Abstract
According to the desmoglein (Dsg) compensation concept, different epidermal cleavage planes observed in pemphigus vulgaris and pemphigus foliaceus have been proposed to be caused by different autoantibody profiles against the desmosomal proteins Dsg 1 and Dsg 3. According to this model, Dsg 1 autoantibodies would only lead to epidermal splitting in those epidermal layers in which no Dsg 3 is present to compensate for the functional loss of Dsg 1. We provide evidence that both pemphigus foliaceus-IgG containing Dsg 1- but not Dsg 3-specific antibodies and pemphigus vulgaris-IgG with antibodies to Dsg 1 and Dsg 3 were equally effective in causing epidermal splitting in human skin and keratinocyte dissociation in vitro. These effects were present where keratinocytes expressed both Dsg 1 and Dsg 3, demonstrating that Dsg 3 does not compensate for Dsg 1 inactivation. Rather, the cleavage plane in intact human skin caused by pemphigus autoantibodies was similar to the plane of keratinocyte dissociation in response to toxin B-mediated inactivation of Rho GTPases. Because we recently demonstrated that pemphigus-IgG causes epidermal splitting by inhibition of Rho A, we propose that Rho GTPase inactivation contributes to the mechanisms accounting for the cleavage plane in pemphigus skin splitting.
Pemphigus is an autoimmune blistering skin disease caused by autoantibodies directed to the cadherin-type adhesion molecules desmoglein (Dsg) 1 and 3.1,2,3,4 Antibodies against other antigens, including cholinergic receptors, have also been implicated in the pathogenesis.5,6,7,8,9 Several theories have been proposed to explain the mechanisms underlying blister formation by Dsg autoantibodies. However, the different clinical phenotypes of pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are still not fully understood.10,11,12 It is well established that PV is characterized by deep (suprabasal) epidermal blistering, whereas PF exhibits superficial blistering (involving granular or spinous cell layers). In PF, mucous membranes are not involved; whereas in PV, mucosal disease is a regular finding, and skin involvement may occur in addition.4 Interestingly, these phenotypes usually correlate with different autoantibody profiles. PV patients with only mucosal lesions show autoantibodies to Dsg 3 but not to Dsg 1. PF patients (skin involvement only) reveal autoantibodies to Dsg 1 but not to Dsg 3. In PV patients with both mucous membrane and skin involvement, autoantibodies to Dsg 3 and Dsg 1 may be detected.4,13,14 These observations, together with studies reporting that Dsg 1 is predominantly expressed in superficial epidermal layers but absent in the suprabasal layer, whereas Dsg 3 is restricted to deep epidermal layers, and that Dsg 1 is less abundantly expressed in mucosal epithelia, have led to the desmoglein compensation hypothesis as an explanation for the different epidermal cleavage planes in PV and PF.3,4,15,16,17 According to this model, in the deep epidermis, Dsg 3 compensates for the functional loss of Dsg 1 induced by Dsg 1-specific autoantibodies, resulting in superficial blistering in PF.
However, more recently, this model has been repeatedly questioned.2,10,11 i) The model does not explain why blistering in patients with mucocutaneous PV, where Dsg 1- and Dsg 3-specific autoantibodies are present, occurs suprabasally and not throughout the epidermis. ii) The desmoglein compensation hypothesis postulates that the different forms of pemphigus and the clinical stages of PV are distinguishable by antibody profiles and histology. This view has recently been challenged.2,18 iii) To validate the desmoglein compensation theory, expression patterns of desmogleins have been evaluated in mouse models.4,16 However, recent studies indicate that the expression patterns of Dsg 1 and Dsg 3 in mice profoundly differ from those in humans.19 iv) To visualize the desmoglein localization pattern in human epidermis, autoantibodies from patients with PV and PF have been applied.15,20 In these studies, either whole sera of PV and PF patients were used without characterization of the autoantibody profile20 or IgG fractions were used after immunosabsorption with recombinant Dsg extracellular domains fused to the Fc portion of human IgG.15 More recently, it has been suggested that immunoabsorption using this fusion protein containing the extracellular domain of desmogleins is not entirely specific. Therefore, the specificity of autoantibodies used for immunolocalization of desmogleins in human skin in previous studies is not entirely clear.9 v) Finally, for desmoglein compensation to be a valid mechanism in human epidermis, only autoantibodies against Dsg 1 and Dsg 3 should cause epidermal splitting, and these antibodies should specifically block the adhesive function of the one desmoglein subtype (Dsg 1 or Dsg 3) to which they are directed.4 Therefore, the desmoglein compensation model has been used to support the hypothesis that autoantibodies directly affect desmoglein binding by steric hindrance.16,17 This has also become a matter of discussion because other antibodies, including those antibodies to cholinergic receptors have been found to be pathogenic.2,9 However, we have shown that PF-IgG causes keratinocyte dissociation and inhibition of Dsg 1 binding by mechanisms different from steric hindrance because PF-IgG was not effective in reducing Dsg 1-mediated transinteraction when probed by single-molecule atomic force microscopy.21 This observation supported the idea that autoantibody-triggered signaling mechanisms are involved in pemphigus pathogenesis. Among others, catenin and Rho protein signaling have been shown to be involved in pemphigus pathogenesis.22,23,24 These aspects led us to reconsider the desmoglein compensation concept and to evaluate the role of Rho GTPase inactivation in pemphigus skin blistering.
Materials and Methods
Cell Culture and Test Reagents
The immortalized human keratinocyte cell line HaCaT was grown in Dulbecco’s modified Eagle’s medium (Life Technologies, Karlsruhe, Germany) supplemented with 50 U/ml penicillin-G, 50 μg of streptomycin, and 10% fetal calf serum (Biochrom, Berlin, Germany) in a humidified atmosphere (95% air/5% CO2) at 37°C. The cultures were used for all experiments that used confluent monolayers and were grown in Dulbecco’s modified Eagle’s medium at 1.8 mmol/L Ca2+. Dsg 1 expression was detected by dot blot analysis and immunostaining (day 7 after plating). All experiments were performed using Dulbecco’s modified Eagle’s medium containing 1.8 mmol/L Ca2+. Cytotoxic necrotizing factor 1 (CNF-1) (a kind gift from Gudula Schmidt, University of Freiburg, Freiburg, Germany) was used at 300 ng/ml for 120 minutes as described previously.23 The cell-permeable C 3 fusion toxin (C3FT) from Clostridium botulinum,23 toxin B from Clostridium difficile, and lethal toxin (LT) from Clostridium sordellii were contributed by Holger Barth (University of Ulm, Ulm, Germany) and used at 300 ng/ml for 180 minutes. For experiments using human epidermis, 800 ng/ml toxin B was required.
Purification of PF-IgG
Purification was performed as described previously.21 Sera from two PF patients and two patients suffering from a mucocutaneous form of PV whose diagnosis was confirmed clinically, histologically, and serologically and from a volunteer without any skin disease (control) were used for the present study. Patients’ sera were tested by enzyme-linked immunosorbent assay for reactivity against Dsg 1 and Dsg 3, respectively. IgG fractions PF-IgG 1 and 2 contained Dsg 1 antibodies but no Dsg 3 antibodies, whereas PV-IgG 1 and 2 contained Dsg 1 and Dsg 3 antibodies (enzyme-linked immunosorbent assay cut-off was 2025). IgG fractions were purified by affinity chromatography using protein A agarose as described previously.21 Concentrations of all IgG fractions were adjusted to 150 μg/ml final concentration for all experiments.
Skin Biopsies from Pemphigus Patients
Skin biopsies were taken from PV patient 1 and PF patient 2 when disease was initially diagnosed. After paraffin embedding, semisectioning (5-μm thickness) was performed, and sections were stained with hematoxylin and eosin (H&E).
Ex Vivo Model of Human Skin Splitting
The model was used as described previously.23 Skin pieces were taken from fresh cadavers of individuals not suffering from any skin disease who had donated their bodies to the Institute of Anatomy and Cell Biology of Würzburg. Specimens were incubated with Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum and 1.8 mmol/L Ca2+ for 24 hours in the presence or absence of PV-IgG, PF-IgG, or toxin B. After brief rinsing with phosphate-buffered saline (PBS; consisting of 137 mmol/L NaCl, 2.7 mmol/L KCl, 8.1 mmol/L Na2HPO4, and 1.5 mmol/L KH2PO4, pH 7.4), skin specimens were mounted on copper plates using Reichert-Jung mounting medium (Cambridge Instruments, Nußloch, Germany) and frozen in liquid nitrogen. Cryosections (5 μm thick) were obtained using a Reichert-Jung 2800 Frigocut (Cambridge Instruments). For each condition, three to five pieces of skin (2 × 2 mm) from at least two different cadavers were used and incubated in the presence or absence of patient IgG separately. After immunostaining as described below, serial sectioning was performed. In three to five skin pieces incubated with PV-IgG, PF-IgG, or control IgG, 39 to 63 different sections were evaluated. After each section was harvested, at least 50 μm of tissue was discarded. In the next section, it was verified by microscopic evaluation that no blistering was found to ensure that each blister measured was counted not more than once. For each blister, localization was analyzed and described as deep splitting when suprabasal splitting or splitting within the lower spinous layer was detected and described as superficial splitting when splitting was located in the upper spinous or granular layer. For studies using toxin B, skin was fixed at room temperature with 2% formaldehyde (freshly prepared from paraformaldehyde) in PBS, dehydrated in ascending concentrations of ethanol (50, 70, and 96% and 3 × 100%; 10 minutes each), equilibrated with propylene oxide (two times for 15 minutes), and embedded in Epon 812. Semithin sections (1-μm thick) were stained with toluidine blue or prepared for immunostaining by incubation (5 minutes each) with sodium-methanolate, sodium-methanolate mixed with toluol (1:1), acetone (2×), and H2O, followed by PBS before immunostaining was performed as described below.
Cytochemistry
HaCaT cells were grown on coverslips to confluence as described above (7 days) and incubated with PF-IgG or PV-IgG for 24 hours at 37°C. After incubation with autoantibodies, culture medium was removed, and monolayers were fixed for 10 minutes at room temperature with 2% formaldehyde (freshly prepared from paraformaldehyde) in PBS. Afterward, monolayers were treated with 0.1% Triton X-100 in PBS for 5 minutes at room temperature. Cryosections of human skin were dried on a heat plate for 30 minutes and fixed with ice-cold acetone (−20°C) for 5 minutes. After rinsing with PBS at room temperature, cryosections/semithin sections of human skin or HaCaT cells were preincubated for 30 minutes with 10% normal goat serum and 1% bovine serum albumin at room temperature and incubated for 16 hours at 4°C with mouse monoclonal antibody directed to the ectodomain of human Dsg 1 (clone p124; Progen, Heidelberg, Germany) or Dsg 3 (Zytomed, Berlin, Germany) or against Rho family GTPases Rho A or Rac1 (Upstate, Hamburg, Germany), respectively (dilution 1:100 in PBS each). After several rinses with PBS (three times for 5 minutes), monolayers were incubated for 60 minutes at room temperature with Cy3-labeled goat anti-mouse IgG (Dianova, Hamburg, Germany). For visualization of filamentous actin (F-actin), ALEXA-phalloidin (diluted 1:60 in PBS; Mobitec, Göttingen, Germany) was used (incubation for 1 hour at room temperature). Cells incubated with antibodies or ALEXA-phalloidin were rinsed with PBS (three times for 5 minutes). Immunostaining of cryosections from skin specimens was performed after incubation with PF-IgG and PV-IgG as described for cell culture monolayers. Finally, coverslips were mounted on glass slides with 60% glycerol in PBS containing 1.5% n-propyl gallate (Serva, Heidelberg, Germany) as antifading compound. Monolayers and cryosections/semithin sections were examined using an LSM 510 (Zeiss, Göttingen, Germany). Images were processed using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Transmission Electron Microscopy
As described previously,21 HaCaT monolayers were fixed in 2.5% glutaraldehyde containing 0.01% ruthenium red in 0.1 mol/L sodium cacodylate, pH 7.35, for 1 hour at 4°C. After rinsing in 0.1 mol/L cacodylate (three times for 5 minutes) cells were postfixed in 2% OSO4 in 0.1 mol/L cacodylate for 1 hour at room temperature. Cells were rinsed again in cacodylate and dehydrated in ascending concentrations of methanol (25, 50, 70, and 80%; 2 × 95%; and 2 × 100%; 10 minutes each). After incubation in 70% methanol, monolayers were placed for 1 hour in saturated uranyl acetate in 70% methanol for 1 hour at dark. After dehydration, cells were equilibrated in propylene oxide (two times for 10 minutes) and embedded in Epon 812. Semithin sections (1 μmol/L) were stained with toluidine blue, and ultrathin sections were contrasted with uranyl acetate and lead citrate and examined with a LEO AB 912 electron microscope (Zeiss, Jena, Germany).
Laser Tweezer
Expression and purification of recombinant Dsg 1, coating of polystyrene beads, and the laser tweezer set-up were described previously in detail.21 Through all experiments, the laser intensity was 42 mW in the focal plane. Coated beads (10 μl of stock solution) were suspended in 200 μl of culture medium and allowed to interact with HaCaT monolayers for 30 minutes at 37°C before measuring the number of bound beads (control values). Beads were considered tightly bound when resisting laser displacement at the 42-mW setting. For every condition, 100 beads were counted. Afterward, control IgG, PV-IgG, or PF-IgG was applied for 120 minutes in the absence or presence of CNF-1; or toxin B, C3FT, or LT was added for 180 minutes before the number of bound beads was counted again. Percentage of beads resisting laser displacement under various experimental conditions was normalized to control values.
Statistics
Differences in bead adhesion and epidermal splitting were assessed using Student’s t-test. In text and bar diagrams, values were expressed as mean ± SEM. Statistical significance was assumed for P < 0.05.
Results
PV-IgG and PF-IgG Caused Epidermal Splitting of Layers Expressing Dsg 1 and Dsg 3 in Intact Human Skin in Vitro
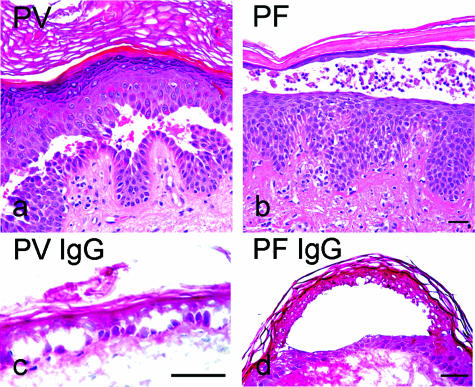
The cleavage planes in skin specimens from the PV and PF patients who contributed IgG fractions for this study clearly differed from each other and were typical for PV and PF, respectively. Representative patterns are shown in Figure 1, a and b. In contrast, in organ cultures of human skin from individuals not suffering from pemphigus disease, incubation for 24 hours with PV-IgG (containing Dsg 1 and Dsg 3 antibodies; Table 1) and PF-IgG (Dsg 1 antibodies only; Table 1) resulted in predominantly deep splitting (Figure 1, c and d; Table 2). For PV, both IgG fractions tested (PV-IgG 1 and PV-IgG 2) caused suprabasal epidermal splitting with frequencies of 87% (n = 23, PV-IgG 1) and 89% (n = 90, PV-IgG 2), respectively. For PF, the cleavage plane of 76% (PF-IgG 1, n = 68) and 64% (PF-IgG 2, n = 22) of splits was also localized in lower epidermal layers. It has to be noted that the mean split length induced by pemphigus IgG ranged from 75.5 ± 14 (PV-IgG 1) to 105.5 ± 21 μm/mm (PV-IgG 2) and from 36.7 ± 6 (PF-IgG 2) to 82.3 ± 11 μm/mm (PF-IgG 1). Thus, the split length induced by pemphigus IgG in vitro in the short time period of 24 hours is substantially smaller than the size of blisters in pemphigus patients.
Figure 1.
Histology from pemphigus patients whose sera were used for this study revealed typical blister localization (a and b). The H&E-stained paraffin sections showed suprabasal epidermal cleavage in the PV histology and superficial blistering in the PF lesion. In human skin in vitro (c and d), PV-IgG-induced splitting was suprabasally located, whereas PF-IgG-induced cleavage was found within the spinous layer. Bar = 50 μm in all panels.
Table 1.
Antibody Profile of Patient’s IgG
| ELISA | Dsg 1 (U/ml) | Dsg 3 (U/ml) |
|---|---|---|
| PV-IgG 1 | 535 | 1098 |
| PV-IgG 2 | 280 | 685 |
| PF-IgG 1 | 95.4 | — |
| PF-IgG 2 | 128.4 | — |
ELISA, enzyme-linked immunosorbent assay.
Table 2.
Location of Splitting in Human Skin in Vitro
| Treatment | Total sections (n) | Splits
|
||
|---|---|---|---|---|
| Total (n) | Deep (%) | Superficial (%) | ||
| PV-IgG 1 | 57 | 23 | 87 | 13 |
| PV-IgG 2 | 39 | 90 | 89 | 11 |
| PF-IgG 1 | 63 | 68 | 76 | 24 |
| PF-IgG 2 | 50 | 22 | 64 | 36 |
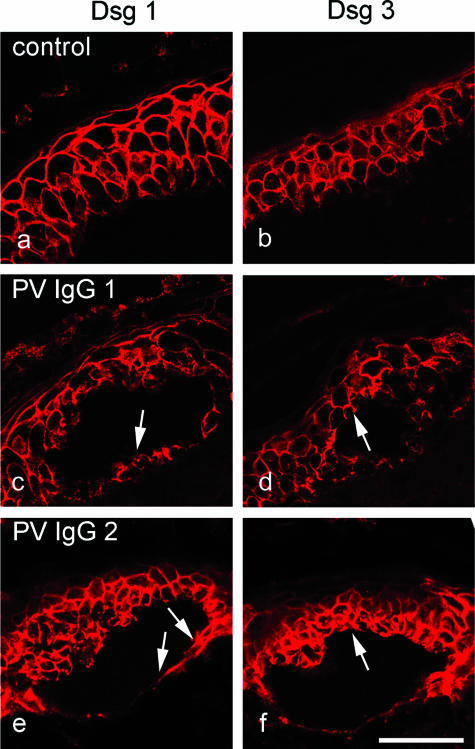
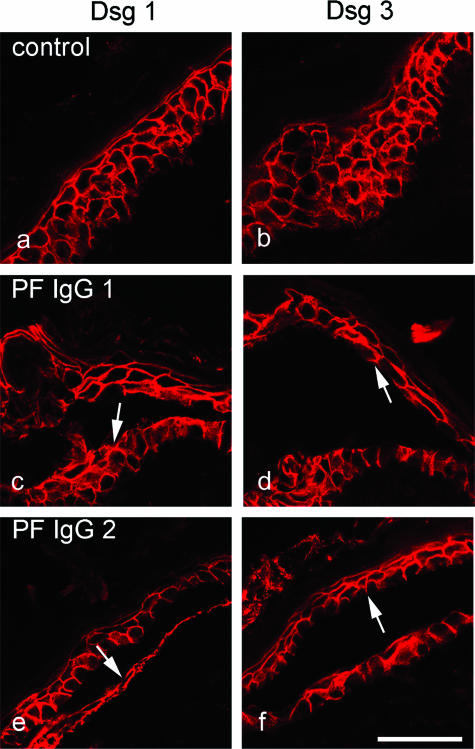
In a second set of experiments, we characterized the expression pattern of Dsg 1 and Dsg 3 in deep layers of human epidermis in which pemphigus IgG-induced splitting occurred in vitro. Immunostaining of cryosections (5 μm) using specific monoclonal antibodies directed against human Dsg 1 and Dsg 3 revealed that these desmogleins display a broad overlapping distribution pattern in human epidermis. Dsg 1 (Figures 2a and 3a) was expressed throughout the entire epidermis with an apicobasally decreasing gradient. Expression was especially strong in the granular layer, whereas in the basal layer, fluorescence was weak but still present. This was even more obvious in semithin sections from plastic-embedded epidermis (1 μm), which showed continuous Dsg 1 staining in the granular and upper spinous layer, whereas individual desmosomes could be detected in the basal layer (compare Figure 5b). Dsg 3 was found in all epidermal layers except the uppermost granular layer, but staining was more prominent in the basal layer compared with upper spinous layers (Figures 2b and 3b). Because both PV-IgG and PF-IgG caused skin splitting primarily in deep epidermal layers in vitro, we next performed immunostaining for Dsg 1 and Dsg 3 in consecutive cryosections from skin incubated with PV-IgG or PF-IgG. Moderate to strong Dsg 1 staining was seen in the basal layer underneath the suprabasally located splitting areas caused by PV-IgG 1 and 2 (arrows in Figure 2, c and e). Similarly, PF-IgG 1 and 2 caused epidermal splitting of spinous layers containing both Dsg 1 (arrows in Figure 3, c and e) and Dsg 3 (arrows in Figure 3, d and f). Taken together, these data indicate that, at least in intact epidermis in vitro, PV-IgG and PF-IgG are capable to induce epidermal splitting where Dsg 1 and Dsg 3 are present at similar immunoreactive levels.
Figure 2.
In cultured intact human epidermis, PV-IgG caused epidermal splitting where both Dsg1 and Dsg3 were present. Skin was immunostained for Dsg 1 (a, c, and e) and Dsg 3 (b, d, and f). In untreated skin, Dsg 1 and Dsg 3 displayed broad overlapping distribution patterns (a and b). Dsg 1 was expressed throughout the epidermis with a decreasing gradient from superficial to basal layers. Despite its basal prominence, Dsg 3 was present in all layers except the upper granular layer. Incubation with PV-IgG containing antibodies against both Dsg 1 and Dsg 3 lead to suprabasal splitting (c–f). Note that Dsg1 was present in the basal layer underneath the split (arrows in c and e) and that Dsg 3 was found above the cleavage plane (arrows in d and f). Bar = 50 μm.
Figure 3.
Similar to PV-IgG, PF-IgG also caused epidermal splitting where both Dsg1 and Dsg3 were present. Skin was immunostained for Dsg 1 (a, c, and e) and Dsg 3 (b, d, and f). Compared with untreated skin (a and b), incubation with PF-IgG containing only Dsg 1-specific antibodies resulted in splitting where expression of Dsg 1 as well as Dsg 3 was found (c–f). Dsg 1 was present underneath the cleavage plane (arrows in c and e), and Dsg 3 immunoreactivity was detected above the split (arrows in d and f). Bar = 50 μm.
Figure 5.
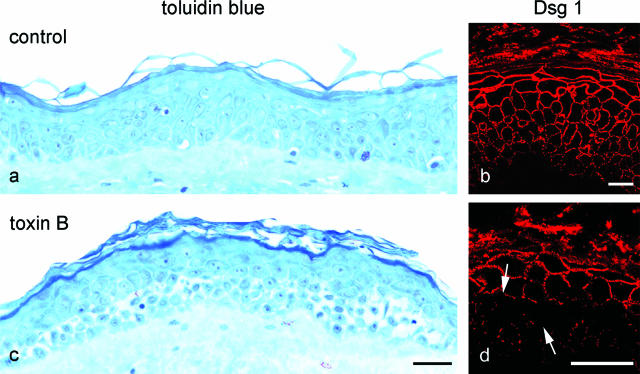
Inhibition of Rho GTPases by toxin B caused keratinocyte dissociation in the deep epidermis. Staining of semithin sections of human skin with toluidine blue (a) revealed profound cell dissociation in deep epidermal layers in response to inhibition of Rho A, Rac 1, and Cdc42 by toxin B (c). Compared with untreated skin (b), immunostaining for Dsg 1 appeared fragmented and reduced in the affected areas (arrows in d). Bar: 100 μm (a and c); 20 μm (b and d).
PF-IgG Is Sufficient to Induce Dissociation of Human Keratinocytes Expressing Both Dsg 1 and Dsg 3
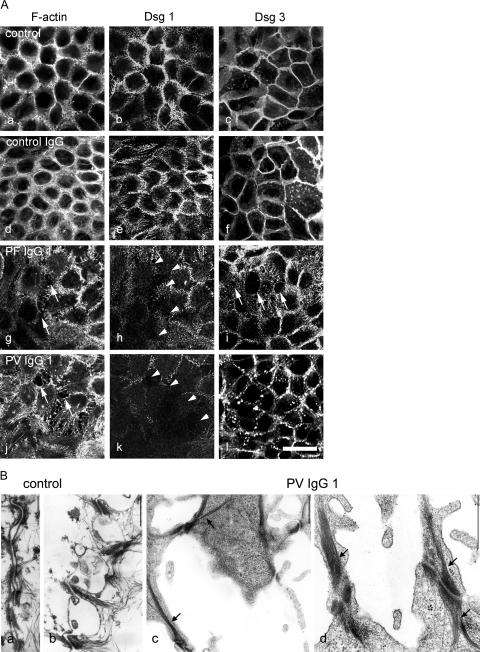
To support this observation further, we performed immunostaining of HaCaT cells after incubation with patients’ IgG. To detect sensitively cell dissociation and to demonstrate that effects of pemphigus IgG on Dsg 1 localization were confined to areas of cell dissociation, cortical cytoplasm was labeled with ALEXA-phalloidin for F-actin, and desmogleins were immunostained with specific monoclonal antibodies. In ALEXA-phalloidin-stained monolayers, even small intercellular gaps can be detected by the lack of green fluorescence.21 In untreated cells, Dsg 1 and Dsg 3 were located along cell junctions that were also labeled strongly for F-actin (Figure 4A, a–c). These distribution patterns were not affected by 24-hour incubation with control IgG from a healthy volunteer (Figure 4A, d–f). However, incubation with PF-IgG 1 and 2 (shown for PF-IgG 1 in Figure 4A, g) as well as with PV-IgG 1 and 2 (shown for PV-IgG 1 in Figure 4A, j) induced profound alterations of the actin cytoskeleton and keratinocyte dissociation, leading to formation of large intercellular gaps (arrows). As revealed by double-labeling, Dsg 1 immunoreactivity was drastically reduced in areas exhibiting cell dissociation in response to treatment with PF-IgG 1 (Figure 4A, h) and PV-IgG 1 (Figure 4A, k). In contrast, the effect of PF-IgG and PV-IgG on Dsg 3 staining differed. After incubation with PF-IgG 1, Dsg 3 immunoreactivity was weak only at margins facing intercellular gaps but was strong elsewhere (Figure 4A, i). These data support the findings from intact epidermis that PF-IgG (containing Dsg 1-specific antibodies only) is sufficient to induce loss of adhesion and keratinocyte dissociation. In contrast, treatment with PV-IgG 1 resulted in fragmentation of the continuous Dsg 3 staining throughout the monolayer, indicating a generalized reduction of desmosomes. This result was confirmed by transmission electron microscopy (Figure 4B). In controls, neighboring cell borders were tightly aligned with numerous desmosomes visible between adjacent cells (Figure 4B, a and b). In contrast, after treatment with PV-IgG 1 (Figure 4B, a and d), large gaps were present (Figure 4B, c), and desmosomes were completely missing in many areas of the monolayers. Where desmosomes were present, they were localized on filopodial processes connecting neighboring cells (Figure 4B, c and d). Notably, these desmosomes were linked to thick bundles of keratin filaments (arrows).
Figure 4.
Pemphigus IgG induced cell dissociation in cultured human keratinocytes (HaCaT cells) expressing both Dsg 1 and Dsg 3. A: Cells were stained for F-actin using ALEXA-phalloidin to detect sensitively cell dissociation and to visualize effects on the actin cytoskeleton (a, d, g, and j) and for Dsg 1 (b, e, h, and k) or for Dsg 3 (c, f, i, and l) to label desmosomes. In untreated monolayers (a–c), after incubation with control IgG from a healthy volunteer (d–f), staining of F-actin and Dsg 1 and Dsg 3 was continuous along the cell borders. Incubation with PF-IgG 1 or PV-IgG 1 resulted in keratinocyte dissociation (arrows in g and j) and profound alteration of the actin cytoskeleton and loss of Dsg 1 immunoreactivity in areas of cell dissociation (arrowheads in h and k). After incubation with PF-IgG 1, staining for Dsg 3 was lost at gap margins (arrows in i), whereas PV-IgG 1 resulted in fragmentation of Dsg 3 immunoreactivity, indicating a general loss of desmosomes. Bar = 20 μm. B: The effect of PV-IgG 1 on desmosomes was characterized by transmission electron microscopy (n = 3). In controls (a and b), keratinocyte cell borders were aligned, and numerous desmosomes were visible. After treatment with PV-IgG 1 (c and d), large gaps were present, and desmosomes were reduced in number and restricted to filopodial processes between neighboring cells. These desmosomes were linked to thick keratin filament bundles. Bar = 600 nm.
Inhibition of Rho GTPases Caused Keratinocyte Dissociation in Deep Epidermal Layers
The observations described above indicate that the localization of epidermal splitting induced in vitro by autoantibodies from pemphigus patients is difficult to explain by the desmoglein compensation concept that is based on different expression patterns of Dsg 1 and Dsg 3 throughout the epidermal layers. Recently, we found that epidermal splitting and keratinocyte dissociation caused by pemphigus IgG was linked to antibody-triggered inactivation of Rho A.23 In an attempt to elucidate whether Rho GTPase inhibition could account for the cleavage plane of epidermal splitting in human skin in vitro, we treated human skin with toxin B (800 ng/ml, 24 hours) to inhibit all Rho family GTPases such as Rho A, Rac 1, and Cdc42. Toluidine blue staining of tissue sections revealed that, compared with untreated skin (Figure 5, a and b), toxin B caused keratinocyte dissociation and fragmentation of Dsg 1 staining specifically in the deep epidermal layers (Figure 5, c and d). Thus, the cleavage plane was located at a similar epithelial level as seen in the epidermis after incubation with pemphigus IgG. Similar experiments using LTs to inhibit Rac 1 did not result in significant keratinocyte dissociation in cultured human epidermis (not shown).
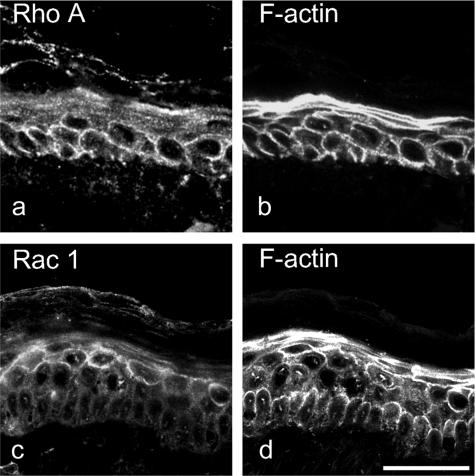
These data indicate that Rho A signaling may be primarily important in the lower epidermis to maintain adhesion of keratinocytes, whereas in superficial layers, the role of Rho A signaling seemed to be less pronounced. To test this hypothesis further, we stained human epidermis for Rho A (Figure 6a) and Rac 1 (Figure 6c) in combination with ALEXA-phalloidin staining of F-actin (Figure 6, b and d). It is well known that active Rho GTPases localize to cell membranes, whereas inactive GTPases cycle back to the cytosol.26 Membrane localization is therefore indicative for significant amounts of active GTPases. As shown in Figure 6, Rho A is primarily found along cell membranes in the lower epidermis (Figure 6a), whereas Rac 1 displays membrane localization in superficial layers (Figure 6c). These data indicate that toxin B-mediated epidermal splitting is more likely due to inactivation of Rho A rather than of Rac 1.
Figure 6.
Distribution of Rho A and Rac 1 is different in human epidermis. Skin was stained for Rho A (a), Rac 1 (c), or for F-actin (b and d) using ALEXA-phalloidin to visualize the entire epidermis. Rho A displayed membrane localization primarily in the deep epidermis (a), whereas Rac 1 was expressed predominantly in superficial epidermal layers (c). Bar = 50 μm.
Inhibition of Rho GTPases Resulted in Cell Dissociation and Loss of Dsg 1- and Dsg 3-Mediated Adhesion in Cultured Keratinocytes
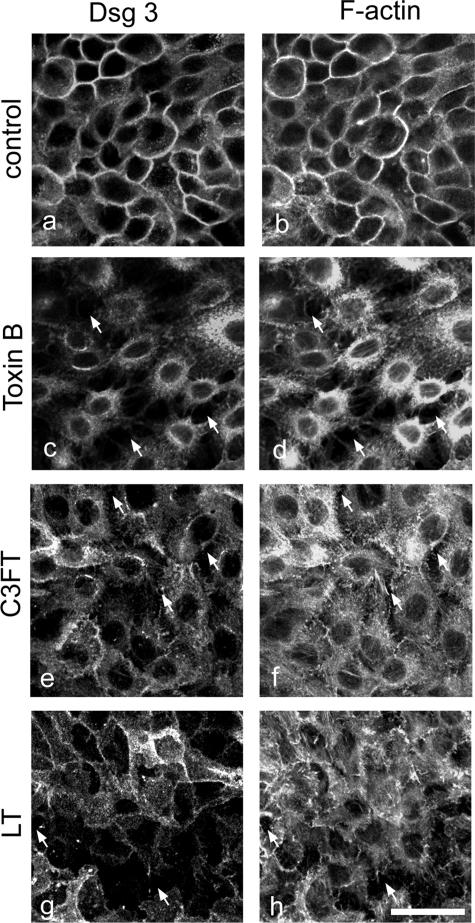
We then addressed the role of Rho GTPases for maintenance of desmosomal adhesion in more detail using HaCaT monolayers. Collective inhibition of Rho GTPases by toxin B resulted in robust keratinocyte dissociation and cell retraction comparable with loss of cell-cell adhesion in cultured human skin (Figure 7c, compare with Figure 5c). In addition, Dsg 3 immunoreactivity was almost completely removed from the cell periphery, and the junction-associated actin belt was missing (Figure 7, c and d). The effects of specific Rho A inhibition by a cell-permeable C3FT (Figure 7, e and f) and inhibition of Rac 1 by LT (Figure 7, g and h) were similar, but keratinocyte dissociation was less pronounced. Therefore, changes in Dsg 3 localization and F-actin organization closely resembled the effects induced by PF-IgG (compare with Figure 4A, g and i).
Figure 7.
Inhibition of Rho GTPases resulted in cell dissociation and loss of desmosomes in keratinocyte monolayers. HaCaT cells were stained for Dsg 3 (a, c, e, and g) to label desmosomes and for F-actin using ALEXA-phalloidin (b, d, f, and h) to detect sensitively cell dissociation and to visualize effects on the actin cytoskeleton. Compared with untreated cells (a and b), inhibition of all three GTPases Rho A, Rac 1, and Cdc42 by toxin B caused cell dissociation (arrows in c and d), loss of Dsg 3 at cell membranes (c), and complete disruption of actin organization (d). Specific inhibition of Rho A by C3FT and Rac 1 inhibition by LT caused similar effects like PF-IgG (compare with Figure 4, g and i) but less pronounced than toxin B (e–h). Bar = 20 μm.
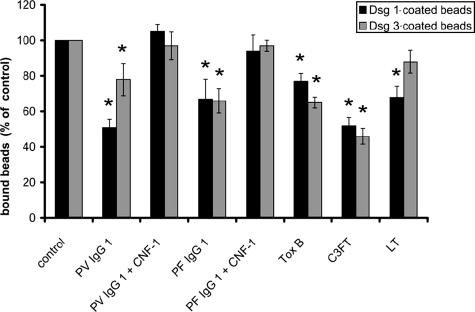
To investigate specifically the role of Rho GTPases in the regulation of Dsg 1 and Dsg 3 and to exclude that keratinocyte dissociation was primarily due to inactivation of the adhesive function of other cadherins, we applied the laser tweezer technique using beads coated with chimeric proteins in which the extracellular domain of Dsg 1 or Dsg 3 was fused to the Fc portion of human IgG. In this assay, microbeads coated with recombinant Dsg 1 or Dsg 3 are allowed to settle on the surface of HaCaT cells for 30 minutes where many beads undergo formation of desmosome-like cell-to-bead contacts as described previously for Dsg 1.21 Within 30 minutes, approximately 60 to 75% of Dsg 1- and Dsg 3-coated beads had formed tight adhesive contacts and could no longer be displaced by the laser beam. These control values were set to 100% (n = 6 for all conditions) (Figure 8). The number of bound Dsg 1-coated beads was significantly reduced by incubation with PV-IgG 1 (containing Dsg 3 and Dsg 1 antibodies) and PF-IgG 1 (Dsg 1 antibodies only) to 51 ± 5 and 67 ± 11% of control levels, respectively. Under the same conditions, PV-IgG 1 and PF-IgG 1 also significantly reduced binding of Dsg 3-coated beads to 78 ± 10 and 66 ± 7% of controls. From the fact that PF-IgG 1 (containing Dsg 1 autoantibodies only) reduced adhesion of Dsg 3-coated beads to a comparable extent as adhesion of Dsg 1-coated beads, it can be concluded that loss of bead binding was not caused by direct steric hindrance. The effects of PV-IgG 1 and PF-IgG 1 on adhesion of beads coated with Dsg 1 and Dsg 3 was completely abolished by activation of Rho GTPases via CNF-1 because numbers of bound beads were not significantly different from controls under these conditions. These data indicate that loss of bead binding was caused by inactivation of Rho GTPases, as we have shown previously.23
Figure 8.
Pemphigus IgG and inhibition of Rho GTPases reduced desmoglein-mediated adhesion. Adhesion of beads coated with Dsg 1 or Dsg 3 was assayed using laser tweezers. Beads were allowed to settle on the cell surface for 30 minutes before binding was probed (control). After incubation with pemphigus IgG for 120 minutes in the absence or presence of CNF-1 to activate Rho GTPases or after incubation with bacterial toxins for 180 minutes to inhibit Rho GTPases, binding was tested again. Compared with controls, PV-IgG 1 and PF-IgG 1 significantly reduced adhesion of Dsg 1- and Dsg 3-coated beads to the cell surface, which was completely blocked by simultaneous activation of Rho GTPases. Similarly, inhibition of Rho A, Rac 1, and Cdc42 by toxin B as well as specific inhibition of Rho A by C3FT resulted in significant loss of bead binding. Inhibition of Rac 1 by LT caused significant reduction of Dsg 1-mediated binding only. Significance compared with controls is indicated by asterisks (P < 0.05).
Inhibition of Rho GTPases by toxin B also resulted in impaired binding of Dsg 1- and Dsg 3-coated beads (significant drop to 77 ± 4 and 65 ± 3% of controls). Specific inhibition of Rho A (C3FT) also drastically reduced adhesion of Dsg 1- (52 ± 5%) and Dsg 3-coated beads (46 ± 5%), whereas inhibition of Rac 1 (LT) only significantly reduced adhesion of Dsg 1 beads (68 ± 6%) but not of Dsg 3-coated beads. Taken together, these data demonstrate that Rho GTPases are important for maintenance of desmoglein-mediated adhesion, and pemphigus IgG-induced inactivation of Rho GTPases could account for the loss of desmoglein binding.23
Discussion
Dsg 3 Does Not Compensate for Dsg 1 in Human Epidermis
In our study, we analyzed Dsg 1 and Dsg 3 distribution patterns in intact human epidermis using specific monoclonal antibodies and correlated these results with the localization of epidermal splitting induced by PV-IgG and PF-IgG in an ex vivo model of human skin. We found that distribution patterns of both desmogleins broadly overlap and that epidermal splitting in this model occurred between epidermal layers expressing Dsg 1 and Dsg 3 at similar immunoreactive levels. Moreover, PV-IgG and PF-IgG caused keratinocyte dissociation in HaCaT monolayers immunoreactive for both Dsg 1 and Dsg 3. Because incubation with PF-IgG (containing Dsg 1-specific autoantibodies only) led to keratinocyte dissociation and epidermal splitting, these results demonstrate that Dsg 3 in these models does not compensate for the functional loss of Dsg 1. Our results on the distribution patterns of Dsg 1 and Dsg 3 in human epidermis are supported by a recent study by Mahoney et al,19 who also found that Dsg 1 and Dsg 3 broadly overlap in the epidermis and that restriction of Dsg 1 to the superficial epidermis and of Dsg 3 to the basal layer is only observed in mouse but not human epidermis. All these data suggest that the clinical phenotype of PV and PF in humans may not be explained by the distribution patterns of Dsg 1 and Dsg 3 alone.16
Splitting in Intact Human Skin Can Be Explained by Inactivation of Rho A
The size of pemphigus IgG-induced splitting in the ex vivo model of human skin was substantially smaller compared with blisters in vivo. Moreover, in contrast to the histological findings in skin of patients from whom the sera were taken, cleavage of the epidermis in vitro was observed primarily in the deep layers for PV-IgG and PF-IgG. Therefore, one may speculate that the splits in our model reflect a precursor lesion that is not equivalent to the final stages of skin blistering in vivo. In view of our recent observation that epidermal splitting caused by pemphigus IgG was associated with antibody-induced inactivation of the GTPase Rho A,23 we investigated whether this mechanism could also account for the cleavage plane of splitting in human donor skin. We found that simultaneous inhibition of all Rho GTPases by toxin B resulted in similar suprabasal cleavage as caused by pemphigus autoantibodies. In support of this observation, inhibition of Rho GTPases by toxin B also caused keratinocyte dissociation in cultured human keratinocytes associated with loss of Dsg 1 and Dsg 3 binding. From these data, we conclude that inhibition of Rho GTPases caused an epidermal splitting pattern similar to that caused by pemphigus IgG and that Rho signaling may be the underlying signaling mechanism triggered by pemphigus IgG.
Skin Blistering As a Multistep Process Involving Different Mechanisms for PV and PF
Our study provides the first evidence that regional prevalence of signaling events involved in pemphigus pathogenesis could account for the cleavage plane in pemphigus. This supports the hypothesis recently proposed that some cellular signaling mechanisms specific for basal cells might be responsible for deep blistering in PV.10 Because activation of Rho A by CNFy and CNF-1 abolished autoantibody-induced epidermal splitting23 and inactivation of Rho GTPases resulted in keratinocyte dissociation in the same epidermal layers as pemphigus IgG, we conclude that inactivation of Rho A is the major mechanism underlying splitting in human skin in vitro. This supports our recent finding that both PV-IgG and PF-IgG reduced activity of Rho A by approximately 50% and also decreased CNF-1-mediated activation of Rho A by 60 to 70%, indicating that some of the signaling mechanisms induced by pemphigus IgG interfered with Rho A signaling.23
Another hint to the notion that signaling mechanisms may be different in superficial versus deep epidermal layers comes from the distribution of some subtypes of muscarinic acetylcholine receptors. For example, muscarinic receptor 3 is found only in basal keratinocytes, whereas muscarinic receptor 4 is expressed exclusively in suprabasal cells.5 It is obvious that in PV, additional mechanisms such as altered plakoglobin signaling leading to c-Myc overexpression,22,24 activation of protein kinase C and of p38 mitogen-activated kinase,27,28 activation of epidermal growth factor receptor and Src,29,30 endocytosis and degradation of Dsg 3,31,32,33 and apoptosis34 are involved in the mechanisms finally leading to skin blistering. In contrast, for PF pathogenesis, such data are lacking at present. Therefore, it is possible that in PV and PF signaling events triggered by pemphigus IgG induce splitting in deep epidermis because of their pivotal role for desmosomal adhesion in deep epidermal layers. Additional mechanisms such as steric hindrance of desmoglein binding, which was not detectable in PF21 but may occur in PV (J.W. and D.D., unpublished observation), could explain why the cleavage plane in PV in vivo is found in the same localization like in our in vitro model. Because steric hindrance of desmoglein binding would not necessarily result in active cell retraction, the contribution of steric hindrance to acantholysis might therefore not be detectable in our model. In PF where no steric hindrance occurs, other secondary mechanisms have to be postulated. However, at present, there is no strong explanation as to why superficial splitting, which is typical for PF in vivo, was only found in 24 to 36% of lesions in our model.
Taken together, our results demonstrate that pemphigus is a complex disease that may not be explained by the mechanistic model of the desmoglein compensation theory. Rather, we found that signaling mechanisms involved in pemphigus disease have different roles for the maintenance of desmosomal adhesion in separate epidermal layers. Further studies on the association of signaling mechanisms with specific epidermal layers will be necessary to further our understanding of pemphigus pathogenesis.
Acknowledgments
We thank Stefanie Imhof and Franziska König for excellent technical assistance. CNF-1 was a kind gift from Gudula Schmidt (University of Freiburg). We are grateful to Holger Barth (University of Ulm) for contributing C3FT, toxin B, and LT.
Footnotes
Address reprint requests to Dr. Jens Waschke, Institute of Anatomy and Cell Biology, Julius-Maximilians-University, Koellikerstrasse 6, D-97070 Würzburg, Germany. E-mail: jens.waschke@mail.uni-wuerzburg.de.
Supported by Deutsche Forschungsgemeinschaft (grant SFB 487 TP B5) and the Interdisziplinäres Zentrum für Klinische Forschung Würzburg (grant TP A-51).
References
- Amagai M. Desmoglein as a target in autoimmunity and infection. J Am Acad Dermatol. 2003;48:244–252. doi: 10.1067/mjd.2003.7. [DOI] [PubMed] [Google Scholar]
- Amagai M, Ahmed AR, Kitajima Y, Bystryn JC, Milner Y, Gniadecki R, Hertl M, Pincelli C, Fridkis-Hareli M, Aoyama Y, Frusic-Zlotkin M, Muller E, David M, Mimouni D, Vind-Kezunovic D, Michel B, Mahoney M, Grando S. Are desmoglein autoantibodies essential for the immunopathogenesis of pemphigus vulgaris, or just “witnesses of disease”? Exp Dermatol. 2006;15:815. doi: 10.1111/j.1600-0625.2006.00499_1.x. [DOI] [PubMed] [Google Scholar]
- Payne AS, Hanakawa Y, Amagai M, Stanley JR. Desmosomes and disease: pemphigus and bullous impetigo. Curr Opin Cell Biol. 2004;16:536–543. doi: 10.1016/j.ceb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med. 2006;355:1800–1810. doi: 10.1056/NEJMra061111. [DOI] [PubMed] [Google Scholar]
- Grando SA. Cholinergic control of epidermal cohesion. Exp Dermatol. 2006;15:265–282. doi: 10.1111/j.0906-6705.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- Grando SA, Pittelkow MR, Shultz LD, Dmochowski M, Nguyen VT. Pemphigus: an unfolding story. J Invest Dermatol. 2001;117:990–995. doi: 10.1046/j.0022-202x.2001.01489.x. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Arredondo J, Chernyavsky AI, Pittelkow MR, Kitajima Y, Grando SA. Pemphigus vulgaris acantholysis ameliorated by cholinergic agonists. Arch Dermatol. 2004;140:327–334. doi: 10.1001/archderm.140.3.327. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Ndoye A, Grando SA. Novel human α9 acetylcholine receptor regulating keratinocyte adhesion is targeted by pemphigus vulgaris autoimmunity. Am J Pathol. 2000;157:1377–1391. doi: 10.1016/s0002-9440(10)64651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Ndoye A, Shultz LD, Pittelkow MR, Grando SA. Antibodies against keratinocyte antigens other than desmogleins 1 and 3 can induce pemphigus vulgaris-like lesions. J Clin Invest. 2000;106:1467–1479. doi: 10.1172/JCI10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystryn JC, Grando SA. A novel explanation for acantholysis in pemphigus vulgaris: the basal cell shrinkage hypothesis. J Am Acad Dermatol. 2006;54:513–516. doi: 10.1016/j.jaad.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Lanza A, Cirillo N, Femiano F, Gombos F. How does acantholysis occur in pemphigus vulgaris: a critical review. J Cutan Pathol. 2006;33:401–412. doi: 10.1111/j.0303-6987.2006.00523.x. [DOI] [PubMed] [Google Scholar]
- Sitaru C, Zillikens D. Mechanisms of blister induction by autoantibodies. Exp Dermatol. 2005;14:861–875. doi: 10.1111/j.1600-0625.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40:167–170. doi: 10.1016/s0190-9622(99)70183-0. [DOI] [PubMed] [Google Scholar]
- Ding X, Aoki V, Mascaro JM, Jr, Lopez-Swiderski A, Diaz LA, Fairley JA. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J Invest Dermatol. 1997;109:592–596. doi: 10.1111/1523-1747.ep12337524. [DOI] [PubMed] [Google Scholar]
- Amagai M, Koch PJ, Nishikawa T, Stanley JR. Pemphigus vulgaris antigen (desmoglein 3) is localized in the lower epidermis, the site of blister formation in patients. J Invest Dermatol. 1996;106:351–355. doi: 10.1111/1523-1747.ep12343081. [DOI] [PubMed] [Google Scholar]
- Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103:461–468. doi: 10.1172/JCI5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udey MC, Stanley JR. Pemphigus: diseases of antidesmosomal autoimmunity. JAMA. 1999;282:572–576. doi: 10.1001/jama.282.6.572. [DOI] [PubMed] [Google Scholar]
- Müller E, Kernland K, Caldelari R, Wyder M, Balmer V, Hunziker T. Unusual pemphigus phenotype in the presence of a Dsg1 and Dsg3 autoantibody profile. J Invest Dermatol. 2002;118:551–555. doi: 10.1046/j.0022-202x.2001.01703.x. [DOI] [PubMed] [Google Scholar]
- Mahoney MG, Hu Y, Brennan D, Bazzi H, Christiano AM, Wahl JK., III Delineation of diversified desmoglein distribution in stratified squamous epithelia: implications in diseases. Exp Dermatol. 2006;15:101–109. doi: 10.1111/j.1600-0625.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Masunaga T, Ishiko A, Kikuchi A, Hashimoto T, Nishikawa T. Pemphigus vulgaris and pemphigus foliaceus sera show an inversely graded binding pattern to extracellular regions of desmosomes in different layers of human epidermis. J Invest Dermatol. 1995;105:153–159. doi: 10.1111/1523-1747.ep12316695. [DOI] [PubMed] [Google Scholar]
- Waschke J, Bruggeman P, Baumgartner W, Zillikens D, Drenckhahn D. Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction. J Clin Invest. 2005;115:3157–3165. doi: 10.1172/JCI23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldelari R, de Bruin A, Baumann D, Suter MM, Bierkamp C, Balmer V, Muller E. A central role for the armadillo protein plakoglobin in the autoimmune disease pemphigus vulgaris. J Cell Biol. 2001;153:823–834. doi: 10.1083/jcb.153.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke J, Spindler V, Bruggeman P, Zillikens D, Schmidt G, Drenckhahn D. Inhibition of Rho A activity causes pemphigus skin blistering. J Cell Biol. 2006;175:721–727. doi: 10.1083/jcb.200605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson L, Raess NA, Caldelari R, Zakher A, de Bruin A, Posthaus H, Bolli R, Hunziker T, Suter MM, Muller EJ. Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. EMBO J. 2006;25:3298–3309. doi: 10.1038/sj.emboj.7601224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagai M, Komai A, Hashimoto T, Shirakata Y, Hashimoto K, Yamada T, Kitajima Y, Ohya K, Iwanami H, Nishikawa T. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol. 1999;140:351–357. doi: 10.1046/j.1365-2133.1999.02752.x. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, Rubenstein DS. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J Biol Chem. 2005;280:23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- Berkowitz P, Hu P, Warren S, Liu Z, Diaz LA, Rubenstein DS. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc Natl Acad Sci USA. 2006;103:12855–12860. doi: 10.1073/pnas.0602973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky AI, Arredondo J, Kitajima Y, Sato-Nagai M, Grando SA. Desmoglein versus non-desmoglein signaling in pemphigus acantholysis: characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. J Biol Chem. 2007;282:13804–13812. doi: 10.1074/jbc.M611365200. [DOI] [PubMed] [Google Scholar]
- Frusić-Zlotkin M, Raichenberg D, Wang X, David M, Michel B, Milner Y. Apoptotic mechanism in pemphigus autoimmunoglobulins-induced acantholysis: possible involvement of the EGF receptor. Autoimmunity. 2006;39:563–575. doi: 10.1080/08916930600971836. [DOI] [PubMed] [Google Scholar]
- Aoyama Y, Kitajima Y. Pemphigus vulgaris-IgG causes a rapid depletion of desmoglein 3 (Dsg3) from the Triton X-100 soluble pools, leading to the formation of Dsg3-depleted desmosomes in a human squamous carcinoma cell line, DJM-1 cells. J Invest Dermatol. 1999;112:67–71. doi: 10.1046/j.1523-1747.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Aoyama Y, Owada MK, Kitajima Y. A pathogenic autoantibody, pemphigus vulgaris-IgG, induces phosphorylation of desmoglein 3, and its dissociation from plakoglobin in cultured keratinocytes. Eur J Immunol. 1999;29:2233–2240. doi: 10.1002/(SICI)1521-4141(199907)29:07<2233::AID-IMMU2233>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Calkins CC, Setzer SV, Jennings JM, Summers S, Tsunoda K, Amagai M, Kowalczyk AP. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. J Biol Chem. 2006;281:7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- Wang X, Bregegere F, Frusic-Zlotkin M, Feinmesser M, Michel B, Milner Y. Possible apoptotic mechanism in epidermal cell acantholysis induced by pemphigus vulgaris autoimmunoglobulins. Apoptosis. 2004;9:131–143. doi: 10.1023/B:APPT.0000018795.05766.1f. [DOI] [PubMed] [Google Scholar]