Abstract
We here examined whether c-Jun NH2 terminal kinase (JNK) might be involved in the progression of urothelial carcinomas. In vitro and in vivo invasion assays using Matrigel and chick embryo chorioallantoic membrane approaches showed constitutive activation of JNK to significantly increase two processes, invasion and angiogenesis, in the human urothelial carcinoma cell line kU-7, this being suppressed by a JNK inhibitor, SP600125, or cell-permeable peptides. In addition, we found that mitogen-activated protein kinase phosphatase (MKP)-1 functions as an endogenous inhibitor of JNK-mediated signals in urothelial carcinoma cells: chorioallantoic membrane assays showed UMUC14 cells with low MKP-1 expression to be more invasive and have pronounced angiogenesis compared to UMUC6 cells with high MKP-1. Furthermore, knockdown of the MKP-1 gene by siRNA transfection enhanced JNK activation in UMUC6 cells to the UMUC14 level. Immunohistochemically, JNK was found to be highly phosphorylated in high-grade and invasive carcinomas (≥pT2) as well as carcinoma in situ but not in low-grade and noninvasive phenotypes (pTa, pT1). In contrast, MKP-1 was much more expressed in low-grade/noninvasive cancers than with the high-grade/invasive phenotype, reversely correlating with phosphorylated JNK. Taken together, JNK activation and decreased expression of MKP-1 may play important roles in progression of urothelial carcinoma.
There are ∼336,000 new cases of urothelial carcinoma and 132,000 deaths annually all throughout the world.1 In Japan, ∼90% of the lesions are derived from the urinary bladder, and cancers are extremely rare in the renal pelvis, ureter, and other sites, as in Western countries.2 Most tumor types progress to a malignant phenotype through a single pathway involving accumulation of genetic and epigenetic abnormalities in key signals that regulate cell growth, survival, cell death, or cell-to-cell interaction.3,4 However, urothelial carcinomas appear to be grouped into two types, markedly differing in their biological behavior and prognoses: low-grade (G1, G2)/noninvasive cancers (pTa, pT1), including papillary urothelial neoplasms of low malignant potential, and high-grade (G3)/muscle invasive lesions (greater than pT2). Approximately 80% of urothelial carcinomas are of noninvasive type, these often being multifocal or recurrent (∼70%) but with limited muscle invasion (∼15%) and a good prognosis.5 This type of tumor is often preceded by simple and nodular papillary urothelial hyperplasia and harbors frequent mutations in ras (30 to 40%) and fibroblast growth factor receptor 3 (∼70%) genes,6,7 suggesting primary involvement of activation of receptor tyrosine kinase and oncogenic ras. In contrast, high-grade and muscle invasive cancers progress to local and distant metastases despite radical cystectomy or systemic chemotherapy. They seem to arise de novo or from high-grade carcinoma in situ and show loss of function of the tumor suppressor genes p53 and/or Rb.8,9 Elucidation of whether other key signals may also differ between the two divergent types of urothelial cancer was the aim of the present study.
c-Jun NH2 terminal kinase (JNK), a member of the mitogen-activated protein (MAP) kinase family including extracellular stress-regulated kinase (ERK) and p38, is well known to play roles in cell death or survival in various neoplasms.10,11 In general, ERK activation favors cell proliferation, whereas JNK and p38 cause cell death or sensitize cancer cells to chemotherapy-induced cytotoxicity.12,13,14 We previously demonstrated that JNK/p38 activation and downstream signaling through p53 or phosphorylation of Fas-associated death domain protein or Bcl-2 are associated with sensitization to anticancer drug-induced apoptosis, with ERK activation reducing the chemosensitivity in human prostate cancer cells.15,16,17 JNK is also reported to have tumor-promoting functions in various types of cancer cells.18,19,20 Cuevas and colleagues21 recently showed an upstream kinase of JNK, MEKK1, to enhance cell migration, cell invasion, and metastasis through up-regulating urokinase-type plasminogen activator in breast cancer cells. Negative regulation of MAP kinases, including JNK, is mediated primarily by MAP kinase phosphatases (MKPs), a group of 11 dual-specificity phosphatases that dephosphorylate the regulatory threonine and tyrosine residues.22 MKP-1 was the first of this family to be associated with cell cycle progression (G0 to G1 transition) and resistance to cytotoxicity by a variety of genotoxic stresses,23,24,25 through modulating the MAP kinase activity,26,27 which contributes to carcinogenetic processes.28,29 However, MKP-1 may cause cellular differentiation through inhibiting ERK, a promoter of the cell cycle and growth, resulting in strong suppression of tumor development. Thus, activation of MAP kinases and the inactivation by MKPs have complicated functions, probably associated with both promotion and suppression of malignant tumors.
In the present study, we therefore focused on biological roles of JNK activation and MKP-1, in two different processes, invasion and angiogenesis, of urothelial carcinoma cells. In addition, an immunohistochemical study of human urinary bladder cancer specimens was included to estimate the pathological interactions between phosphorylated JNK/MKP-1 expression and tumor grade, invasion depth, and tumor-related microvascular proliferation.
Materials and Methods
Cell Culture, Plasmids, and Chemicals
Human urothelial carcinoma cell lines, kU-7, UMUC6, and UMUC14, were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum as previously described.30,31 His- and Myc-tagged plasmids encoding human JNK1 (SRα-HA-JNK1) and the upstream kinase MKK7 (SRα-3XMyc-MKK7) were constructed as described previously.17,32 The hygromycin-resistant gene (pTK-Hyg) was from Clontech Laboratories (Tokyo, Japan) and the JNK inhibitor SP600125 and the JNK inhibitor cell-permeable peptide were from Calbiochem (Tokyo, Japan). Rabbit polyclonal anti-phosphorylated JNK antibodies were from Cell Signaling (Danvers, MA); goat polyclonal anti-matrix metalloproteinase (MMP)-9 and rabbit anti-Myc tag antibody from Abcam Ltd. (Cambridge, UK), and mouse monoclonal anti-His, rabbit polyclonal anti-JNK1, anti-MKP-1, anti-green fluorescence protein (GFP), anti-vascular endothelial growth factor (VEGF), and anti-actin antibodies were from Santa Cruz Biotechnologies (Santa Cruz, CA). We have estimated the specificities of antibodies against JNK, phosphorylated JNK, and MKP-1. KU7 cells were stained with anti-JNK antibody, which was canceled by siRNA transfection. KU7 cells stably overexpressing MKK7 (constructed in the current study), by which JNK is constitutively phosphorylated (Figure 1), were strongly stained with phosphorylated JNK but negative in control clones. KU7 cells overexpressing MKP-1 were strongly positive for anti-MKP-1 antibody, which was canceled by knockdown of the MKP-1 gene. These cells were similarly fixed and processed as per the clinical specimens (data not shown). Taken together, specificities of antibodies used in the current study are enough to assess the level of protein expression.
Figure 1.
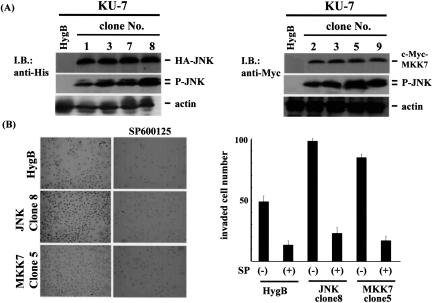
JNK activation enhances invasiveness of urothelial carcinoma cells in vitro. A: A hygromycin-resistant gene was co-transfected with or without plasmids encoding HA-tagged JNK or Myc-tagged MKK7 gene into the human urothelial carcinoma cell line kU-7. After cultivation in the presence of hygromycin B, resistant clones were selected: HygB denotes cells transfected with hygromycin-resistant gene alone (for control), clones 1, 3, 7, and 8 were for HA-JNK, and clones 2, 3, 5, and 9 were for Myc-MKK7. Expression of HA-JNK, Myc-MKK7, and phosphorylated JNK were confirmed by immunoblotting using anti-HA, anti-Myc, and anti-phosphorylated JNK antibodies. Actin expression was used as an internal control. B: Control clone (HygB), HA-JNK clone 8, and Myc-MKK7 clone 5 were pretreated with or without 25 μmol/L SP500125 for 1 hour and then were placed in inserts of Matrigel chambers. After cultivation for 36 hours, invading cells were stained (left), and their numbers were counted under a light microscope (right). The experiment was repeated three times, and the values are means ± SE.
Preparation of Cell Lysates and Immunoblotting Analysis
Immunoblotting using cell lysates was conducted as previously described.16,33 In brief, cells were washed with phosphate-buffered saline and suspended in lysis buffer [40 mmol/L HEPES, pH 7.4, with 10% glycerol, 1% Triton X-100, 0.5% Nonidet P-40, 150 mmol/L NaCl, 50 mmol/L NaF, 20 mmol/L β-glycerol phosphate, 1 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1 mmol/L phenylmethyl sulfonyl fluoride, and 0.1 mmol/L vanadate] containing a protease inhibitor mixture (1 μg/ml aprotinin, leupeptin, and pepstatin). Cell lysates were cleared by centrifugation at 15,000 rpm for 30 minutes and then resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membranes (Millipore Ltd., Bedford, MA). The membranes were blocked in TBST buffer (20 mmol/L Tris-HCl, pH 7.5, containing 150 mmol/L NaCl and 0.1% Tween 20) with 5% skim milk at room temperature for 1 hour, and then incubated with indicated primary antibodies for 16 hours at 4°C. After washing with TBST, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-sheep IgG (GE Biosciences, Tokyo, Japan). After further washing with TBST, peroxidase activity was detected using an enhanced chemiluminescence detection system.
Stable Transfection of Expression Vectors
To establish cell lines stably expressing vectors, 105 cells per well were seeded in six-well plates, cultured in fresh medium for 24 hours, and then co-transfected with the pTK-Hyg vector harboring the hygromycin-resistant gene and an expression vector encoding His-tagged JNK1 or Myc-tagged MKK7 using Lipofectamine (Invitrogen, Tokyo, Japan) in accordance with the manufacturer’s protocol. The transfectants were grown for 1 month in the presence of hygromycin, and several viable clones for each gene were selected.
Tissue Samples, Histology, and Immunohistochemistry
Specimens of human urinary bladder cancers diagnosed as urothelial carcinomas (n = 73) were obtained from patients undergoing transurethral resection or radical cystectomy without radiation or chemotherapy at Nara Medical University Hospital. Informed consent was obtained from all patients before the collection of specimens as appropriate and tumor stage and grade were noted at the time of diagnosis. Fixation and tissue processing were as described in a previous report.34 Sections were incubated with the indicated primary antibodies for 16 hours at 4°C and binding was visualized using a streptavidin-peroxidase kit with diaminobenzidine as the chromogen (Nichirei, Tokyo, Japan), with hematoxylin counterstaining. Percentages of cells positive for phospho-JNK or MKP-1 were estimated from counts of at least 1000 cells examined. Microvessel count was quantified using immunohistochemical staining for CD34 and CD31 as markers for endothelial cells. The most vascularized area was identified by scanning the sections at a magnification of ×40. Then, the number of microvessels positive for CD31 or CD34 was counted in 10 selected spots at a magnification of ×200 (0.95 mm2 field area). The mean count of 10 spots was defined as the microvessel counts per field area, to avoid the bias.
Matrigel Invasion Assays
In vitro invasion assays were performed using Matrigel invasion chambers (BD Biosciences, Bedford, MA). Briefly, 2 × 104 KU7 cells of the control clone (transfected with hygromycin-resistant gene encoding vector only) and the stable clones overexpressing HA-tagged JNK or Myc-tagged MKK7 were placed in the insert. After 36 hours of incubation at 37°C, the chambers were scrubbed with a cotton swab to remove noninvading cancer cells, and invading cells were fixed and stained with the Diff Quick staining kit (Sysmex, Kobe, Japan) and then counted under a light microscope. The experiment was repeated three times.
Chick Chorioallantoic Membrane Assays
In vivo cancer cell invasion and intravasation assays were performed using 11-day-old chick embryos wherein 106 cancer cells were transfected with GFP-encoding vector, pEGFP (Clontech, Mountain View, CA). After 24-hour cultivation, cells were seeded on chorioallantoic membranes (CAMs) for 3 days.35 After collection of CAM samples, tissues were fixed and stained with anti-GFP to allow the numbers of invading cancer cells positive for GFP to be quantified in three or more randomly selected fields. Depth of invasion from the CAM surface was defined as the leading front of three or more invading cells in five randomly selected fields. Angiogenesis was assessed as the number of visible blood vessel branch points within a defined area of the filter disk.35,36 At least three CAMs were used for each experiment.
Transfection of MKP-1 siRNA
UC6 cells were seeded at 106 cells per well in 6-cm dish plates and then transfected with 100 nmol/L of control or MKP-1 siRNA (Santa Cruz Biotechnology) in the presence of the GFP-carrying vector pEGFP. After 16 hours of cultivation, cells were harvested and cultured on a CAM for 3 days. The grafts and the underlying CAM tissues were removed and homogenized, and their lyses were used for immunoblotting to analyze MKP-1 expression.
Gelatin Zymography
Activity and expression of MMP-9 were analyzed by gelatin zymography and immunoblotting, respectively. Equal amounts of concentrated conditioned medium obtained from cell culture experiments or equal amounts of sample protein (30 mg) of CAM tissue lysates were mixed with sample buffer (0.25 mol/L Tris-HCl, pH 6.8, 0.4% sodium dodecyl sulfate, 40% glycerol, and bromphenol blue) and loaded onto 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis containing 1 mg/ml gelatin (Wako Pure Chemical Industries, Ltd., Osaka, Japan). After electrophoresis, the gel was stained with Coomassie Blue solution using Quick-CBB plus (Wako Pure Chemical Industries, Ltd.) in accordance with the manufacturer’s protocol, followed by destaining with destaining buffer (5% acetic acid and 10% ethanol in distilled water) until bands of gelatinolytic activity could be visualized. The proteins were transferred to polyvinylidene difluoride membranes and then immunoblotting using anti-MMP-9 antibody was performed.
Statistical Analysis
The Wilcoxon’s rank sum test was used to analyze the distribution of percentages of phosphorylated JNK- or MKP-1-positive cells. Statistical analyses were performed using the Fisher’s exact test supplemented by the Bonferroni procedures as described previously.37 Results were considered significant if the P was <0.05.
Results
Overexpression of JNK and MKK7 Enhances in Vitro Invasion of Urothelial Carcinoma Cells
To examine the role of JNK activation in invasion by urothelial carcinoma cells, we constructed the urothelial carcinoma cell line KU7 stably overexpressing JNK or the specific upstream kinase MKK7 by which JNK can be constitutively phosphorylated. As shown in Figure 1A, several clones of stable transfectants were obtained, and single clones with highly phosphorylated JNK were selected, clone 8 for HA-JNK and clone 5 for Myc-MKK7. Cell invasion through Matrigel was enhanced by JNK/MKK7 overexpression, and this was canceled by treatment with the JNK inhibitor SP600125. SP600125 is known to be cytotoxic, but there were no significant effects on survival of urothelial carcinoma cells for up to 5 days as assessed by cell viability assay (data not shown). Even in a control clone, invasion was significantly inhibited by JNK inhibition (Figure 1B).
JNK Activation in Urothelial Carcinoma Cells Contributes to Two Different Processes, Invasion and Angiogenesis, in Vivo
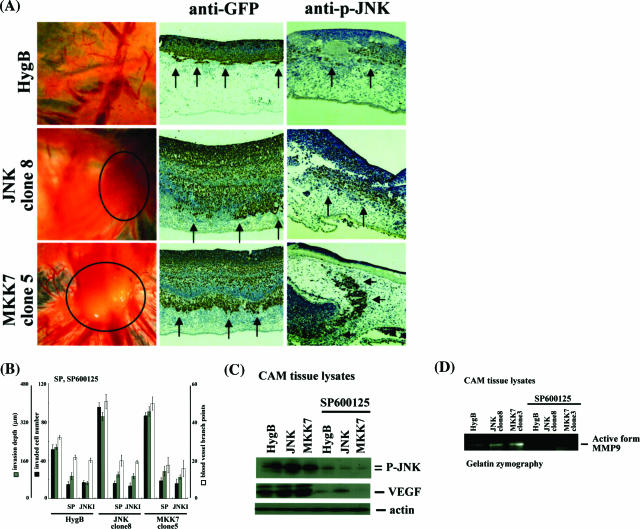
We next performed CAM assays to examine whether JNK affects in vivo invasion and angiogenesis. Macroscopic findings showed numerous allantoic vessels radiating in a wheel pattern toward the grafts of cancer cells stably overexpressing JNK/MKK7, and blood vessel branch points were up-regulated. Because cancer cells were transfected with GFP-encoding vector before plating on a CAM, cancer invasion was immunohistochemically assessed using anti-GFP antibodies. Invasion depth and invading cell numbers were significantly increased by constitutive JNK activation in KU7 cells (Figure 2, A and B). Interestingly, JNK was also highly activated in the control clone, especially in the invasive areas, even though the activity was less than that of stable clones expressing JNK/MKK7 (Figure 2A). We used two JNK inhibitors, SP600125 and JNK inhibitor cell-permeable peptide (JNKI), which strongly canceled both processes, invasion and angiogenesis (Figure 2B). To estimate downstream signals, we examined activities of matrix metalloproteinases (MMPs) and expression of VEGF using gelatin zymography or immunoblotting. Activity of MMP-9 and expression of VEGF were much higher with constitutive activation of JNK (Figure 2, A and B). Induction of both MMP-9 and VEGF was completely canceled by the JNK inhibitor SP600125. Other MMPs such as MMP-1, -2, -7, and -13 and other factors that contribute to tumor angiogenesis, like the fibroblast growth factor and interleukin-6, were also examined, but these molecules were not significantly changed by JNK activation or inhibition (data not shown). Taken together, JNK activation plays an essential role in both invasion and angiogenesis, through increasing MMP-9 and VEGF in urothelial carcinoma cells.
Figure 2.
Effects of JNK activation in urothelial carcinoma cells on invasion and angiogenesis. A and B: Control clone (HygB), HA-JNK clone 8, and Myc-MKK7 clone 5 derived from the human urothelial carcinoma cell line kU-7 were transfected with pTK-GFP. After 24 hours, cells were pretreated with or without 25 μmol/L SP600125 or 1 μmol/L JNK inhibitory cell-permeable peptide for 1 hour and then were seeded on top of the CAMs of 11-day-old chicks and cultured for 3 days. Macroscopic findings (left column) and results of immunohistochemical staining of CAM cross sections using anti-GFP or anti-phosphorylated JNK antibodies (middle and right columns) are demonstrated in A. Invasion was assessed by counting the number of invading cells per high-power field and quantifying front depth of invasion (three or more cells). Vessels were enumerated by counting vessel branch points in a double-blinded manner. B: Each value is the mean ± SE of at least three experiments. C and D: After 3 days of culture, grafting tumor masses (control, HA-JNK, Myc-MKK7 with or without SP600125 pretreatment for 1 hour) on CAMs were collected, homogenized, and lysed with lysis buffer. Expression of phosphorylated JNK, VEGF (C), or active form of MMP-9 (D) were examined by immunoblotting using anti-phosphorylated JNK, anti-VEGF antibodies, or gelatin zymography as described in Materials and Methods.
MKP-1 Is an Endogenous Inhibitor of JNK-Dependent Signaling in Two Processes, Invasion and Angiogenesis
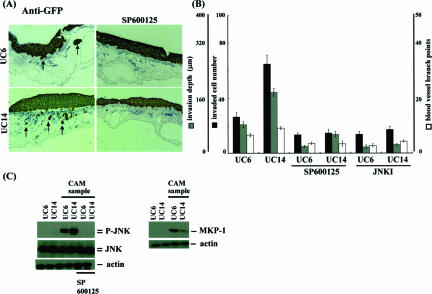
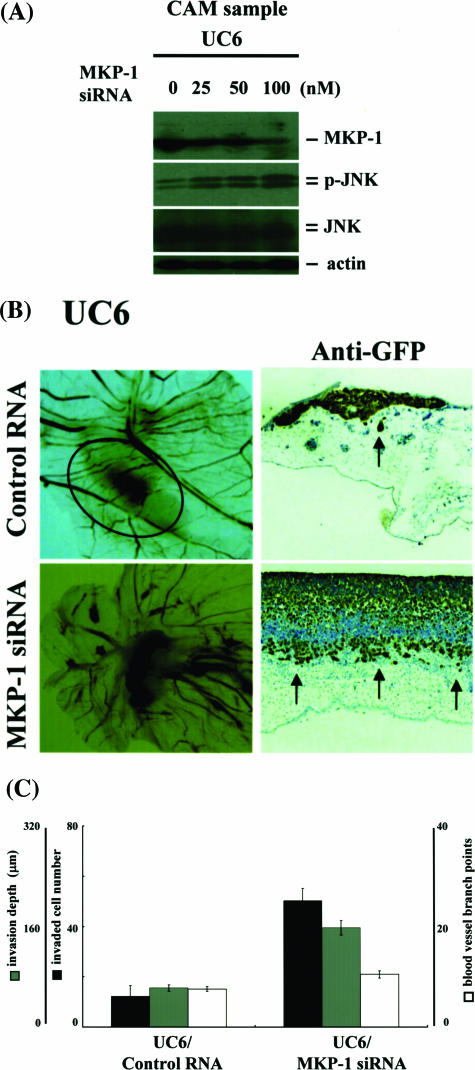
To estimate endogenous molecules that regulate JNK activity, we compared the less-invasive urothelial carcinoma cell line UMUC6 with the highly invasive UMUC14. CAM assays showed clear differences between the two cell lines: invading cell number, invasion depth and angiogenesis of UMUC6 were less than those of UMUC14 (Figure 3A). Consistently, phosphorylated JNK levels in UMUC14 were much higher than in UMUC6, even though JNK was not activated before grafting on CAM in both lines (Figure 3B). Invasion and angiogenesis were strongly inhibited by JNK inhibition (SP600125 or JNKI) in UMUC6 and UMUC14, so that the differences between the two cell lines were canceled (Figure 3, A and B). Interestingly, expression of an endogenous inhibitor of MAP kinases, MKP-1, was more up-regulated in UMUC6 compared with UMUC14 (Figure 3C). Other isoforms of MKPs were not significantly induced (data not shown). We next examined the effects of knockdown of MKP-1 on JNK activation and the dependent invasion/angiogenesis. UMUC6 cells were transfected with MKP-1 siRNA ranging from 0 to 100 nmol/L for 24 hours and then cells were seeded on top of CAMs and incubated for 3 days. MKP-1 expression was reduced, whereas JNK was phosphorylated by siRNA transfection in a dose-dependent manner (Figure 4A). Macroscopically, tumor mass formation and growth of allantoic vessels toward the grafts were enhanced by MKP-1 knockdown, as supported by quantitative assessment of invading cell number, invasion depth, and blood vessel branch points (Figure 4, B and C). Total protein expression level of JNK was not significantly changed between UMUC6 and UMUC14 cells. Moreover, the amount of JNK activation or MKP-1 induction by nonspecific extracellular stresses, such as high osmotic pressure, radiation exposure, and so forth, was almost equal between UMUC6 and UMUC14 cells. No promoter methylation of JNK or MKP-1 genes was observed in these lines. We concluded UMUC6 and UMUC14 cells are useful cell lines for evaluation of the physiological roles of JNK activation and MKP-1 induction or the interactions between JNK and MKP-1 in invasion and angiogenesis processes of human urothelial carcinoma cells.
Figure 3.
Phosphorylated JNK and the dependent invasion/angiogenesis are inversely correlated with MKP-1 expression in human urothelial carcinoma cells. A and B: The human urothelial carcinoma cell lines UMUC-6 and UMUC-14 were transfected with pTK-GFP and after a 24-hour cultivation were treated with or without 25 μmol/L SP600125 or 1 μmol/L JNK inhibitory cell-permeable peptide for 1 hour then they were seeded on top of CAMs and incubated for 3 days. A: Immunohistochemical findings of cross sections using anti-GFP antibody are shown. Invasion was assessed by counting the number of invading cells per high-power field and quantifying front depth of invasion (three or more cells), whereas vessels were enumerated by counting vessel branch points in a double-blinded manner. B: Each value is the mean ± SE from at least three experiments. C: Samples obtained from UMUC6 and UMUC14 cells cultured in vitro or grafted on top of CAMs were lysed with lysis buffer as described in Materials and Methods, and expression of phosphorylated JNK, JNK, and MKP-1 was examined by immunoblotting using anti-phosphorylated JNK, anti-JNK, and anti-MKP-1 antibodies.
Figure 4.
Reduction of MKP-1 enhances JNK activation and the dependent invasion/angiogenesis in human urothelial carcinoma cells. A: The human urothelial carcinoma cell line UMUC6 was co-transfected with pTK-GFP and MKP-1 siRNA ranging from 0 to 100 nmol/L. After a 24-hour cultivation, cells were seeded on top of CAMs and incubated for 3 days. Grafting tissue samples were collected and lysed with lysis buffer. Knockdown efficacy of MKP-1 and expression of phosphorylated JNK were examined by immunoblotting using anti-MKP-1, anti-phosphorylated JNK, and anti-JNK antibodies. B and C: Cells were transfected with 100 nmol/L of control or MKP-1 siRNA. After a 24-hour cultivation, they were seeded on top of CAMs and incubated for 3 days. Macroscopic (left column) and immunohistochemical findings (right column) using anti-GFP antibodies for cross sections are demonstrated in B. Invasion was assessed by counting the number of invading cells per high-power field and quantifying front depth of invasion (three or more cells), whereas vessels were enumerated by counting vessel branch points in a double-blinded manner. C: Each value is the mean ± SE of at least three experiments.
Expression of Phosphorylated JNK and MKP-1 in Human Urothelial Carcinoma of the Urinary Bladder
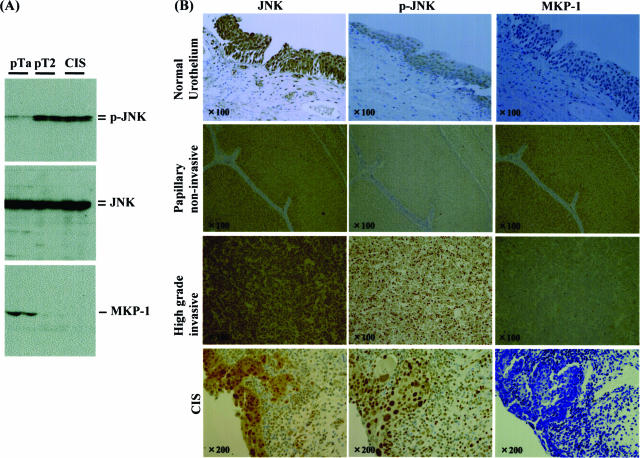
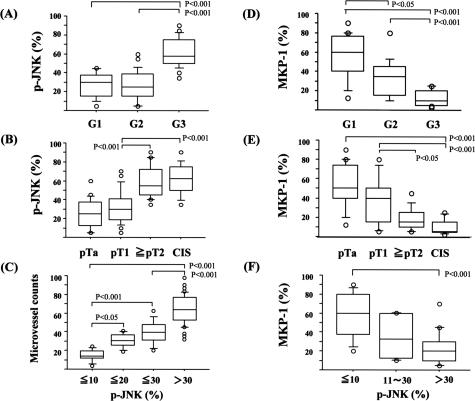
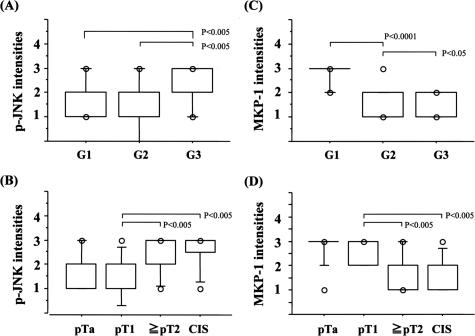
Finally, immunohistochemical analysis of phosphorylated JNK (p-JNK) and MKP-1 expression with reference to grade, invasiveness, and angiogenesis was examined using human bladder cancer specimens. To examine the specificities of antibodies used in this study, immunoblotting was performed using surgical specimens of low-grade/noninvasive, high-grade/invasive, and carcinoma in situ, all of which were diagnosed by two urological pathologists. JNK, phosphorylated JNK, and MKP-1 were observed as a single or double bands, and nonspecific ones were not found very often, suggesting antibodies used in the current study are of high specificities. The expression pattern of these molecules demonstrated in Figure 5A was consistent with the immunohistochemical analysis: as shown in Figure 5B and Figure 6, A and B, the percent positivity of p-JNK was much higher in high-grade (G3) and invasive urothelial carcinomas (≥pT2) including CIS (G3) than in low-grade (G1 and G2) lesions with a noninvasive phenotype (pTa and pT1), even though total expression of JNK was unchanged [G1, 26.6 ± 3.3%; G2, 26.7 ± 2.8% versus G3, 61.2 ± 2.8% (P < 0.01) and pTa, 25.4 ± 2.8%; pT1, 31.9 ± 3.8% versus ≥pT2, 59.4 ± 4.3%, CIS, 62.5 ± 4.4% (P < 0.01)]. Microvessel density assessed by CD34 or CD31 immunostaining correlated with the positive percentage for p-JNK (Figure 6C). In contrast, MKP-1 was highly expressed in low-grade and noninvasive cancers but to a much lesser extent in high-grade, CIS, and invasive cancers [G1, 54.7 ± 5.8%; G2, 34 ± 5.3% versus G3, 12.4 ± 2.0% (P < 0.01) and pTa, 25.4 ± 2.8%; pT1, 31.9 ± 3.8% versus ≥pT2, 59.4 ± 4.3%; CIS, 62.5 ± 4.4% (P < 0.01)] (Figures 5 and 6, D and E). Interestingly, the positive percentage for MKP-1 was higher in cancer cells with lower p-JNK (%MKP-1: 58.1 ± 9.2% in p-JNK less than 10% versus 22.7 ± 3.8% in p-JNK more than 30% (P < 0.05)] (Figures 5 and 6F). The intensities for phosphorylated JNK and MKP-1 were assessed by 0, +1 (weak), +2 (moderate), +3 (high), and +4 (very high), which showed almost similar results to the data of positive percentages (Figure 7). In normal urothelial cells, JNK was highly expressed, but expression of phosphorylated JNK and MKP-1 was very low (Figure 5B).
Figure 5.
Immunohistochemical findings for JNK, phosphorylated JNK, and MKP-1 in human urothelial carcinomas of the urinary bladder. A: Tissue samples of urothelial carcinoma obtained by transurethral resection or radical cystectomy were divided into three groups, low-grade (G1, 2)/noninvasive (pTa, pT1) phenotype, high-grade (G3)/invasive (more than pT2) phenotype, and G3/carcinoma in situ. Two cases for each were collected and lysed and then immunoblotting using anti-JNK, anti-phosphorylated JNK, and anti-MKP-1 antibodies was performed. B: All samples and normal urothelial cells distant from cancer foci were stained with these antibodies.
Figure 6.
Box plot figures of immunohistochemical analyses (positive percentages) of human urothelial carcinomas. A, B, D, and E: Relationships between percentages of positive cells for phosphorylated JNK or MKP-1 and grade [G1, 2, and 3)/stage pTis (CIS), pTa, pT1, and ≥pT2]. C: The relationship between microvessel counts and percentages of cells positive for phosphorylated JNK. F: The relationship between percentages of positive cells for MKP-1 and phosphorylated JNK.
Figure 7.
Box plot figures of immunohistochemical analyses (intensities) of human urothelial carcinomas. A–D: Relationships between intensities of positive cells for phosphorylated JNK or MKP-1 and grade [G1, 2, and 3)/stage pTis (CIS), pTa, pT1, and ≥pT2].
Discussion
In the present study, we demonstrated for the first time that JNK activation could account for highly invasive phenotypes of urothelial carcinoma cells and contribute to cancer development through MMP-9 and VEGF induction in vitro and in vivo. Interestingly, JNK-mediated enhancement of cancer invasion or angiogenesis was confirmed by immunohistochemical analyses using human urothelial carcinoma specimens: JNK was found to be highly phosphorylated in high-grade (G3) and muscular invasive phenotypes (≥pT2) but to only a much lower extent in low-grade and noninvasive ones, even though total expression of JNK was similar. In addition, tumor vessel counts were increased in bladder urothelial carcinoma with higher positive percentages for phosphorylated JNK. Evidence has accumulated of essential roles of upstream and downstream pathways of MAP kinases in cancer invasion or metastasis, but most attention has been focused on the function of ERK38,39,40,41,42,43,44 without any pathological evidence using human cancer specimens. A number of authors have investigated the biological significance of JNK in detail: MAP kinase kinase kinase (MEKK) 1, upstream kinase of JNK, has been reported to exert positive effects on cancer invasion or metastasis through induction of MMPs or urokinase type tissue plasminogen activator (u-PA), FAK activation via co-localization-dependent mechanism, or Rho family GTPases, and so forth.42,43,44 Recently, Lijnen and colleagues45 indicated that u-PA, upstream of MMP-1, -2, -3, -9, -10, or -13,46,47 destroys basement membrane integrity, resulting in metastasis in vivo.21 The promoter regions of MMP-2 and MMP-9 feature a binding motif of AP-1 that can be activated by MAP kinases. Taken together, JNK activation appears involved in cancer progression, especially invasion, as indicated in our present study. JNK phosphorylation is well known to be a downstream signal of Ras.48,49,50 In addition, recent reports demonstrated that JNK activation sensitizes cancer cells including urothelial carcinomas to anticancer drug-induced cytotoxicity.15,37,51 The studies emphasized anti-tumor functions of JNK, which seem to be inconsistent with our present results. There are no critical explanations for this discrepancy, but Igaki and colleagues52 found that JNK activation switches its proapoptotic effects to survival effects on tumor cells in the presence of oncogenic Ras in Drosophila genetic models. If their theory fits with urothelial carcinoma cells, this raises the possibility that activated JNK switches a noninvasive phenotype with frequently mutated Ras to an invasive one, conferring resistance to chemotherapy via prosurvival effects. Whatever the case, it should be emphasized that activated JNK has positive and negative effects on urothelial carcinoma development. As to the role of JNK in tumor angiogenesis, Kim and colleagues53 reported thrombospondin production can be mediated through JNK activation, whereas Ennis and colleagues54 found JNK inhibition to suppress endothelial cell migration and development of vasculature. Similarly to MMP-2 and -9, the promoter region of VEGF has an AP-1-binding motif,55,56 and VEGF expression has been reported to be increased in urothelial carcinomas with malignant potential.57,58,59 The results support tumor angiogenesis via activation of the JNK/VEGF axis as suggested in our study. Because angiogenesis can be frequently seen even in low-grade, noninvasive urothelial carcinomas, JNK-mediated cancer progression might be mainly mediated through enhancement of invasion rather than VEGF-associated angiogenesis. It is well known that VEGF has a variety of biological functions as well as angiogenesis; therefore, we should further evaluate the roles of JNK-dependent induction of VEGF in urothelial carcinoma development.
One more important finding is that decreased expression of MKP-1 can lead to enhancement of JNK activation in urothelial carcinoma cells. CAM assays showed high invasion of the urothelial carcinoma cell line UMUC14, which has more activated JNK and lower expression of MKP-1 than the less invasive line UMUC6, and that knockdown of MKP-1 resulted in enhancement of two processes, invasion and angiogenesis, through increased JNK activity. These in vivo results clearly indicate that MKP-1 inhibits JNK-mediated invasiveness and angiogenesis in urothelial carcinoma cells, as supported by the immunohistochemical analysis showing MKP-1 expression: it was increased in lower-grade and noninvasive bladder cancer but strongly decreased in advanced cases. Loda and colleagues29 previously showed MKP-1 to be overexpressed in prostate, colon, and bladder cancers as compared to normal epithelium but also found loss of expression with higher histological grade and metastases on assessing 164 human epithelial tumors. Our present research suggested that JNK can be activated by decreased expression of its endogenous MKP-1, resulting in enhanced invasiveness and angiogenesis. JNK phosphorylation and MKP-1 expression were not constitutively induced in either UMUC14 or UMUC6 cells, and growth capacity of these lines was not significantly different in vitro (data not shown). However, they were activated or induced during incubation on CAM, and the levels differed between the two cases. JNK activation or MKP-1 expression is well known to be induced by growth factors, UV stimulation, or oxidative stresses, all of which affect cell migration. Therefore, JNK activation or MKP-1 expression might be induced in response to various growth factors during the invasion step. Moreover, CAM analyses might give important pointers to novel mechanisms of two processes, invasion and angiogenesis, of urothelial carcinomas. Some investigators have documented that MKP-1 stimulates progression through the cell cycle43,59,60 and leads to chemoresistance.61,62,63 Therefore, we proposed MKP-1 might contribute to carcinogenesis and resistance to therapy by itself, whereas it could inhibit urothelial carcinoma development.
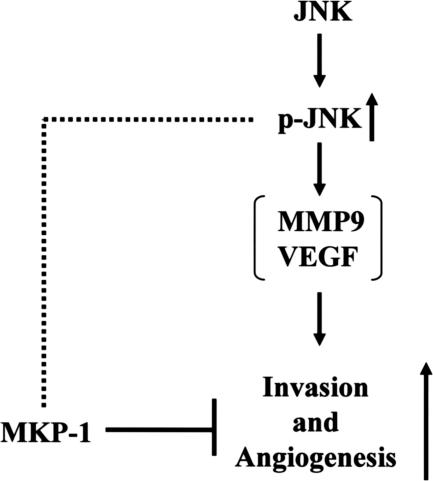
In summary, up-regulation of JNK and the associated induction of MMP-9 and VEGF were found to be novel pathways associated with high-grade muscle invasive urothelial carcinomas. Phosphorylated JNK-mediated invasiveness and angiogenesis were physiologically inhibited by the phosphatase, MKP-1, that is highly expressed in low-grade and noninvasive urothelial carcinoma (Figure 8). We conclude that MKP-1 induction and decreased JNK activity contribute to determination of the noninvasive or invasive phenotypes of urothelial carcinoma cells. Moreover, it is necessary to examine not only MKP-1 expression but also phosphorylated JNK for prediction of malignant behavior or prognosis in urothelial carcinoma, and MKP-1 and activated JNK might be key targets for cancer therapy.
Figure 8.
The biological and pathological roles of phosphorylated JNK and MKP-1 in invasion and angiogenesis of human urothelial carcinomas. Reduction of JNK phosphatase, MKP-1, and activated JNK (p-JNK) enhance invasion and angiogenesis, and low MKP-1/high p-JNK is associated with high-grade/muscle-invasive urothelial carcinoma. In contrast, up-regulation of MKP-1 and decreased p-JNK suppress invasion and angiogenesis, and high MKP-1/low p-JNK expression is associated with low-grade/noninvasive phenotype. Therefore, examination of p-JNK and MKP-1 expression may predict the malignant potential and prognosis of urothelial carcinomas.
Footnotes
Address reprint requests to Dr. Noboru Konishi, Department of Pathology, Nara Medical University School of Medicine, Shijo-cho, Kashihara city, Nara, 634-8521, Japan. E-mail: nkonishi@naramed-u.ac.jp.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- Kakizoe T. Development and progression of urothelial carcinoma. Cancer Sci. 2006;97:821–828. doi: 10.1111/j.1349-7006.2006.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- Jebar AH, Hurst CD, Tomlinson DC, Johnston C, Taylor CF, Knowles MA. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24:5218–5225. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- Mhawech-Fauceglia P, Cheney RT, Schwaller J. Genetic alterations in urothelial bladder carcinoma: an updated review. Cancer. 2006;106:1205–1216. doi: 10.1002/cncr.21743. [DOI] [PubMed] [Google Scholar]
- Steinberg GD, Trump DL, Cummings KB. Metastatic bladder cancer. Natural history, clinical course, and consideration for treatment. Urol Clin North Am. 1992;19:735–746. [PubMed] [Google Scholar]
- Liebert M, Seigne J. Characteristics of invasive bladder cancers: histological and molecular markers. Semin Urol Oncol. 1996;14:62–72. [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Shimada K, Nakamura M, Ishida E, Konishi N. Molecular roles of MAP kinases and FADD phosphorylation in prostate cancer. Histol Histopathol. 2006;21:415–422. doi: 10.14670/HH-21.415. [DOI] [PubMed] [Google Scholar]
- MacKeigan JP, Collins TS, Ting JP. MEK inhibition enhances paclitaxel-induced tumor apoptosis. J Biol Chem. 2000;275:38953–38956. doi: 10.1074/jbc.C000684200. [DOI] [PubMed] [Google Scholar]
- Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, Law PY, Hebbel RP. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247:495–504. doi: 10.1006/excr.1998.4359. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Shimada K, Matsuyoshi S, Nakamura M, Ishida E, Kishi M, Konishi N. Phosphorylation of FADD is critical for sensitivity to anticancer drug-induced apoptosis. Carcinogenesis. 2004;25:1089–1097. doi: 10.1093/carcin/bgh130. [DOI] [PubMed] [Google Scholar]
- Shimada K, Nakamura M, Ishida E, Kishi M, Konishi N. Roles of p38- and c-jun NH2-terminal kinase-mediated pathways in 2-methoxyestradiol-induced p53 induction and apoptosis. Carcinogenesis. 2003;24:1067–1075. doi: 10.1093/carcin/bgg058. [DOI] [PubMed] [Google Scholar]
- Shimada K, Nakamura M, Ishida E, Kishi M, Yonehara S, Konishi N. c-Jun NH2-terminal kinase-dependent Fas activation contributes to etoposide-induced apoptosis in p53-mutated prostate cancer cells. Prostate. 2003;55:265–280. doi: 10.1002/pros.10227. [DOI] [PubMed] [Google Scholar]
- Collins CS, Hong J, Sapinoso L, Zhou Y, Liu Z, Micklash K, Schultz PG, Hampton GM. A small interfering RNA screen for modulators of tumor cell motility identifies MAP4K4 as a promigratory kinase. Proc Natl Acad Sci USA. 2006;103:3775–3780. doi: 10.1073/pnas.0600040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC, Tan TH, Colburn NH. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26:1297–1306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Murakami A, Kawabata K, Ohigashi H. (−)-Epigallocatechin-3-gallate promotes pro-matrix metalloproteinase-7 production via activation of the JNK1/2 pathway in HT-29 human colorectal cancer cells. Carcinogenesis. 2005;26:1553–1562. doi: 10.1093/carcin/bgi104. [DOI] [PubMed] [Google Scholar]
- Cuevas BD, Winter-Vann AM, Johnson NL, Johnson GL. MEKK1 controls matrix degradation and tumor cell dissemination during metastasis of polyoma middle-T driven mammary cancer. Oncogene. 2006;25:4998–5010. doi: 10.1038/sj.onc.1209507. [DOI] [PubMed] [Google Scholar]
- Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Magi-Galluzzi C, Mishra R, Fiorentino M, Montironi R, Yao H, Capodieci P, Wishnow K, Kaplan I, Stork PJ, Loda M. Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Lab Invest. 1997;76:37–51. [PubMed] [Google Scholar]
- Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xu J, Zhou JY, Liu Y, Wu GS. Mitogen-activated protein kinase phosphatase-1 is required for cisplatin resistance. Cancer Res. 2006;66:8870–8877. doi: 10.1158/0008-5472.CAN-06-1280. [DOI] [PubMed] [Google Scholar]
- Lau LF, Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985;4:3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles CH, Abler AS, Lau LF. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992;7:187–190. [PubMed] [Google Scholar]
- Vicent S, Garayoa M, Lopez-Picazo JM, Lozano MD, Toledo G, Thunnissen FB, Manzano RG, Montuenga LM. Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients. Clin Cancer Res. 2004;10:3639–3649. doi: 10.1158/1078-0432.CCR-03-0771. [DOI] [PubMed] [Google Scholar]
- Loda M, Capodieci P, Mishra R, Yao H, Corless C, Grigioni W, Wang Y, Magi-Galluzzi C, Stork PJ. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am J Pathol. 1996;149:1553–1564. [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Gee JR, De La Cerda J, Rosser CJ, Zhou JH, Benedict WF, Grossman HB. Noninvasive detection of bladder cancer in an orthotopic murine model with green fluorescence protein cytology. J Urol. 2003;170:975–978. doi: 10.1097/01.ju.0000073209.65128.c1. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Koul D, Davies MA, Liebert M, Steck PA, Grossman HB. MMAC1/PTEN inhibits cell growth and induces chemosensitivity to doxorubicin in human bladder cancer cells. Oncogene. 2000;19:5406–5412. doi: 10.1038/sj.onc.1203918. [DOI] [PubMed] [Google Scholar]
- Shimada K, Nakamura M, Ishida E, Kishi M, Yonehara S, Konishi N. Phosphorylation of Fas-associated death domain contributes to enhancement of etoposide-induced apoptosis in prostate cancer cells. Jpn J Cancer Res. 2002;93:1164–1174. doi: 10.1111/j.1349-7006.2002.tb01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Matsuyoshi S, Nakamura M, Ishida E, Konishi N. Phosphorylation status of Fas-associated death domain-containing protein (FADD) is associated with prostate cancer progression. J Pathol. 2005;206:423–432. doi: 10.1002/path.1791. [DOI] [PubMed] [Google Scholar]
- Konishi N, Nakamura M, Kishi M, Nishimine M, Ishida E, Shimada K. Heterogeneous methylation and deletion patterns of the INK4a/ARF locus within prostate carcinomas. Am J Pathol. 2002;160:1207–1214. doi: 10.1016/S0002-9440(10)62547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA. Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J Cell Biol. 2003;162:933–943. doi: 10.1083/jcb.200304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi N, Tsujikawa K, Yamamoto H, Ishida E, Nakamura M, Shimada K, Yane K, Yamashita H, Noguchi S. Overexpression of leucocyte common antigen (LAR) P-subunit in thyroid carcinomas. Br J Cancer. 2003;88:1223–1228. doi: 10.1038/sj.bjc.6600876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- Glading A, Chang P, Lauffenburger DA, Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Yujiri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, Zaitsu Y, Clarke P, Tyler K, Oka Y, Fanger GR, Henson P, Johnson GL. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-kappa B activation. Proc Natl Acad Sci USA. 2000;97:7272–7277. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas BD, Abell AN, Witowsky JA, Yujiri T, Johnson NL, Kesavan K, Ware M, Jones PL, Weed SA, DeBiasi RL, Oka Y, Tyler KL, Johnson GL. MEKK1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. EMBO J. 2003;22:3346–3355. doi: 10.1093/emboj/cdg322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger GR, Johnson NL, Johnson GL. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen HR, Van Hoef B, Lupu F, Moons L, Carmeliet P, Collen D. Function of the plasminogen/plasmin and matrix metalloproteinase systems after vascular injury in mice with targeted inactivation of fibrinolytic system genes. Arterioscler Thromb Vasc Biol. 1998;18:1035–1045. doi: 10.1161/01.atv.18.7.1035. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Hattori T, Kimura G, Nakazawa N. Tumor progression and expression of matrix metalloproteinase-2 (MMP-2) mRNA by human urinary bladder cancer cells. Urol Res. 1998;26:371–376. doi: 10.1007/s002400050071. [DOI] [PubMed] [Google Scholar]
- Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- Uchida T, Wada C, Ishida H, Egawa S, Ao T, Yokoyama E, Koshiba K. Infrequent involvement of mutations on neurofibromatosis type 1, H-ras, K-ras and N-ras in urothelial tumors. Urol Int. 1995;55:63–67. doi: 10.1159/000282753. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JM, Ramchurren N, Rieger K, Levesque P, Silverman M, Libertino JA, Summerhayes IC. Identification of H-ras mutations in urine sediments complements cytology in the detection of bladder tumors. J Natl Cancer Inst. 1995;87:129–133. doi: 10.1093/jnci/87.2.129. [DOI] [PubMed] [Google Scholar]
- Burchill SA, Neal DE, Lunec J. Frequency of H-ras mutations in human bladder cancer detected by direct sequencing. Br J Urol. 1994;73:516–521. doi: 10.1111/j.1464-410x.1994.tb07636.x. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Nishiyama H, Matsui Y, Ito M, Kawanishi H, Kamoto T, Ogawa O. Dicoumarol potentiates cisplatin-induced apoptosis mediated by c-Jun N-terminal kinase in p53 wild-type urogenital cancer cell lines. Oncogene. 2006;25:2500–2508. doi: 10.1038/sj.onc.1209162. [DOI] [PubMed] [Google Scholar]
- Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol. 2006;16:1139–1146. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim C, Kim TS, Bang SI, Yang Y, Park H, Cho D. IL-18 enhances thrombospondin-1 production in human gastric cancer via JNK pathway. Biochem Biophys Res Commun. 2006;344:1284–1289. doi: 10.1016/j.bbrc.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Ennis BW, Fultz KE, Smith KA, Westwick JK, Zhu D, Boluro-Ajayi M, Bilter GK, Stein B. Inhibition of tumor growth, angiogenesis, and tumor cell proliferation by a small molecule inhibitor of c-Jun N-terminal kinase. J Pharmacol Exp Ther. 2005;313:325–332. doi: 10.1124/jpet.104.078873. [DOI] [PubMed] [Google Scholar]
- Krejsgaard T, Vetter-Kauczok CS, Woetmann A, Lovato P, Labuda T, Eriksen KW, Zhang Q, Becker JC, Odum N. Jak3- and JNK-dependent vascular endothelial growth factor expression in cutaneous T-cell lymphoma. Leukemia. 2006;20:1759–1766. doi: 10.1038/sj.leu.2404350. [DOI] [PubMed] [Google Scholar]
- Chang HJ, Park JS, Kim MH, Hong MH, Kim KM, Kim SM, Shin BA, Ahn BW, Jung YD. Extracellular signal-regulated kinases and AP-1 mediate the up-regulation of vascular endothelial growth factor by PDGF in human vascular smooth muscle cells. Int J Oncol. 2006;28:135–141. [PubMed] [Google Scholar]
- Zu X, Tang Z, Li Y, Gao N, Ding J, Qi L. Vascular endothelial growth factor-C expression in bladder transitional cell cancer and its relationship to lymph node metastasis. BJU Int. 2006;98:1090–1093. doi: 10.1111/j.1464-410X.2006.06446.x. [DOI] [PubMed] [Google Scholar]
- Kim B, Oh S, van Golen CM, Feldman EL. Differential regulation of insulin receptor substrate-1 degradation during mannitol and okadaic acid induced apoptosis in human neuroblastoma cells. Cell Signal. 2005;17:769–775. doi: 10.1016/j.cellsig.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Yang CC, Chu KC, Yeh WM. The expression of vascular endothelial growth factor in transitional cell carcinoma of urinary bladder is correlated with cancer progression. Urol Oncol. 2004;22:1–6. doi: 10.1016/S1078-1439(03)00015-2. [DOI] [PubMed] [Google Scholar]
- Brondello JM, McKenzie FR, Sun H, Tonks NK, Pouyssegur J. Constitutive MAP kinase phosphatase (MKP-1) expression blocks G1 specific gene transcription and S-phase entry in fibroblasts. Oncogene. 1995;10:1895–1904. [PubMed] [Google Scholar]
- Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci USA. 1998;95:3014–3019. doi: 10.1073/pnas.95.6.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Pérez I, Martinez-Gomariz M, Williams D, Keyse SM, Perona R. CL100/MKP-1 modulates JNK activation and apoptosis in response to cisplatin. Oncogene. 2000;19:5142–5152. doi: 10.1038/sj.onc.1203887. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Bennett AM. Essential role for mitogen-activated protein (MAP) kinase phosphatase-1 in stress-responsive MAP kinase and cell survival signaling. J Biol Chem. 2005;280:16461–16466. doi: 10.1074/jbc.M501762200. [DOI] [PubMed] [Google Scholar]