovin, 0.22 μg/vial) was from Dade Behring (Deerfield, IL). The primers were synthesized by the core facility of the University of Oklahoma Health Science Center. Triton X-100, pepstatin A, leupeptin, pefabloc SC, calpain inhibitor I, calpain inhibitor II, aprotinin, benzamidine, sodium o-vanadate, 1,10-phenanthroline, o-phenylenediamine, n-octyl β-d-glucopyranoside, native protein deglycosylation kit, and normal goat serum were obtained from Sigma Chemical (St. Louis, MO). p-Amidinophenylmethylsulfonyl fluoride was from Calbiochem. Bovine serum albumin was obtained from Equitech-Bio (Kerrville, TX). E. coli organisms (serotype B7-086a:K61; American Type Culture Collection, Rockville, MD), stored in the lyophilized state at 4°C after growth in tryptic soybean agar, were reconstituted and used as described previously.24 To eliminate differences due to E. coli strain variations, all animals were infused with E. coli from this single isolate.
Experimental Procedures
The study protocol received prior approval by the Institutional Animal Care and Use Committees of both Oklahoma Medical Research Foundation and the University of Oklahoma Health Science Center. Papio cyanocephalus baboons were held for 30 days at the University of Oklahoma Health Science Center animal facility, and only animals with a negative blood culture were included in the study. Two experimental E. coli groups were studied. One group of 13 animals was infused with live E. coli, using mostly sublethal doses (Supplemental Table 1 at http://ajp.amjpathol.org) as described previously.24 The time point at which the infusion was started is further indicated as T+0, a time point of n hours thereafter referred to as T+n hours. Time points before the start of the challenge are indicated as T−n hours. Three animals per time point were sacrificed at T+2, T+8, and T+24 hours after infusion. A subgroup of E. coli-treated animals received inhibitory antibodies anti-TFPI or anti-PAI-1. Two animals received 5 mg/kg mAb anti-human TFPI administered intravenously 10 minutes before the start of the E. coli challenge, followed by a second injection with the same amount at T+6 hours after E. coli infusion, and the animals were sacrificed at T+24 hours. Another two animals were injected with mAb anti-human PAI-1 (2C8) at T−30 minutes before E. coli challenge. The control group comprising three animals received saline infusion only. Lung tissue samples were snap frozen in liquid nitrogen and stored at −80°C.
Preparation of Lung Homogenates
Lung tissue was homogenized on ice with 1% Triton X-100 and 60 mmol/L n-octyl β-d-glucopyranoside in 50 mmol/L Tris-HCl and 150 mmol/L NaCl, pH 8.5, containing protease inhibitors (1.5 μmol/L pepstatin A, 10.5 μmol/L leupeptin, 0.25 mmol/L pefabloc SC, 20.9 μmol/L calpain inhibitor I, 20 μmol/L calpain inhibitor II, 1.5 μmol/L aprotinin, and 1 mmol/L each of benzamidine, sodium o-vanadate, and 1,10-phenanthroline). The extracts were centrifuged at 14,500 × g for 15 minutes, and the supernatants, representing the lung lysates, were stored at −80°C.
TFPI Antigen and Anticoagulant Activity Assays
For TFPI antigen measurement in the lung extracts, we developed a sandwich-type enzyme-linked immunosorbent assay (ELISA), using a cocktail of mAbs against r-TFPI as capturing layer and the rabbit anti-human TFPI IgG for detection. The concentration of TFPI was extrapolated from a standard curve made of serial dilutions of human full-length r-TFPI.
For the TFPI activity assay, homogenates were dialyzed overnight against 50 mmol/L Tris-HCl buffer, pH 7.4, to remove the detergents. Next, p-amidinophenylmethylsulfonyl fluoride (2.7 mmol/L final concentration, pH 5.0) was added to inhibit endogenous serine proteinase activities. TFPI activity was then measured, by evaluating the ability of TFPI to inhibit the activation of FX by TF-FVIIa. In selected experiments, TFPI activity was measured on tissue cryosections.25 TFPI activity was extrapolated from a standard curve constructed with serial dilutions of human full-length r-TFPI.
TF Antigen and Activity Assays
TF antigen was determined by ELISA using a matched-pair antibody set from Affinity Biological Inc. (Ancaster, ON, Canada). TF activity was measured through the ability of TF in lysates to shorten the clotting time of plasma. Briefly, tissue homogenates were dialyzed to remove the detergents as above, and a clotting time assay was performed using a STArt 4 coagulometer (Diagnostica Stago, Asnieres, France) after adding normal pooled citrated plasma and 25 mmol/L CaCl2. TF activity of the samples was determined by reference to a standard curve made with known amounts of recombinant TF (Innovin; Dade Behring, Marburg, Germany).
Bleeding Time
To determine the time required for the bleeding to stop in the animals treated with anti-TFPI antibodies, a lancet was used to make a standardized 3-mm stab wound on the skin of the forearm. A stopwatch was started immediately on skin incision and blood drops were removed every 15 seconds with the use of a paper filter. If bleeding did not recur within 30 seconds of cessation, it was considered stopped. Bleeding time was measured at T0 and then every 30 minutes, until 2 hours after treatment with the anti-TFPI antibody.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blotting
Lung lysates treated or not with the deglycosydases contained in the E-DEGLY kit according to Piro and Broze26 were subjected to nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (NuPAGE MES 4 to 12% polyacrylamide gradient gels; Invitrogen, Carlsbad, CA), and TFPI was detected by Western blotting using rabbit anti-TFPI antibodies, as described previously.27
Quantitative Reverse Transcriptase-Polymerase Chain Reaction-Based Gene Expression Analysis
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was used to determine the relative amount of TF, TFPIα, TFPIβ, and β-actin mRNA in the lung. Primers were designed by using Primer Express software (Applied Biosystems, Foster City, CA). The sequences of the primers are listed in Table 1.
Table 1.
Sequences Primers for TF, TFPI-α, TFPI-β, and β-Actin
| Name | Forward primers | Reverse primers |
|---|---|---|
| TF | 5′-TGCTTTTACACAGCAGACACAGAGT-3′ | 5′-AAGACCCGTGCCAAGTACGT-3′ |
| TFPI-α | 5′-TGAGGGCATGTAAAAAAGGTTTC-3′ | 5′-TTCACTCTCTGCTTCTTTCTTTTTCTT-3′ |
| TFPI-β | 5′-TGGAAGAATGCGGCTCATATT-3′ | 5′-TGCTATCCAATCCTAGAAAGAACATG-3′ |
| β-Actin | 5′-TGGAAGAATGCGGCTCATATT-3′ | 5′-TGCTATCCAATCCTAGAAAGAACATG-3′ |
Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer. For each sample, 2 μg of total RNA was reverse-transcribed by using the Omniscript Reverse Transcription System (Qiagen, Valencia, CA) with oligo-dT primers. Real-time PCR was performed in duplicate with 1 μl of the 20-μl RT reaction products by using iTaq SYBR Green Supermix with ROX kit (Bio-Rad, Hercules, CA) in an ABI Prism 5700 sequence detector (Applied Biosystems). Relative quantification of gene expression with the standard curve method was performed following the manufacturer’s protocol (Applied Biosystems). The relative expression of target genes was normalized with β-actin mRNA level as housekeeping gene.
Morphological Analysis
For immunofluorescence, tissues were fixed in 4% paraformaldehyde, washed with phosphate-buffered saline containing 20% sucrose, embedded in ornithine carbamoyltransferase, snap-frozen, and stored at −80°C.
Staining with hematoxylin and eosin, double immunolabeling for TFPI or TF and cell markers (CD68 for macrophages, myeloperoxidase or elastase for neutrophils, and gpIIb-IIIa for activated platelets) and fibrin were performed as described previously.25 As negative control for polyclonal antibody staining, the primary antibodies were replaced with equivalent amount of rabbit nonimmune serum. mAb anti-digoxigenin (IgG1; Roche Diagnostics, Indianapolis, IN), a hapten antigen that occurs only in plants, was used as isotype-matched control for mAb staining.25
Specimens were examined by epifluorescence confocal imaging using a Nikon C1 confocal microscope (Nikon USA, Melville, NY). The measurement of fluorescence intensity was done as previously described.25 In brief, 10 to 15 single-channel grayscale images (12-bit, 4095 gray levels/pixel) were collected for each experimental condition, and the average fluorescence intensity of each whole image was integrated using the EZ-C1 software (Nikon). Image collection parameters (neutral density filters, pinhole, and detector gains) were kept constant during image acquisition, to make reliable comparisons between specimens.
Statistical Analysis
For statistical analyses, we used InStat (GraphPad Software, Inc., San Diego, CA). Values are given as mean ± SEM. The differences between E. coli-challenged groups were compared against the control by one-way analysis of variance, followed by single comparison with control by using Dunnett test. Differences were considered as significant when P < 0.05. All experiments were performed in duplicate.
Results
Clinical Evidence of Sepsis after E. coli Challenge
The main clinical and hematological parameters of the animals before E. coli challenge (T0) and at the time of sacrifice (Ts) are summarized in Supplemental Table 1 (available at http://ajp.amjpathol.org). The challenge led to progressive systemic hypotension, paralleled by increased heart rate (tachycardia) and respiratory rate (tachypnea).
Alterations of the hemostatic and hematological parameters include variable decrease in fibrinogen levels, slight prolongation of the activated partial thromboplastin time, and increased levels of fibrin-degradation products, which reached maximum levels at T+24 hours. White blood cell counts dropped at T+2 and T+8 hours and recovered or increased at T+24 hours. Platelet counts gradually decreased and reached the lowest level at T+24 hours.
Sepsis Induces Structural Changes in the Lung
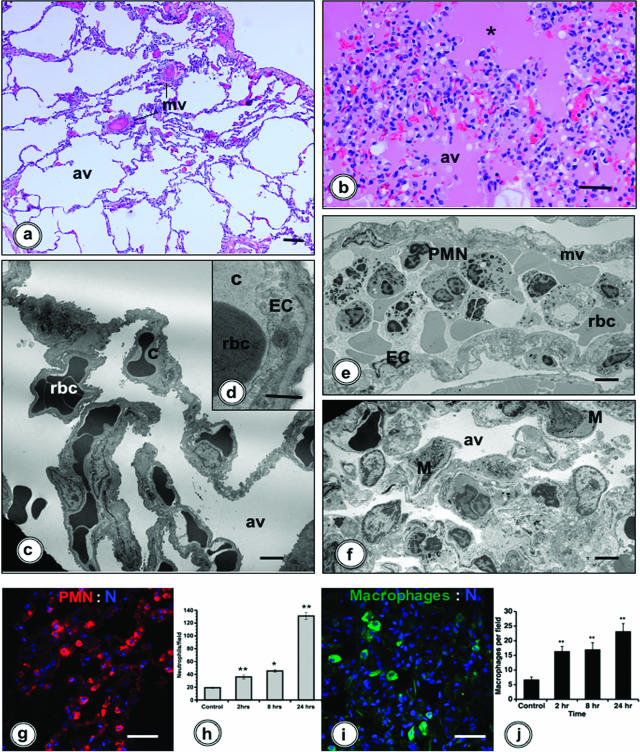
Morphological evaluation of lungs was performed by histology and electron microscopy. The lung structure changed gradually and reached the most extensive alterations at T+24 hours. In contrast to the normal architecture of the alveolar septae in healthy baboons (Figure 1, a, c, and d), the lungs of E. coli-challenged animals showed thickened septae (Figure 1, b, e, and f) and marked accumulation of inflammatory cells (especially neutrophils and monocytes) within the microvasculature (Figure 1e).
Figure 1.
Morphological analysis and dynamics of inflammatory cell accumulation in the lung of E. coli-challenged baboons. a and b: Light microscopy of the lung in control (a) and septic (b; 24 hours) baboons. The lung from a healthy baboon shows normal morphology, whereas the organ from a septic animal displays massive interstitial edema, intra-alveolar accumulation of eosin-stained proteinaceous material (asterisk), and inflammatory cell infiltrates within the intra- and extravascular compartments. c–f: Electron microscopy images reveal that, in contrast to normal ultrastructure of the lung from control animals (c and d), E. coli-challenged baboons (e and f) showed massive accumulation of leukocytes (predominantly neutrophils) in the microvasculature (e). The alveolar spaces collapsed, and the alveolar walls are thick and rich in interstitial fibroblasts, extracellular matrix, and inflammatory cells (f, M). g–j: Immunofluorescence detection of neutrophils (g, red, rabbit anti-elastase IgG/donkey anti-rabbit IgG-Cy3) and monocyte/macrophages (i, green, mAb anti-CD68/donkey anti-mouse IgG-fluorescein isothiocyanate). Nuclei (N) were counterstained with TOPRO-3 (blue). h and j: Histograms showing the time course of neutrophils and macrophages accumulation in the lung during sepsis. Twelve microscopic fields per time point were counted, and data were analyzed by analysis of variance (*P < 0.05; **P < 0.01). av, alveolae; c, capillaries; EC, endothelial cells; M, macrophages; mv, microvessels; PMN, neutrophils; rbc, red blood cells. Magnification bars: 100 μm (a, b, g, and i); 10 μm (c, d, e, and f).
Patchy edema due to capillary leakage into the alveolar space and accumulation of eosinophilic proteinaceous material in the alveolae were observed at T+24 hours (Figure 1b), especially in the animals with poor clinical prognosis. The same animals also displayed inflammatory cells and hemorrhage in the alveolar space (Figure 1f).
Quantitative Analysis of Neutrophil and Macrophage Accumulation in the Lung
Quantification performed on lung cryosections after staining for neutrophil elastase and CD68 revealed that both neutrophils (Figure 1, g and h) and macrophages (Figure 1, i and j) gradually accumulated in the lung, attaining maximum numbers at T+24 hours.
TF-Dependent Coagulation Increases in the Lung of Septic Baboons
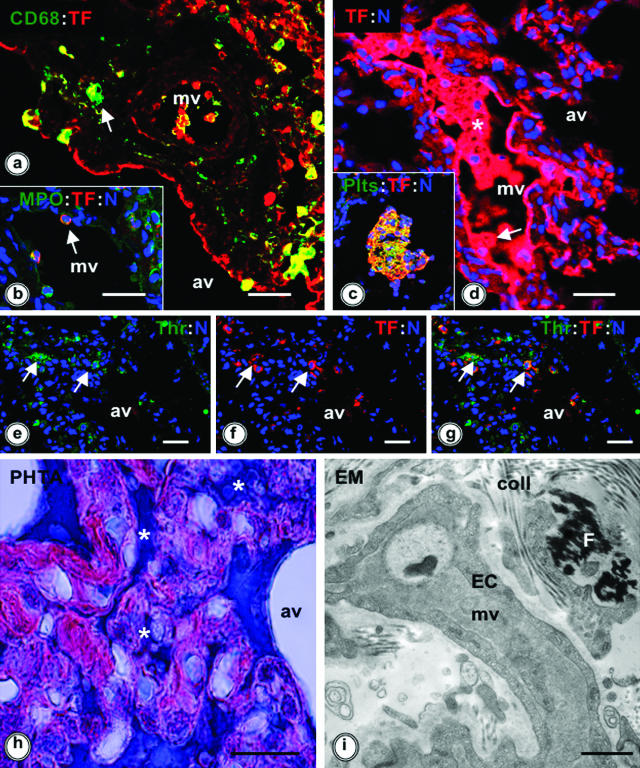
Cell type-specific localization of TF was investigated by double immunofluorescence labeling for TF and cell markers. In septic baboon lungs, TF was mainly detected in macrophages (Figure 2a) and EC (Figure 2, a and d) and to a lesser extent in neutrophils (Figure 2b).
Figure 2.
TF expression, thrombin generation, and fibrin deposition in the baboon lung at 24 hours after E. coli challenge. a–c: Double immunostaining for TF (red, rabbit anti-human TF or mAb 10H10 anti-TF, followed by the appropriate donkey secondary IgG conjugated with Cy3) and cell markers (green, donkey secondary IgGs conjugated with fluorescein isothiocyanate): mAb anti-CD68 for monocyte/macrophages (a), rabbit anti-myeloperoxidase IgG for neutrophils (b), and mAb anti-gpIIb/IIIa for platelets (c). Colocalization of the red and green fluorophores is shown in yellow (arrows). d: TF immunostaining (red, as for a) of an intravascular microthrombus (asterisk) and of the vascular endothelium (arrow). e–g: Double immunostaining for hirudin, used as thrombin probe (arrows, green, rabbit anti-hirudin IgG/donkey anti-rabbit IgG-fluorescein isothiocyanate) and TF (arrows, red, mAb 10H10 as above). Colocalization of thrombin and TF appears as yellow (g, arrows). For a–g, TOPRO-3 nuclear counterstaining is shown in blue. h and i: Phosphotungstic acid-hematoxylin (PHTA) staining (h) and transmission electron microscopy (EM) (i) show the presence of intravascular and interstitial fibrin deposits in the lung (h: asterisks, dark blue; i: F, black staining). av, alveolae; coll, collagen; EC, endothelial cells; MPO, myeloperoxidase; mv, microvessels. Magnification bars: 100 μm (a–h); 10 μm (i).
Strong TF staining was associated with intravascular microthrombi (Figure 2, c and d), especially colocalizing with activated platelets, as shown by staining with anti-gpIIb-IIIa IgG (Figure 2c). The activation of coagulation in the lung parenchyma led to local generation of thrombin, revealed through binding of its specific inhibitor hirudin, followed by immunodetection with rabbit anti-hirudin IgG (Figure 2, e–g). The presence of intravascular and/or interstitial fibrin deposits was detected by immunostaining with fibrin-specific antibodies, by phosphotungstic acid staining (Figure 2h), or by electron microscopy (Figure 2i).
TF mRNA expression was increased by 4.5-fold over controls at T+2 hours (P < 0.01) and stayed slightly elevated at T+8 and T+24 hours (Figure 3a). The levels of both TF antigen and activity were significantly higher than in controls at T+8 (P < 0.05) and T+24 hours (P < 0.01) (Figure 3, b and c). The specificity of the activity assay was confirmed through incubation of the samples with inhibitory antibodies, which produced almost total inhibition of TF clotting activity (Figure 3c).
Figure 3.
Analysis of the expression and activity of TF in lung extracts from control or E. coli-challenged baboons. a: TF mRNA levels determined by real-time RT-PCR. b: TF antigen assayed by ELISA. c: TF activity measured through clotting time assay. *P < 0.05; **P < 0.01.
TFPI Inhibition with Blocking Antibodies Increased Fibrin Deposition in the Lung
The baboons treated with blocking mAb anti-human TFPI plus E. coli survived the first 24 hours after challenge and were sacrificed for tissue analysis. The treatment resulted in massive intravascular deposition of fibrin (Figure 4a), compared with the animals treated with E. coli alone (Figure 4b). No fibrin staining was detected in nonchallenged animals (Figure 4c). Isotype-matched control immunostaining (mAb anti-digoxigenin, IgG1) was negative (Figure 4d).
Figure 4.
Immunostaining for fibrin in lungs of baboons challenged with E. coli plus mAb anti-TFPI or mAb anti-PAI1, E. coli alone, and nonchallenged controls. a–d: The lungs of animals treated with E. coli and mAb anti-TFPI display much stronger staining for fibrin (a) compared with animals exposed to E. coli only (b). No fibrin was detected in the nonchallenged animals (c). Tissue sections incubated with mAb anti-digoxigenin, a hapten antigen that does not exist in animal tissues, was used as negative control for immunological specificity of the mouse mAbs (d). e and f: Massive fibrin deposition was detected in the lungs of animals challenged with E. coli and anti-PAI-1 antibody (e, phosphotungstic acid staining; f, fibrin-specific immunostaining). Magnification bars = 100 μm.
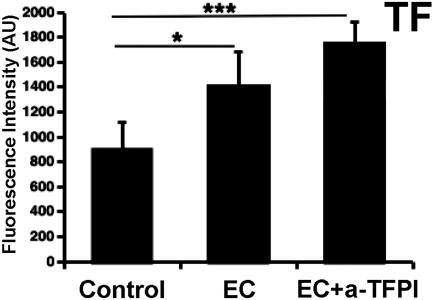
Quantitative analysis of TF immunostaining revealed significant increase of TF in the lung of the baboons treated with anti-TFPI plus E. coli versus E. coli alone or controls (Figure 5). TFPI activity in lung homogenates was almost completely abolished by the treatment with TFPI blocking antibody: 3.06 ± 1.26 ng/mg total protein (n = 2 animals; each in duplicate) compared with 59.4 ± 5.36 ng/mg (n = 3 animals, each in duplicate) in controls and 39.7 ± 11.37 ng/mg (n = 3 animals, each in duplicate) in animals challenged with E. coli only. In addition, the animals receiving the blocking mAb exhibited consumption of fibrinogen and decreased platelet count at T+8 hours compared with T0 (Supplemental Table 1 at http://ajp.amjpathol.org). Bleeding time was reduced to zero during the first hours after treatment with anti-TFPI antibody (0 from T+30 minutes to T+2 hours versus 2.5 ± 0.5 minutes at T0), suggesting full abrogation of TFPI function, and was increased to more than 9 minutes at T+24 hours, probably reflecting the decreased platelet counts.
Figure 5.
Quantitative analysis of the fluorescence intensity of TF staining in lungs of baboons challenged with E. coli and mAb anti-TFPI, E. coli alone, and nonchallenged controls. Fifteen randomly selected microscopic fields [five images per animal, three animals per group for nonchallenged control and EC groups and two animals per group for E. coli plus anti-TFPI mAb (EC+anti-TFPI)] were collected, and the mean fluorescence intensity values of each image were averaged. The values are expressed as arbitrary units (AU) of gray levels on a 12-bit scale (*P < 0.05; ***P < 0.001).
TFPI Expression and Activity Are Decreased in the Lung of Septic Baboons
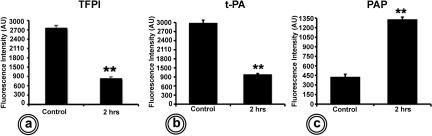
Immunofluorescence labeling showed large amounts of TFPI in the normal baboon lung (Supplemental Figure 1a at http://ajp.amjpathol.org). In contrast, TFPI in EC baboon lungs was considerably decreased (Supplemental Figure 1b at http://ajp.amjpathol.org), showing and approximately threefold decrease of TFPI fluorescence intensity at T+2 hours (Figure 6a). This coincided with a concurrent decrease in t-PA immunostaining (Supplemental Figure 1, c and d, at http://ajp.amjpathol.org; Figure 6b) and was paralleled by a peak of plasmin generation, as identified by immunostaining with an antibody directed to a neo-epitope of plasmin-α2 antiplasmin (Supplemental Figure 1, e and f, at http://ajp.amjpathol.org; Figure 6c).
Figure 6.
Quantitative analysis of the fluorescence intensity after immunolabeling of lungs from control and septic baboons. a: TFPI. b: t-PA. c: Plasmin-α2 antiplasmin. Twelve randomly selected microscopic fields (four images per animal, three animals per group) and the mean fluorescence intensity values of each image were averaged. Values are expressed as arbitrary units (AU) of gray levels on a 12-bit scale (**P < 0.01).
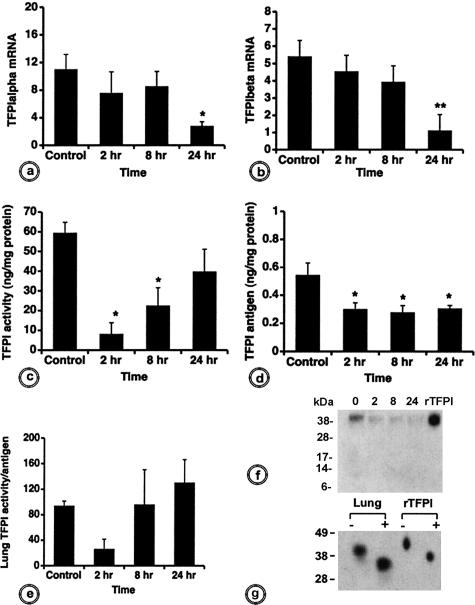
TFPI-α mRNA levels were decreased at T+2 and T+8 hours and reached a significant difference (P < 0.05) at T+24 hours (Figure 7a). Although expressed at lower levels than TFPI-α, TFPI-β mRNA followed the same temporal pattern after E. coli challenge (Figure 7b).
Figure 7.
Biochemical analysis of the time course of TFPI expression and activity in lung extracts from control and E. coli-challenged baboons. Analysis of TFPI-α (a) and TFPI-β (b) mRNA expression performed by real-time RT-PCR shows steady decrease in septic baboons over time. c: TFPI antigen determined by ELISA also decreases. d: TFPI activity, measured through its capability to inhibit FX activation by pre-formed TF-FVIIa, drops dramatically at T+2 hours, after which it recovers gradually. e: The ratio between TFPI activity and antigen defines the specific activity of TFPI at different time points. For a–e, *P < 0.05; **P < 0.01. f: Western blot for TFPI in lung extracts from baboons challenged with E. coli at T+2, T+8, or T+24 hours compared with healthy animals (T0). TFPI was probed with a rabbit antibody anti-TFPI. g: Western blot for TFPI in lung extracts at T+24 hours. Tissue lysates were treated (+) or not (−) with deglycosylation enzymes before sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transfer, and probing with rabbit anti-TFPI IgG, as above. Full-length human r-TFPI was used as control.
The time course of TFPI activity, measured as the ability of TFPI to inhibit activation of FX by TF-FVIIa, was different (Figure 7c). At T+2 hours, the inhibitory ability of TFPI dropped to 14% of the control levels (P < 0.01). The initial decrease was followed by a recovery trend, reaching levels of 38% (P < 0.05) and 67% of the control values at T+8 and T+24 hours (Figure 7c). TFPI antigen measured by ELISA decreased significantly in the septic animals versus controls for all of the time points examined (P < 0.05; Figure 7d). Plotting the above results as TFPI activity-to-antigen ratio showed that the inhibitory potential of the lung-associated TFPI decreased dramatically at T+2 hours (Figure 7e).
The decrease of TFPI activity was confirmed in TF clotting assays, in which we preincubated the samples with inhibitory anti-TFPI IgG. The differences between values measured after neutralizing the available TFPI and the ones determined for noninhibited samples should give the measure of the functional potency of TFPI to inhibit TF-FVIIa procoagulant activity. In controls, TF activity was approximately three times higher in the presence of anti-TFPI IgG than in its absence (data not shown), thus confirming that TFPI had high inhibitory potential in normal conditions. In contrast, we did not find equivalent differences in the T+2-hour lung samples, which substantiates the loss of inhibitory capability of the available TFPI at this time point.
Western blot analysis of TFPI from lung lysates by probing with a rabbit polyclonal anti-full-length TFPI showed a decrease in intensity of TFPI at all time points after E. coli challenge, as compared with controls, without detectable degradation bands (Figure 7f). Deglycosylation of lung extracts from control animals with an enzyme cocktail containing PNGase, α2-neuroaminidase, and o-glycosidase induces a shift of TFPI band from an apparent molecular mass of ∼45 to ∼37 kd, typical for TFPI-α (Figure 7g).26 No other smaller molecular weight bands potentially corresponding to TFPI-β26 were observed, suggesting that the α-spliced form is the major TFPI isoform in the baboon lung.
Effect of Increased Plasmin Generation Achieved through Blocking of PAI-1 with Inhibitory Antibodies
Treatment with blocking mAb anti-human PAI-1 plus E. coli resulted in massive fibrin deposition in the lung, as demonstrated by phosphotungstic acid staining (Figure 4e) and immunostaining with anti-human fibrin antibodies (Figure 4f). Histopathology showed marked congestion, leukocyte influx, and massive capillary leak, characterized by edema and hemorrhage (not shown). These lesions were incompatible with animal survival, and the two animals died after 11 and 51 hours after challenge, respectively. Quantitative analysis of TFPI immunostaining revealed a significant decrease of lung-associated TFPI in animals treated with anti-PAI-1 antibody and E. coli compared with E. coli alone, and both were significantly decreased compared with unchallenged animals (Supplemental Figure 2, a–c, at http://ajp.amjpathol.org; Figure 8a). TF staining was significantly increased in E. coli-challenged animals compared with controls but was not affected by anti-PAI-1 antibody treatment (Figure 8b).
Figure 8.
Quantitation of the fluorescence intensity for TFPI (a) and TF (b) in the lung of baboons challenged with E. coli and anti-PAI-1 blocking antibody, E. coli alone, and nonchallenged controls. Fifteen randomly selected microscopic fields [five images per animal, three animals per group for nonchallenged control and EC, and two animals per group for E. coli plus anti-PAI-1 mAb (EC+anti-PAI-1), and the mean fluorescence intensity values of each image were averaged. The values are expressed as arbitrary units (AU) of gray levels on a 12-bit scale (*P < 0.05; ***P < 0.001).
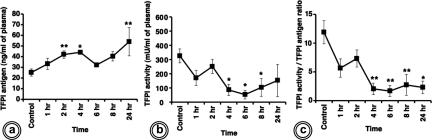
Functional Activity of Plasma TFPI Is Decreased in Sepsis
In contrast to the lung, plasma TFPI antigen was found increased T+2 (P < 0.01) and T+4 hours (P < 0.05) (Figure 9a). After showing a temporary reversal at T+6 hours, the TFPI antigen increased again at T+24 hours. However, the activity of TFPI in the same plasma samples did not mirror the changes of the antigen (Figure 9b). TFPI activity-to-antigen ratio demonstrated that TFPI in the plasma of septic baboons was significantly less functionally potent than in control animals (Figure 9c).
Figure 9.
TFPI antigen and activity in plasma from control and E. coli-challenged baboons. The time course of TFPI antigen determined by ELISA (a) and TFPI activity assayed against added TF-FVIIa and FX (b) indicate opposite tendencies of the parameters studied. c: Specific activity of TFPI, calculated as described for Figure 6e, decreased significantly throughout the time course (*P < 0.05; **P < 0.01).
Discussion
The pathology of sepsis results, in part, from a complex deregulation of normal hemostasis leading to activation of the procoagulant pathways concurrently with impairment of both anticoagulant28 and fibrinolytic systems.29
Analysis of coagulation plasma markers revealed that baboons infused with 108 to 109 cfu/kg E. coli exhibit a two-stage procoagulant response30: an early stage (T0 to T+6 hours) dominated by thrombin and plasmin production and a second stage (T+12 to T+24 hours) dominated by peak elevation of soluble fibrin monomer, elastase, and soluble thrombomodulin.
Here, we used this model of non-human primate sepsis to study, for the first time, the regulation of TF-dependent coagulation both in the lung tissue and in plasma at three key time points: T+2 hours (first stage), T+8 hours (transition from first to second stage), and T+24 hours (second stage) after E. coli infusion.
The highest increase in TF mRNA detected by us at 2 hours matches the peak plasma levels of lipopolysaccharide, tumor necrosis factor-α, and other inflammatory cytokines,31 which are potent TF-inducers in monocytes,32 EC,33 and neutrophils,34 and could generate procoagulant microparticles.35 The second TF mRNA increase observed at 24 hours (second stage) coincides with the second plasma peak of TF antigen.36 Lung TF protein and its procoagulant potential increased during the 24-hour period, reflecting the gradual accumulation of leukocytes and platelets and TF production by EC and epithelial cells.37 The detection of platelet-associated TF supports a recent report demonstrating TF pre-mRNA splicing and translation in activated platelets.38 TF up-regulation results in thrombin generation, as reflected by increased plasma levels of TAT29 and tissue-bound active thrombin, platelet activation, and fibrin deposition in the lung of septic baboons.
A major finding of our study is the significant impairment of TFPI anticoagulant activity in the lungs of septic baboons. Both TFPI-α and TFPI-β mRNA expression levels decreased during the first and mid-stages and reached their lowest levels during the second stage. Similarly, TFPI antigen decreased steadily during the time course of the experiment. Interestingly, however, the abrupt decrease of TFPI activity detected during the first stage of E. coli challenge did not match entirely the changes in the antigen levels. Because the TFPI inhibitory activity toward TF-FVIIa-FXa reflects mainly the amount of full-length TFPI, the difference between TFPI antigen and activity may indicate the presence in the tissue of truncated, functionally inactive forms of TFPI. Although the activity recovered in part at 8 and 24 hours after E. coli challenge, both the antigen and the activity of TFPI remained significantly lower in the septic animals than in the controls.
To confirm the effect of decreased TFPI on the hemostatic function of the lung during sepsis, two E. coli-challenged baboons were injected with an inhibitory mAb anti-human TFPI. This led to almost complete blocking of TFPI activity and to strong increase of TF and fibrin levels in the lung.
The decrease of tissue-associated TFPI during sepsis may result from multiple causes: i) proteolytic release from the cell surface into the plasma; ii) consumption during anticoagulation through formation of the FXa-TFPI-FVIIa/TF complex; or iii) decreased expression in the endothelium, either because of EC dysfunction or through inhibition of gene expression, as supported by our mRNA results. Most likely, all three processes are probably involved at different time points in the TFPI down-regulation.
We observed that the decrease of TFPI content in the lung was paralleled by an increase of plasma levels of TFPI antigen,12 suggesting an enhanced release from the endothelium, possibly because of local thrombin generation.39,40 However, increased plasma TFPI may not necessarily reflect equivalent enhancement of plasma anticoagulant properties, because most of the TFPI in plasma has low activity, presumably because of C terminus truncation.41 This may explain why the increased plasma TFPI antigen found in the septic baboons actually had poor anticoagulant activity. So far, there are conflicting data about how sepsis mediators can affect the expression of TFPI by EC in vitro42 or in vivo43 or levels of TFPI protein in the plasma.16 It was suggested that increased TFPI in plasma reflects endothelial injury and release of endothelial-bound TFPI into the plasma, where the protein is truncated, and as a result, has reduced anticoagulant activity.44 The available TFPI may inadequately balance the increased TF-dependent coagulation in sepsis patients.45 The resulting imbalance perpetuates the procoagulant state and predicts a poor outcome. Because TFPI could bind lipopolysaccharide directly and thus block the binding of the endotoxin to CD14 and prevent adverse cellular effects,46 decreased levels of TFPI may also mean less protective anti-inflammatory effects in addition to the loss of anticoagulant function.
It has been shown that TFPI can be degraded, with loss of function potency by several proteases, including leukocyte elastase,11,47 metalloproteinases,48 or plasmin.49,50,51 We showed that the loss of TFPI activity at 2 hours coincides with the release of t-PA from the tissue and the consequent peak of plasmin generation but not with the time course of neutrophil elastase release or neutrophil accumulation into the tissue. Net pulmonary release of t-PA could reach 15-fold increase during the first 2 hours.52 Because we have not detected elastase11,47 and/or MMP-specific48 degradation products by Western blot analysis and because it was shown that plasmin can completely cleave TFPI without leaving degradation products,50 we believe that plasmin, rather than elastase, may be responsible for proteolytic degradation of TFPI during the early stages of sepsis. Actually, it was shown that plasmin decreases the surface-associated TFPI on circulating monocytes after thrombolytic therapy and may contribute to thrombotic complications after fibrinolysis in acute myocardial infarction.51 Concordantly, we show here that enhanced plasmin generation by blocking of PAI-1 with inhibitory antibody also significantly decreased the amount of lung-associated TFPI without increasing the TF levels and, paradoxically, promoted massive fibrin deposition in the lung, among other pathological effects that were incompatible with animal survival.
Our data suggest that the decrease of lung-associated TFPI in the advanced stages of sepsis could be responsible for the vascular and perivascular fibrin deposition that may lead to microthrombotic organ failure. We further speculate that the dysfunction of TFPI that occurs in the lung of septic animals may explain the association of this disease with pulmonary intravascular coagulation and acute respiratory distress syndrome.
In view of these observations, restoration of the TF-TFPI balance is likely to correct the derangement of coagulation in sepsis. In fact, it was shown that TFPI infusion in animals with severe sepsis modulates the development of TF-induced disseminated intravascular coagulation and can reduce mortality.46,53 These observations apparently contradict the results of the Phase III multicenter trial that found no substantial impact of r-TFPI administration on survival at 28 days. Some of the factors that could explain why the trial failed were recently reviewed.54 We believe, however, that the main problem resides with the major differences existing between r-TFPI and the endogenous form of the inhibitor with regard to clearance from the circulation,55 distinct membrane anchorage and subsequent mechanism of action on cell surfaces,56,57,58 or control of protease-activated receptor signaling. These differences could not only explain why r-TFPI has limited therapeutic effects in systemic inflammation but may also point toward the necessity of developing strategies to enhance the expression and activity of endogenous TFPI as a more promising approach to improve the outcome of patients with sepsis.
Supplementary Material
Acknowledgments
We thank Dr. James H. Morrissey (University of Illinois, Urbana-Champaign, IL) for the mAb anti-TF, Dr. W. Ruf (Scripps Research Institute, La Jolla, CA) for the rabbit anti-TF IgG, Dr. T. Hamuro (The Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan) for the mAb anti- TFPI and for the human full-length r-TFPI, Todd Ham (Oklahoma Medical Research Foundation, Oklahoma City, OK) for technical support with the electron microscopy, Scott Freeman (Oklahoma Medical Research Foundation) for technical support with animal experimentation, and Dr. Charles T. Esmon (Oklahoma Medical Research Foundation) for critical reading of the manuscript.
Footnotes
Address reprint requests to Florea Lupu, Ph.D., Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St., Oklahoma City, OK 73104. E-mail: florea-lupu@omrf.ouhsc.edu.
Supported by National Institutes of Health grant 5RO1GM037704-17 (to F.L. and F.B.T.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Kaplan RL, Sahn SA, Petty TL. Incidence and outcome of the respiratory distress syndrome in gram-negative sepsis. Arch Intern Med. 1979;139:867–869. [PubMed] [Google Scholar]
- Moore FA, Moore EE, Read RA. Postinjury multiple organ failure: role of extrathoracic injury and sepsis in adult respiratory distress syndrome. New Horiz. 1993;1:538–549. [PubMed] [Google Scholar]
- Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Régnier B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: a multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–974. [PubMed] [Google Scholar]
- Liaw PC, Esmon CT, Kahnamoui K, Schmidt S, Kahnamoui S, Ferrell G, Beaudin S, Julian JA, Weitz JI, Crowther M, Loeb M, Cook D. Patients with severe sepsis vary markedly in their ability to generate activated protein C. Blood. 2004;104:3958–3964. doi: 10.1182/blood-2004-03-1203. [DOI] [PubMed] [Google Scholar]
- Carraway MS, Welty-Wolf KE, Miller DL, Ortel TL, Idell S, Ghio AJ, Petersen LC, Piantadosi CA. Blockade of tissue factor: treatment for organ injury in established sepsis. Am J Respir Crit Care Med. 2003;167:1200–1209. doi: 10.1164/rccm.200204-287OC. [DOI] [PubMed] [Google Scholar]
- Crawley J, Lupu F, Westmuckett AD, Severs NJ, Kakkar VV, Lupu C. Expression, localization, and activity of tissue factor pathway inhibitor in normal and atherosclerotic human vessels. Arterioscler Thromb Vasc Biol. 2000;20:1362–1373. doi: 10.1161/01.atv.20.5.1362. [DOI] [PubMed] [Google Scholar]
- Broze GJ, Jr, Girard TJ, Novotny WF. Regulation of coagulation by a multivalent Kunitz-type inhibitor. Biochemistry. 1990;29:7539–7546. doi: 10.1021/bi00485a001. [DOI] [PubMed] [Google Scholar]
- Chang JY, Monroe DM, Oliver JA, Roberts HR. TFPIβ, a second product from the mouse tissue factor pathway inhibitor (TFPI) gene. Thromb Haemost. 1999;81:45–49. [PubMed] [Google Scholar]
- Zhang J, Piro O, Lu L, Broze GJ., Jr Glycosyl phosphatidylinositol anchorage of tissue factor pathway inhibitor. Circulation. 2003;108:623–627. doi: 10.1161/01.CIR.0000078642.45127.7B. [DOI] [PubMed] [Google Scholar]
- Li Y, Spencer FA, Becker RC. Plasmin-mediated proteolysis of vascular endothelial cell heparin releasable tissue factor pathway inhibitor. J Thromb Thrombolysis. 2003;15:19–23. doi: 10.1023/a:1026136216869. [DOI] [PubMed] [Google Scholar]
- Higuchi DA, Wun TC, Likert KM, Broze GJ., Jr The effect of leukocyte elastase on tissue factor pathway inhibitor. Blood. 1992;79:1712–1719. [PubMed] [Google Scholar]
- Sabharwal AK, Bajaj SP, Ameri A, Tricomi SM, Hyers TM, Dahms TE, Taylor FB, Jr, Bajaj MS. Tissue factor pathway inhibitor and von Willebrand factor antigen levels in adult respiratory distress syndrome and in a primate model of sepsis. Am J Respir Crit Care Med. 1995;151:758–767. doi: 10.1164/ajrccm/151.3_Pt_1.758. [DOI] [PubMed] [Google Scholar]
- Sandset PM, Roise O, Aasen AO, Abildgaard U. Extrinsic pathway inhibitor in postoperative/posttraumatic septicemia: increased levels in fatal cases. Haemostasis. 1989;19:189–195. doi: 10.1159/000215916. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Sandset PM, Joo GB, Ovstebo R, Abildgaard U, Kierulf P. The quantitative association of plasma endotoxin, antithrombin, protein C, extrinsic pathway inhibitor and fibrinopeptide A in systemic meningococcal disease. Thromb Res. 1989;55:459–470. doi: 10.1016/0049-3848(89)90054-6. [DOI] [PubMed] [Google Scholar]
- Novotny WF, Brown SG, Miletich JP, Rader DJ, Broze GJ., Jr Plasma antigen levels of the lipoprotein-associated coagulation inhibitor in patient samples. Blood. 1991;78:387–393. [PubMed] [Google Scholar]
- Bajaj MS, Rana SV, Wysolmerski RB, Bajaj SP. Inhibitor of the factor VIIa-tissue factor complex is reduced in patients with disseminated intravascular coagulation but not in patients with severe hepatocellular disease. J Clin Invest. 1987;79:1874–1878. doi: 10.1172/JCI113030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr TA, Rao LV, Rapaport SI. Disseminated intravascular coagulation in rabbits induced by administration of endotoxin or tissue factor: effect of anti-tissue factor antibodies and measurement of plasma extrinsic pathway inhibitor activity. Blood. 1990;75:1481–1489. [PubMed] [Google Scholar]
- Broze GJ, Jr, Warren LA, Novotny WF, Higuchi DA, Girard JJ, Miletich JP. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 1988;71:335–343. [PubMed] [Google Scholar]
- Broze GJ., Jr The rediscovery and isolation of TFPI. J Thromb Haemost. 2003;1:1671–1675. doi: 10.1046/j.1538-7836.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- Hack CE, Zeerleder S. The endothelium in sepsis: source of and a target for inflammation. Crit Care Med. 2001;29:S21–S27. doi: 10.1097/00003246-200107001-00011. [DOI] [PubMed] [Google Scholar]
- Ameri A, Kuppuswamy MN, Basu S, Bajaj SP. Expression of tissue factor pathway inhibitor by cultured endothelial cells in response to inflammatory mediators. Blood. 1992;79:3219–3226. [PubMed] [Google Scholar]
- Nakahara Y, Miyata T, Hamuro T, Funatsu A, Miyagi M, Tsunasawa S, Kato H. Amino acid sequence and carbohydrate structure of a recombinant human tissue factor pathway inhibitor expressed in Chinese hamster ovary cells: one N-and two O-linked carbohydrate chains are located between Kunitz domains 2 and 3 and one N-linked carbohydrate chain is in Kunitz domain 2. Biochemistry. 1996;35:6450–6459. doi: 10.1021/bi9524880. [DOI] [PubMed] [Google Scholar]
- Biemond BJ, Levi M, Coronel R, Janse MJ, ten Cate JW, Pannekoek H. Thrombolysis and reocclusion in experimental jugular vein and coronary artery thrombosis: effects of a plasminogen activator inhibitor type 1-neutralizing monoclonal antibody. Circulation. 1995;91:1175–1181. doi: 10.1161/01.cir.91.4.1175. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Jr, Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang AC, Laszik Z, Kosanke S, Peer G, Esmon CT. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- Lupu C, Westmuckett AD, Peer G, Ivanciu L, Zhu H, Taylor FB, Jr, Lupu F. Tissue factor-dependent coagulation is preferentially up-regulated within arterial branching areas in a baboon model of Escherichia coli sepsis. Am J Pathol. 2005;167:1161–1172. doi: 10.1016/S0002-9440(10)61204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piro O, Broze GJ., Jr Comparison of cell-surface TFPIα and β. J Thromb Haemost. 2005;3:2677–2683. doi: 10.1111/j.1538-7836.2005.01636.x. [DOI] [PubMed] [Google Scholar]
- Lupu C, Hu X, Lupu F. Caveolin-1 enhances tissue factor pathway inhibitor exposure and function on the cell surface. J Biol Chem. 2005;280:22308–22317. doi: 10.1074/jbc.M503333200. [DOI] [PubMed] [Google Scholar]
- Faust SN, Levin M, Harrison OB, Goldin RD, Lockhart MS, Kondaveeti S, Laszik Z, Esmon CT, Heyderman RS. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med. 2001;345:408–416. doi: 10.1056/NEJM200108093450603. [DOI] [PubMed] [Google Scholar]
- de Boer JP, Creasy AA, Chang A, Roem D, Brouwer MC, Eerenberg AJ, Hack CE, Taylor FB., Jr Activation patterns of coagulation and fibrinolysis in baboons following infusion with lethal or sublethal dose of Escherichia coli. Circ Shock. 1993;39:59–67. [PubMed] [Google Scholar]
- Taylor FB, Jr, Wada H, Kinasewitz G. Description of compensated and uncompensated disseminated intravascular coagulation (DIC) responses (non-overt and overt DIC) in baboon models of intravenous and intraperitoneal Escherichia coli sepsis and in the human model of endotoxemia: toward a better definition of DIC. Crit Care Med. 2000;28:S12–S19. doi: 10.1097/00003246-200009001-00004. [DOI] [PubMed] [Google Scholar]
- Creasey AA, Stevens P, Kenney J, Allison AC, Warren K, Catlett R, Hinshaw L, Taylor FB., Jr Endotoxin and cytokine profile in plasma of baboons challenged with lethal and sublethal Escherichia coli. Circ Shock. 1991;33:84–91. [PubMed] [Google Scholar]
- Nilsen DW, Almdahl SM, Svensson B, Vaage J, Rasmussen K, Østerud B. Lipopolysaccharide induced monocyte thromboplastin synthesis and coagulation responses in patients undergoing coronary bypass surgery after preoperative supplementation with n-3 fatty acids. Thromb Haemost. 1993;70:900–902. [PubMed] [Google Scholar]
- Colucci M, Balconi G, Lorenzet R, Pietra A, Locati D, Donati MB, Semeraro N. Cultured human endothelial cells generate tissue factor in response to endotoxin. J Clin Invest. 1983;71:1893–1896. doi: 10.1172/JCI110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todoroki H, Nakamura S, Higure A, Okamoto K, Takeda S, Nagata N, Itoh H, Ohsato K. Neutrophils express tissue factor in a monkey model of sepsis. Surgery. 2000;127:209–216. doi: 10.1067/msy.2000.103027. [DOI] [PubMed] [Google Scholar]
- Østerud B, Bjorklid E. The tissue factor pathway in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27:605–617. doi: 10.1055/s-2001-18866. [DOI] [PubMed] [Google Scholar]
- Taylor FB., Jr The inflammatory-coagulant axis in the host response to gram-negative sepsis: regulatory roles of proteins and inhibitors of tissue factor. New Horiz. 1994;2:555–565. [PubMed] [Google Scholar]
- Drake TA, Cheng J, Chang A, Taylor FB., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993;142:1458–1470. [PMC free article] [PubMed] [Google Scholar]
- Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss LW, Mackman N, Zimmerman GA, Weyrich AS. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu C, Lupu F, Dennehy U, Kakkar VV, Scully MF. Thrombin induces the redistribution and acute release of tissue factor pathway inhibitor from specific granules within human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1995;15:2055–2062. doi: 10.1161/01.atv.15.11.2055. [DOI] [PubMed] [Google Scholar]
- Lupu C, Kruithof EK, Kakkar VV, Lupu F. Acute release of tissue factor pathway inhibitor after in vivo thrombin generation in baboons. Thromb Haemost. 1999;82:1652–1658. [PubMed] [Google Scholar]
- Novotny WF, Girard TJ, Miletich JP, Broze GJ., Jr Purification and characterization of the lipoprotein-associated coagulation inhibitor from human plasma. J Biol Chem. 1989;264:18832–18837. [PubMed] [Google Scholar]
- Bajaj MS, Bajaj SP. Tissue factor pathway inhibitor: potential therapeutic applications. Thromb Haemost. 1997;78:471–477. [PubMed] [Google Scholar]
- Shimokawa T, Yamamoto K, Kojima T, Saito H. Down-regulation of murine tissue factor pathway inhibitor mRNA by endotoxin and tumor necrosis factor-α in vitro and in vivo. Thromb Res. 2000;100:211–221. doi: 10.1016/s0049-3848(00)00332-7. [DOI] [PubMed] [Google Scholar]
- Shimura M, Wada H, Nakasaki T, Hiyoyama K, Mori Y, Nishikawa M, Deguchi H, Deguchi K, Gabazza EC, Shiku H. Increased truncated form of plasma tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol. 1999;60:94–98. doi: 10.1002/(sici)1096-8652(199902)60:2<94::aid-ajh2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Gando S, Kameue T, Morimoto Y, Matsuda N, Hayakawa M, Kemmotsu O. Tissue factor production not balanced by tissue factor pathway inhibitor in sepsis promotes poor prognosis. Crit Care Med. 2002;30:1729–1734. doi: 10.1097/00003246-200208000-00009. [DOI] [PubMed] [Google Scholar]
- Park CT, Creasey AA, Wright SD. Tissue factor pathway inhibitor blocks cellular effects of endotoxin by binding to endotoxin and interfering with transfer to CD14. Blood. 1997;89:4268–4274. [PubMed] [Google Scholar]
- Petersen LC, Bjorn SE, Nordfang O. Effect of leukocyte proteinases on tissue factor pathway inhibitor. Thromb Haemost. 1992;67:537–541. [PubMed] [Google Scholar]
- Belaaouaj AA, Li A, Wun TC, Welgus HG, Shapiro SD. Matrix metalloproteinases cleave tissue factor pathway inhibitor. Effects on coagulation. J Biol Chem. 2000;275:27123–27128. doi: 10.1074/jbc.M004218200. [DOI] [PubMed] [Google Scholar]
- Li A, Wun TC. Proteolysis of tissue factor pathway inhibitor (TFPI) by plasmin: effect on TFPI activity. Thromb Haemost. 1998;80:423–427. [PubMed] [Google Scholar]
- Stalboerger PG, Panetta CJ, Simari RD, Caplice NM. Plasmin proteolysis of endothelial cell and vessel wall associated tissue factor pathway inhibitor. Thromb Haemost. 2001;86:923–928. [PubMed] [Google Scholar]
- Ott I, Malcouvier V, Schomig A, Neumann FJ. Proteolysis of tissue factor pathway inhibitor-1 by thrombolysis in acute myocardial infarction. Circulation. 2002;105:279–281. doi: 10.1161/hc0302.103591. [DOI] [PubMed] [Google Scholar]
- Nyberg A, Fagerberg A, Ahlqvist M, Jern C, Seeman-Lodding H, Aneman A. Pulmonary net release of tissue-type plasminogen activator during porcine primary and secondary acute lung injury. Acta Anaesthesiol Scand. 2004;48:845–850. doi: 10.1111/j.1399-6576.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- Creasey AA, Chang AC, Feigen L, Wun TC, Taylor FB, Jr, Hinshaw LB. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest. 1993;91:2850–2856. doi: 10.1172/JCI116529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa SP, Opal SM. Tissue factor pathway inhibitor and antithrombin trial results. Crit Care Clin. 2005;21:433–448. doi: 10.1016/j.ccc.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Narita M, Bu G, Olins GM, Higuchi DA, Herz J, Broze GJ, Jr, Schwartz AL. Two receptor systems are involved in the plasma clearance of tissue factor pathway inhibitor in vivo. J Biol Chem. 1995;270:24800–24804. doi: 10.1074/jbc.270.42.24800. [DOI] [PubMed] [Google Scholar]
- Mast AE, Acharya N, Malecha MJ, Hall CL, Dietzen DJ. Characterization of the association of tissue factor pathway inhibitor with human placenta. Arterioscler Thromb Vasc Biol. 2002;22:2099–2104. doi: 10.1161/01.atv.0000042456.84190.f0. [DOI] [PubMed] [Google Scholar]
- Lupu C, Goodwin CA, Westmuckett AD, Emeis JJ, Scully MF, Kakkar VV, Lupu F. Tissue factor pathway inhibitor in endothelial cells colocalizes with glycolipid microdomains/caveolae: regulatory mechanism(s) of the anticoagulant properties of the endothelium. Arterioscler Thromb Vasc Biol. 1997;17:2964–2974. doi: 10.1161/01.atv.17.11.2964. [DOI] [PubMed] [Google Scholar]
- Sevinsky JR, Rao LV, Ruf W. Ligand-induced protease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J Cell Biol. 1996;133:293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.