Abstract
During microbial infection, neutrophils (polymorphonuclear leukocytes; PMNs) activate dendritic cells (DCs). However, early reports illustrated that neutrophil-derived mediators may suppress responses to mitogens. In the present study, we investigated the mechanism used by PMNs to modulate the immunostimulatory ability of DCs. Autologous syngeneic PMNs decreased T-cell proliferation induced by allogeneic DCs. Culture supernatant (CS) derived from PMNs also decreased allostimulation ability of immature DCs and increased the expression of transforming growth factor (TGF)-β1 on DCs. A TGF-β1 monoclonal antibody, a CD40 monoclonal antibody, or a serine protease inhibitor reversed the effect of PMN CS on DC allostimulatory ability. Furthermore, elastase reproduced the inhibitory effect of PMN CS on DC allostimulatory ability and the TGF-β1 production. The role of elastase was confirmed by examining PMN CS from two patients with cyclic neutropenia, a disease due to mutations in the neutrophil elastase gene. These PMN CS samples had reduced elastase activity and were unable to increase DC TGF-β1 production. Moreover, elastase and PMN CS induced IκBα degradation in DCs. We conclude that PMNs decrease DC allostimulatory ability via production of elastase leading to a switch of immature DCs into TGF-β1-secreting cells.
Human polymorphonuclear leukocytes (neutrophils or PMNs) constitute the first line of defense against most classes of pathogenic microorganisms and contribute significantly to inflammation.1,2 In response to pathogens, neutrophils are activated and migrate along chemoattractant gradients to sites of infections, where they engulf pathogens by phagocytosis or kill extracellular pathogens in the absence of phagocytosis.3,4 For the former mechanism, they eliminate pathogens within intracellular phagocytic vacuoles by releasing proteolytic enzymes, antimicrobial peptides, and toxic oxygen radicals from granules.5 For the latter mechanism, PMNs generate extracellular fibers composed of DNA, histones, and granule proteins such as elastase, cathepsin G, defensins, and reactive oxygen species.4 Thus, their effector functions at sites of infection include not only phagocytosis but also production of toxic metabolites and the release of proteolytic enzymes. Although these functions facilitate the elimination of invading organisms, they can also cause severe tissue damage.6 Once at the site of infection, PMNs may interact with pathogens but also with surrounding tissues and cells of the immune system including dendritic cells (DCs).7,8
Distributed throughout the body, DCs are a heterogeneous group of cells that play a critical role in the induction of acquired immune responses.9 DC precursors and progenitors exit the bone marrow and circulate via blood until they seed many tissues and non-lymphoid organs as immature cells. As immature DCs in the tissues, they express low levels of major histocompatibility complex and costimulatory molecules, and they are very effective in capturing and processing antigens. Once DCs encounter local inflammatory mediators, they become activated and undergo a maturation process. This process involves their mobilization from the periphery to the lymph node and spleen T-cell areas, the down-regulation of their antigen capture capacity as well as the up-regulation of the costimulatory molecules to become potent immunostimulatory cells.10,11
In infection or tissue injury, DC activation and maturation occur rapidly, typically noted within 1 to 4 hours,12 often preceding the peak of PMN accumulation at the site. DCs indeed have the capacity to recruit and activate cells of the innate immune system, even PMNs and immature DCs.13 Once in the inflammatory site, PMNs may interact with DCs to modulate their function and the induced T-cell responses. Recently it has been shown that during microbial infection, PMNs affect DC activation leading in turn to Th1 cell activation.7,14 It was suggested that this effect is mediated by the interaction between DC-SIGN and Mac-1 on DCs and PMNs, respectively.14 However, early reports illustrated that neutrophil-derived mediators may suppress responses to mitogens.15 In this study, we further examined the interaction between PMNs and DCs. We hypothesized that PMNs are able to differentially modulate the immune response depending on the density of the cells found in the inflammatory microenvironment.
Materials and Methods
Monoclonal Antibodies
A number of monoclonal antibodies (mAbs) that recognize antigens present on DCs were used in vitro. These included mAbs anti-CD1b (Wm25, IgG1 from Chemicon International, Temecula, CA), anti-HLA-DR (HB-55, IgG2a from the American Type Culture Collection, Manassas, VA), anti-CD86 (FUN-1, IgG1; PharMingen, San Diego, CA), and CD83 (HB15A, IgG2b; Immunotech, Marseille, France). Other mAbs used were anti-CD14 (3C10, IgG2b from the American Type Culture Collection), anti-CD11b (2LPM19c IgG1 from DAKO, Carpinteria, CA), anti-CD54 (84H10, IgG1; Immunotech), and anti-CD40 (PharMingen). A neutralizing mouse mAb (clone 9016, IgG1; R&D Systems Inc., Minneapolis, MN) against human transforming growth factor (TGF)-β1 was used to block TGF-β1 activity. Other antibodies used for TGF-β1 enzyme-linked immunosorbent assay (ELISA) were clones mAB240 and BAf240 from R&D Systems.
Patients and Healthy Blood Donors
Two patients were enrolled for these studies after giving informed consent according to the Declarations of Helsinki. Patient 1 (15-year-old female) was diagnosed with cyclic neutropenia based on at least three blood neutrophil counts of <500/ml and the typical clinical manifestations. Patient 2 (25-year-old male) had typical 21-day cycles of neutropenia, mouth ulcers, chronic gingivitis, and fever and is the father of two children (one deceased, one alive) with the diagnosis of cyclic neutropenia. Both patients were studied while off granulocyte colony-stimulating factor treatment for at least 3 weeks and had been observed clinically by one of the investigators for 2 years before these studies. Blood samples were obtained during the recovery phase of the neutrophils by routine venipuncture. Furthermore, 30 healthy volunteers donated blood for these studies.
Blood Monocyte Isolation and Monocyte-Derived DC (moDC) Culture
Human DCs were generated from monocytes as previously described with minor modifications.16 In brief, blood was obtained from healthy donors, and the peripheral blood mononuclear cells (PBMCs) and monocytes were isolated by a Ficoll-Hypaque (Pharmacia LKB Biotech, Piscataway, NJ) gradient, followed by a Percoll (Pharmacia Fine Chemicals, Dorval, PQ, Canada) gradient.17 Once the monocyte fraction was recovered, these cells were cultured (5 × 106 cells/ml) for 5 days in RPMI 1640 medium supplemented with 2 mmol/L l-glutamine, 50 μg/ml gentamicin, 50 μmol/L 2-mercaptoethanol, 800 U/ml granulocyte macrophage colony-stimulating factor, 500 U/ml interleukin (IL)-4 (Sigma Chemical Co., St. Louis, MO) and 10% heat-inactivated fetal calf serum (Gibco, Grand Island, NY). MoDCs were identified by immunofluorescence staining of the cells. In some experiments, moDC maturation was induced by treating the cells with 50 ng/ml lipopolysaccharide (LPS) for 18 hours (from Escherichia coli 0111:B4; Sigma).
Human PMN Leukocyte Purification from Normal Donors and Patients with Cyclic Neutropenia
Human PMNs were purified as described previously18 from acid citrate dextrose-heparin-anticoagulated venous blood of healthy do citrate dextrosenors and patients with cyclic neutropenia. Briefly, red cells were sedimented with 6% dextran-saline (Rivero, Buenos Aires, Argentina), leukocyte-rich plasma was collected, and PMN leukocytes were purified by discontinuous Percoll gradient centrifugation,18 washed, and resuspended to 2.5 × 106 PMN/ml in RPMI 1640 medium, 0.5% human serum albumin (HSA), and 10 mmol/L HEPES, pH 7.4.18 This method yielded PMNs of >95% purity with essentially no red cell contamination and >98% cell viability.
Flow Cytometric Analysis
The characterization of the DCs was performed by immunofluorescence as previously described.16 DCs were incubated with primary mAb at optimal concentrations (5 to 10 μg/ml) for 45 minutes. Finally, cells were treated with R-phycoerythrin-conjugated goat anti-mouse Ig (DAKO, Glostrup, Denmark) for 45 minutes. For negative controls, cells were labeled with irrelevant isotype-matched primary mAb. Fluorescence intensity was analyzed using a FACStar Plus (Becton Dickinson, Mountain View, CA), and dead cells were excluded by gating with propidium iodide.
Neutrophil and DC Culture Supernatants (CS)
To obtain PMN CS, PMNs were primed (pPMNs) with IL-8 (50 ng/ml), LPS (100 ng/ml), or formyl-Met-Leu-Phe (10−8 U/ml) for 10 or 60 minutes or used unprimed (uPMNs). Next, the cells (107 PMN/ml) were washed with phosphate-buffered saline and incubated in serum-free RPMI 1640 medium with 5 mg/ml HSA at 37°C for 3 hours. Thereafter, supernatants were centrifuged at 1200 rpm for 10 minutes and immediately dispensed in aliquots and frozen at −70°C.
To generate DC CS, the cells were untreated or treated with 100 μl of PMN CS or elastase (0.5 to 50 nmol/L) for 3 hours. Thereafter, the cells (2 × 106 moDC/ml) were washed with phosphate-buffered saline and incubated for 80 hours in RPMI 1640 medium supplemented with 2 mmol/L l-glutamine, 50 mg/ml gentamicin, 50 mmol/L β-mercaptoethanol, 800 U/ml granulocyte macrophage-colony stimulating factor, and 500 U/ml IL-4. Supernatants were centrifuged at 1200 rpm for 10 minutes and immediately dispensed in aliquots and frozen at −70°C. In some experiments PMN CS were pretreated with recombinant human serine leukocyte protease inhibitor (rhSLPI; 5 to 3000 nmol/L, 37°C, 10 minutes) to block the elastase activity, and then supernatants plus rhSLPI were incubated with DCs for another 3 hours.
Mixed Lymphocyte Reaction (MLR)
DCs incubated with PMNs or treated with PMN CS were tested for allostimulatory ability. For the first group of experiments, 105 PBMCs were cultured in 96-well microplates (round-bottomed) with different concentrations of allogeneic DCs and different concentrations of primed or unprimed autologous syngeneic PMNs (103 to 105). For the second group of experiments, 105 PBMCs were cultured in 96-well microplates with uPMN- or pPMN-CS-treated stimulator DCs. Thymidine incorporation was measured on day 5 by 18-hour pulse with [3H]thymidine (1 μCi/well, specific activity 5 mCi/mmol; Amersham Life Sciences). In some experiments, TGF-β1 or a blocking mAb to TGF-β1 was added at the beginning of MLR assay. For some experiments, and to avoid cell-cell interaction, PMNs and PBMCs were separated by a polyvinylpyrrolidone-free polycarbonate filter bearing 0.4-μm pores in Transwell culture plate inserts (6.5-mm diameter, Transwell 3413; Costar, Cambridge, MA).
TGF-β1 Bioassay (Mink Lung Cell Proliferation Assay)
To assay for TGF-β1 activity, we performed a growth inhibition assay essentially as described by Fukushima et al.19 In brief, Mv1Lu cell line (CCL-64; American Type Culture Collection) was seeded at 2 × 104 cells/50 μl/well in Dulbecco’s modified Eagle’s medium supplemented with 0.4% fetal bovine serum. An hour later, 50 μl of CS derived from untreated DCs or from DCs pretreated with PMN CS were added to measure the active TGF-β1. Cells were also assayed with CS heated for 5 minutes at 80°C to activate latent TGF-β1 and evaluate total TGF-β1 secreted. A standard curve with recombinant TGF-β1 (R&D Systems) (0.02 to 3 ng/ml) was performed in each assay. After 20 hours, the plate was pulsed with 1 μCi/well of [3H]thymidine for 18 additional hours and harvested using a multiwell cell harvester.
Reverse Transcription-Polymerase Chain Reaction
To determine whether DCs express mRNA for TGF-β1, we performed reverse transcription-polymerase chain reaction. Total cellular RNA was isolated from 106 purified untreated and PMN-CS-treated DCs by the guanidinium isothiocyanate phenol method using TRIzol LS Reagent (Gibco BRL, Grand Island, NY).20 Equal starting concentrations of total RNA were used as the template for the reverse transcriptase reaction. cDNA synthesis was performed according to the manufacturer’s recommendations (Superscript II; Gibco BRL). Reverse transcription-synthesized cDNA (2 μl) was then mixed with 5 μl of 10× polymerase chain reaction buffer (500 mmol/L KCl and 200 mmol/L Tris-HCl, pH 8.3), 1.5 mmol/L MgCl2, 1 μl of the relevant 10 mmol/L oligonucleotide primer set, and 1 U of Taq polymerase (Gibco BRL) to a final volume of 50 μl. The primers had the following sequence: sense 5′-GCCCTGGACACCAACTATTGCT-3′, and antisense 5′-AGGCTCCAAATGTAGGGGCAGG-3′, with the predicted product of 162 bp. The cDNA was amplified for one cycle of 5 minutes at 94°C, 5 minutes at 59°C, and 2.5 minutes at 72°C; and 22 cycles of 1 minute at 94°C, 1 minute at 59°C, and 1 minute at 72°C. β-Actin cDNA was amplified for each sample to serve as a normalized control. Amplified products together with a DNA ladder as a size standard were resolved on a 2% agarose gel, and products were visualized with ethidium bromide staining. Densitometric analysis and quantification were done using an Image Quant program (MD ImageQuant software, version 3.3; Molecular Dynamics, Sunnyvale, CA).
Intracellular Cytokine Staining of DCs
DCs treated with PMN CS were recovered and incubated for another 4 hours at 37°C with brefeldin A (10 μg/ml; Sigma). After washing, cells were fixed with fixation solution, permeabilized with permeabilization solution, and stained with anti-IL-12 R-phycoerythrin (R-PE) to detect p70 (IQ Products, Groningen, The Netherlands), anti-TGF-β1 (IQ Products), or isotype negative control antibody (Invitrogen, Carlsbad, CA) in the presence of the permeabilizing agent. The cells were then analyzed by flow cytometry.
Detection of TGF-β1 and IL-10 by ELISA
Cytokine concentrations in DC CS were detected by sandwich ELISA. In brief, ELISA plates (Falcon, Franklin Lakes, NJ) were coated with primary antibody for IL-10 (1 μg/ml) or TGF-β1 (5 μg/ml). The next day, the nonspecific binding sites were blocked with phosphate-buffered saline-2% bovine serum albumin (30 minutes, 37°C). Serially diluted cytokine standards and samples were then added and incubated overnight at 4°C. Wells were then washed, and secondary goat anti-IL-10 or anti-TGF-β1 antibody was added. After 90 minutes of incubation, the wells were washed, and peroxidase anti-goat IgG was added (60 minutes, 37°C). Then, O-phenylenediamine (Sigma) substrate solution was added and incubated for 10 to 30 minutes. The absorbance was read at 492 nm. The assay was performed using matched paired antibodies specific for TGF-β1 (R&D Systems) and IL-10 (Endogen, Woburn, MA). The sensitivity of assays was 0.5 ng/ml for TGF-β1 and 30 pg/ml for IL-10.
Cloning and Expression of rhSLPI cDNA
Human SLPI mRNA was extracted from HeLa cells, reverse transcribed to cDNA, and cloned using standard techniques. Sequencing was performed by DNA automatic sequencing facility at the National University of La Plata, Buenos Aires, Argentina. For sequence comparison and analysis, the EMBL, Swiss-Prot, and GenBank molecular biology databases were searched using the network service (National Center for Biotechnology Information, National Library of Medicine, Bethesda, MD). E. coli strain BL21 CodonPlus (DAE)-RIL (Novagen, EMD Biosciences, Inc., Darmstadt, Germany) was transformed with the SLPI-pET22b+ expression vector (Novagen) and grown at 37°C with ampicillin (100 μg/ml) and chloramphenicol (50 μg/ml). Cells were induced with 1 mmol/L IPTG (3 hours, 28°C), harvested, and sonicated. rhSLPI was purified with a Ni-NTA resin column as described by the manufacturer (Qiagen). To evaluate the activity of rhSLPI, the trypsin inhibitory activity was assayed with the colorimetric substrate N-succinyl-Ala-Ala-Pro-Phe q-nitroanilide (Sigma), and the absorbance was monitored at 405 nm in a microplate reader.
Elastase Activity Assay
PMN CS was diluted 1:3 in Tris-buffered saline (pH 7.5) containing the substrate N-methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (Sigma) to a final concentration of 0.6 mmol/L. At the same time, reaction wells with a known concentration (20 nmol/L) of human leukocyte elastase (Sigma) instead of PMN CS were assayed, and the kinetics of the enzyme reaction was measured in an ELISA plate reader at 1-minute intervals over a 20-minute period. Then the velocity of the enzyme reaction was calculated from an optical density/time plot, and the constant for this reaction (k) was deduced from the standard reaction with a known concentration of elastase. The formula used for the determination of neutrophil elastase concentration was: V = [Et] k2[S]/[S] +Km. If [S] ≫Km, then Vmax = k2 [Et], where Km elastase = 0.21 mmol/L ([Et], elastase concentration; and [S], substrate concentration).
Western Blot
For polyacrylamide gel electrophoresis and Western blot, cells were incubated for 1 hour in ice with an extraction buffer: Tris-buffered saline (1×), protease inhibitor (1×), and 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate (1%) (Sigma). After centrifugation at 12,000 × g for 15 minutes at 4°C, the detergent supernatant was removed and the protein quantified by the Micro BCA Protein Assay Reagent Kit (Pierce, Rockford, IL). The samples were boiled in sodium dodecyl sulfate-sample buffer containing dithiothreitol (0.2 mol/L) (Sigma) and analyzed by electrophoresis on a 6% polyacrylamide gel in the presence of sodium dodecyl sulfate. After electrophoresis, gels were transferred to nitrocellulose membrane and subjected to Western blot analysis using enhanced chemiluminescence (Amersham Corp., Arlington Heights, IL) with a polyclonal antibody to IκB-α (C-21; Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:1000 and goat-rabbit IgG (Chemicon International).
Statistical Analysis
Analysis of variance was used for statistical analysis of the data, with individual group means compared using post hoc Student-Newman-Keuls analysis and Dunnett’s multiple comparisons test as indicated in the figure legends. P > 0.05 was considered not significant.
Results
Effect of PMNs on MLR Induced by Allogeneic DCs
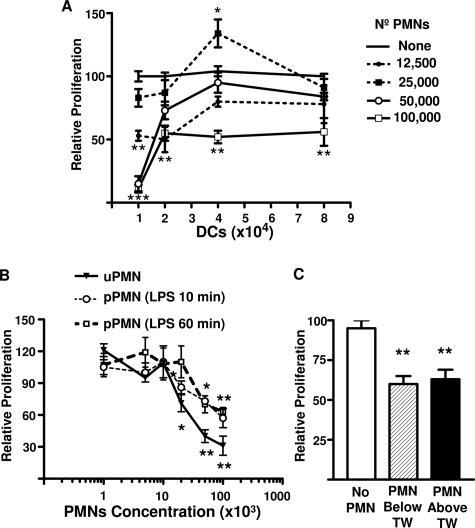
The effect of PMNs on allostimulatory ability of DCs was determined by performing MLR in the presence of different amounts of PMNs. Figure 1A shows that the proliferation of lymphocytes induced by graded doses of viable allogeneic stimulator DCs in the presence of autologous (syngeneic) PMNs were influenced by the number of PMNs added. A significant decrease in the allogeneic lymphoproliferation induced by 104 and 2 × 104 allogeneic DCs was seen with 1.25 × 104, 5 × 104, and 105 PMNs, but not with 2.5 × 104 added PMNs. A decrease was also observed with 105 added PMNs for proliferation induced by 4 × 104 and 8 × 104 allogeneic DCs. In contrast, 2.5 × 104 PMNs increased the lymphocyte proliferation induced by 4 × 104 allogeneic DCs. It is important to note that for these experiments (n = 4), the mean of proliferation induced by DCs in the absence of PMNs was 21,246 ± 740, 29,164 ± 839, 32,836 ± 909, and 36,894 ± 599 cpm for 104, 2 × 104, 4 × 104, and 8 × 104 DCs, respectively (100% relative proliferation for each DC concentration used). Furthermore, the possibility of induction of apoptosis or necrosis of DCs by PMNs was excluded by performing annexin V-fluorescein isothiocyanate and propidium iodide staining (see Supplemental Figure 1 at http://ajp.amjpathol.org).
Figure 1.
Effect of PMNs on DC’s immunostimulatory ability. A: MLR induced by graded doses of viable allogeneic DCs in the presence of different concentrations of autologous (syngeneic) PMNs. Stimulator allogeneic DCs (1 to 8 × 104) were incubated with 105 responder PBMCs and 1.25 to 10 × 104 syngeneic PMNs. Thus, responder cells (PBMCs) and PMNs were related to the donor and unrelated to DCs. B: MLR induced by viable allogeneic DCs (2 × 104) in the presence of different concentrations of unprimed or LPS autologous (syngeneic) PMN-primed at two different time points. C: Allogeneic DCs (2 × 104) were incubated with 105 PBMCs, and 105 syngeneic PMNs were added above or below a Transwell (TW) bare 0.4-μm polycarbonate filter. After 4 days, cells were pulsed with 1 μCi of [3H]thymidine per well for the last 16 hours of culture, harvested, and counted in a beta counter. Proliferation is depicted relative to the proliferation of MLR cultures in the absence of PMNs (100% = 30,739 ± 664 cpm). Relative proliferation is depicted as average means of triplicates ± SEM from a series of four experiments for A and three experiments for B and C. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control group, using Dunnett’s multiple comparisons test.
Bacterial infections or the endotoxin LPS (bacterial cell wall components of gram-negative bacteria) prime, activate, and induce neutrophil migration. Therefore, the effect of PMN-LPS priming was next examined on allostimulatory ability of DCs. For this, PMNs were primed with LPS for 10 minutes and 1 hour followed by thorough washes to remove the LPS. Then, PMNs were incubated with allogeneic DCs and syngeneic lymphocytes. Figure 1B shows that both 5 × 104 and 105 PMNs primed with LPS either for 10 minutes or 1 hour decreased the allogeneic lymphoproliferation induced by DCs. However, the decrease observed with uPMNs was more pronounced and occurred with as few as 2 × 104 cells. From here on, the value of 100% relative proliferation was the mean of proliferation induced by 2 × 104 DCs in the absence of PMNs (30,739 ± 664 cpm for n = 34). The decrease in the allogeneic lymphoproliferation was due to soluble factor(s) released by the PMNs, because the effect was also observed with DCs that had been incubated with PMNs separated by a polyvinylpyrrolidone-free polycarbonate Transwell filter bearing 0.4-μm pores (Figure 1C).
Effect of PMN-Derived CS on MLR Induced by Allogeneic DCs
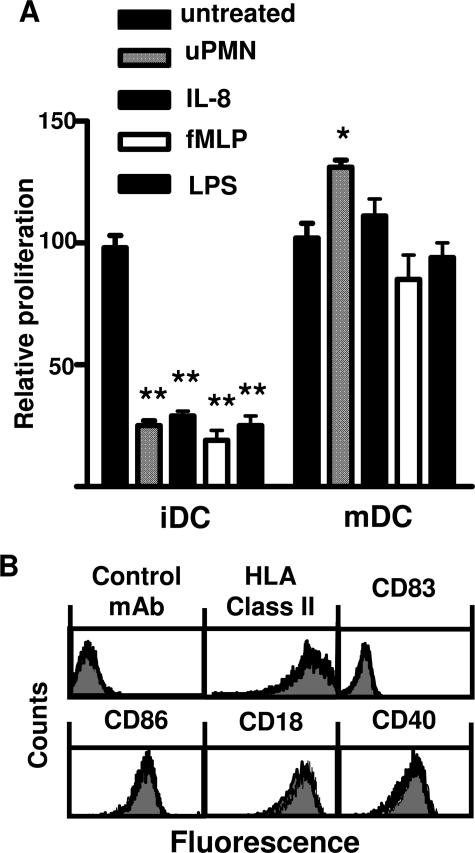
Next, we assessed the lymphocyte proliferation induced by CS derived from allogeneic immature and mature DCs previously treated for 3 hours with uPMNs. Figure 2A shows that CS derived from DCs preincubated with uPMNs decreased the immunostimulatory ability of immature but not mature (18-hour LPS-treated) DCs. Furthermore, mature DCs treated with uPMN-derived CS slightly increased the allogeneic response. PMN CS derived from pPMNs treated with IL-8, LPS, or formyl-Met-Leu-Phe was also examined. Figure 2A shows that CS from pPMNs treated with IL-8, LPS, or formyl-Met-Leu-Phe for 1 hour was able to decrease the lymphocyte proliferation induced by DCs. At least 50% volume of uPMN- or pPMN-derived CS was necessary to produce the effect on DC allostimulatory capacity (data not shown). Furthermore, the CS has to be conditioned for at least 90 and 180 minutes with pPMNs and uPMNs, respectively, to observe the described effect (data not shown). Due to the lack of significant differences between uPMN and pPMN CS, thereafter we used 50% of uPMN or pPMN CS conditioned for 180 minutes to treat DCs.
Figure 2.
Effect of PMN CS on DC’s immunostimulatory ability and surface molecule expression. A: MLR induced by allogeneic immature (iDCs) or previously LPS-treated DCs for 3 hours with unprimed PMN (uPMN) CS or pPMN CS. Priming was performed with IL-8, formyl-Met-Leu-Phe, or LPS followed by thorough washing. CS samples were harvested after 3 hours of culture of the PMNs. Next, CS samples were incubated with untreated or LPS-treated DCs for another 3 hours, and then the DCs were washed and cultured with 105 PBMCs. After 4 days, cells were pulsed with 1 μCi of [3H]thymidine per well for the last 16 hours of culture, harvested, and counted in a beta counter. Proliferation is depicted relative to the proliferation of MLR cultures in the absence of PMNs (see Materials and Methods). Relative proliferation is depicted as average means of quadruplicates ± SEM from a series of five experiments *P < 0.05, **P < 0.01 compared with control group, using Dunnett’s multiple comparisons test. B: HLA class II, CD83, CD86, CD18, and CD40 expression on immature DCs untreated or treated with IL-8 pPMN CS. Immature DCs treated with IL-8 pPMN CS were stained with primary mAb and analyzed by flow cytometry as described in Materials and Methods. Shaded histograms are untreated DCs, which overlapped nearly completely the histogram of pPMN-CS-treated DCs. Data are representative of three independent experiments.
To elucidate the mechanisms by which PMN CS decreased the immunostimulatory ability of DCs, we first excluded the induction of apoptosis or necrosis of DCs by PMN CS. The treatment of DCs with pPMN CS for 24 hours did not induce apoptosis or necrosis of DCs based on the staining of DCs with annexin V and propidium iodide (see Supplemental Figure 2 at http://ajp.amjpathol.org).
The expression of costimulatory and adhesion molecules was also investigated. The treatment of immature DCs with IL-8 pPMN CS did not modify the expression of MHC class I (not shown) and II, CD83, CD86, CD40, and CD18 (Figure 2B). Furthermore, the experiment allowed us to characterize and reconfirm the immature phenotype of immature DCs used in the assays.
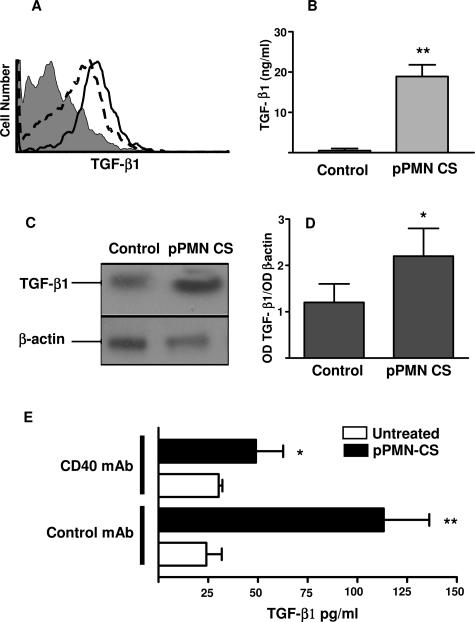
The pattern of cytokines produced by immature DCs after treatment with uPMN CS or IL-8 pPMN CS was next examined. The production by immature DCs of IL-10 and IL-12 (p70) was almost undetectable, and the CS treatment did not modify results (data not shown). However, IL-8 pPMN-CS-treated DCs showed a higher amount of intracellular TGF-β1 staining compared with untreated DCs (Figure 3A). This was confirmed by ELISA measuring TGF-β1 on DC-derived CS previously treated with IL-8 pPMN CS (Figure 3B). We excluded that the TGF-β1 was derived from the IL-8 pPMN CS used to treat the DCs, because we were unable to detect it in pPMN CS. Furthermore, the expression of TGF-β1 mRNA by DCs treated with IL-8 pPMN CS was also increased (Figure 3, C and D). Interestingly, the TGF-β1 production induced by IL-8 pPMN-CS-treated DCs was reversed by treating DCs with mAb against CD40, which mimics binding with CD40L on T cells (Figure 3E).
Figure 3.
Expression of TGF-β1 on DCs treated with pPMN CS. A: Intracellular levels of TGF-β1 in untreated (dotted line histograms) and IL-8 pPMN-CS-treated (thick line histogram) DCs. Cells were treated as described for Figure 2. Afterward, DCs were incubated with brefeldin A (10 μg/ml, 3 hours, 37°C), fixed, and stained with anti-TGF-β1 R-phycoerythrin or control isotype Ab (filled histogram) and analyzed by flow cytometry. Similar results were obtained in three additional experiments. B: TGF-β1 concentration in DC-derived CS. After 3 hours of DC treatment with PMN CS, the cells were washed and incubated for another 24 hours in RPMI-HSA medium. Then the supernatants were recovered, diluted, and assessed for TGF-β1 by an ELISA. Data represent the mean ± SEM of five experiments. **P < 0.01 compared with untreated DCs using post hoc Student-Newman-Keuls analysis. C and D: Reverse transcription-polymerase chain reaction analysis for TGF-β1 and β-actin mRNA in untreated and IL-8 pPMN-CS-treated DCs. After 3 hours of treatment with pPMN CS, RNA was extracted from DCs. Data represent one representative experiment of three for C and the mean ± SEM for D. E: Effect of mAb to CD40 on TGF-β1 induced by DCs treated with IL-8 pPMN CS. After DCs were treated with IL-8 pPMN CS, the cells were incubated with CD40 mAb for another 3 hours. Then the supernatants were recovered, diluted, and assessed for TGF-β1 by ELISA. Data represent the mean ± SEM of four experiments. *P < 0.05; **P < 0.01 compared with untreated DC group using post hoc Student-Newman-Keuls analysis.
Role of TGF-β1 Produced by PMN-CS-Treated DCs
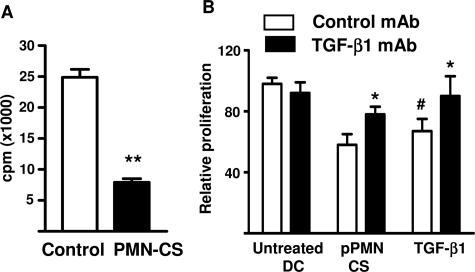
The biological activity of TGF-β1 produced by IL-8 pPMN-CS-treated DCs was confirmed by bioassay (Figure 4A). To confirm definitively the role of TGF-β produced by PMN-CS-treated DCs, we repeated the allostimulatory assays in the presence of a TGF-β1 and a blocking TGF-β1 mAb. Figure 4B shows that TGF-β1 reproduced the inhibitory effect of PMN-CS-treated DCs on lymphocyte proliferation. Furthermore, the mAb against TGF-β1 partially reversed the depressed lymphocyte proliferation seen with IL-8 pPMN-CS-treated DCs or added TGF-β1 (Figure 4B) but not with untreated DCs.
Figure 4.
TGF-β1 activity. A: TGF-β1 bioactivity was assessed using the mink lung cell assay as described in Materials and Methods. Mink lung cells (20 × 103 or 40 × 103 cells/well) were incubated with DC-derived CS. After 22 hours, the incorporated [3H]thymidine (used as a marker of DNA synthesis) was measured. Data represent the mean ± SEM of five experiments; **P < 0.01 Student’s t-test. B: Effect of TGF-β1 and a mAb against TGF-β1 on DC-induced allogenic lymphoproliferation. DCs were untreated or treated for 3 hours with IL-8 pPMN CS. Afterward, the cells were washed and incubated with allogenic lymphocyte in the presence of 20 μg of TGF-β1 mAb, isotype control mAb, or 10 μg of TGF-β1. After 4 days, cells were pulsed with 1 μCi of [3H]thymidine per well for the last 16 hours of culture, harvested, and counted in a beta counter. Proliferation is depicted relative to the proliferation of MLR cultures induced by untreated DCs (100%). Relative proliferation is depicted as average means of quadruplicates ± SEM from a series of four experiments. #P < 0.05 compared with untreated DC group, and *P < 0.05 compared with isotype control mAb, using Dunnett’s multiple comparisons test.
Role of Neutrophilic Elastase
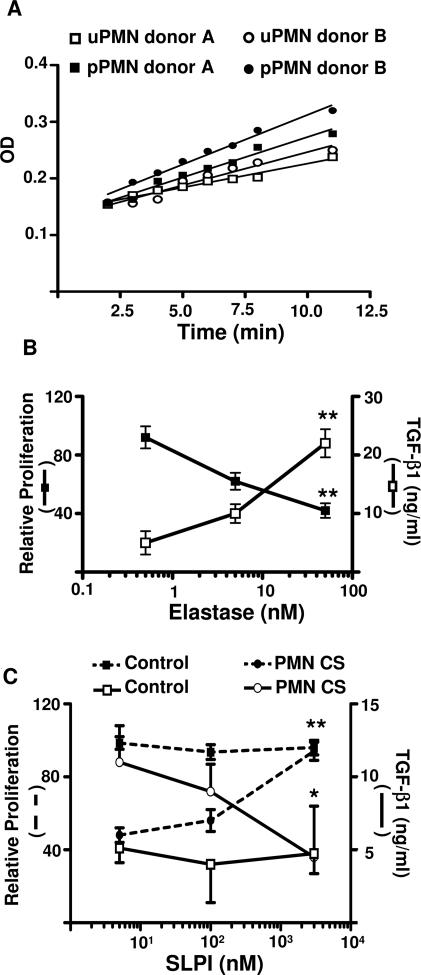
To examine the putative factors present in PMN CS that may affect DC physiology, we assessed the effect of protease inhibitors. From all of the protease inhibitors tested, only serine protease inhibitor (aprotinin) partially reversed the effect of PMN CS on DCs (data not shown). Because elastase is a main serine protease present in PMN granules, we examined the presence of elastase activity in the uPMN CS. Figure 5A shows that uPMN and IL-8 pPMN CS from two healthy donors (A and B) had elastase activity.
Figure 5.
Effect of elastase on DC lymphocyte allostimulatory ability. A: Elastase activity of PMN CS. PMNs from two healthy donors were primed or not primed with IL-8 for 5 minutes. Afterward, the cells were thoroughly washed and cultured in RPMI-HSA medium for another 3 hours. Then the supernatants were recovered, diluted, and assessed for elastase activity. B: TGF-β production and MLR induced by allogeneic immature DCs previously treated with elastase. Different concentration of elastase was incubated with DCs for 3 hours and then thoroughly washed and cultured in RPMI-HSA medium for 48 hours for assay of TGF-β or incubated with 105 PBMCs for the MLR. Proliferation is depicted relative to the proliferation of MLR cultures induced by untreated DCs (100%). Relative proliferation is depicted as average means of quadruplicates ± SEM from a series of four experiments. *P < 0.05; **P < 0.01 compared with control group, using Dunnett’s multiple comparisons (cpm and TGF-β1 from untreated DCs was 30,739 ± 664 cpm and 3 ± 0.2 ng/ml, respectively). C: To evaluate the effect of SLPI on PMN-CS-treated DC lymphocyte allostimulatory ability and TGF-β1 production, DCs (3 × 104) were incubated for 3 hours with PMN CS and different concentrations of SLPI. Then, cells were thoroughly washed and incubated with 105 PBMCs for the MLR (dotted line) or cultured in RPMI-HSA medium for 48 hours for assay of TGF-β1 production (solid line). Proliferation is depicted relative to the proliferation of MLR cultures induced by untreated DCs (100%). Relative proliferation is depicted as average means of quadruplicates ± SEM from a series of four experiments *P < 0.05 and **P < 0.01 compared with control group, using Dunnett’s multiple comparisons test.
Whether elastase was able to reproduce the PMN CS effect on DC allostimulatory ability and TGF-β1 production was next examined. Figure 5B shows that immature DCs treated with 50 nmol/L elastase for 3 hours had decreased lymphocyte allostimulatory ability and increased DC TGF-β1 production.
To confirm that elastase from PMN CS was responsible for the effect, we then preincubated PMN CS with rhSLPI, a serine protease inhibitor. Figure 5C shows that rhSLPI reversed the effect of PMN CS on allostimulatory ability and TGF-β production by immature DCs treated with PMN CS.
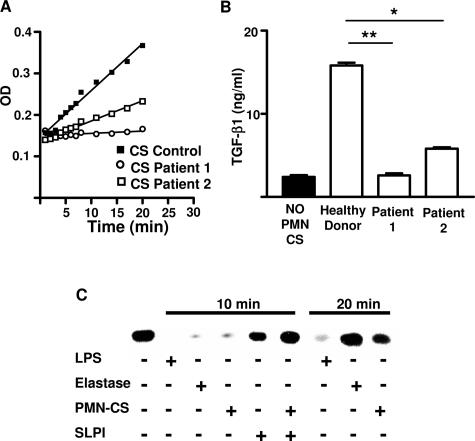
To confirm definitively the role of elastase, we examined the ability of PMN CS from two patients with cyclic neutropenia, a disease characterized by mutations in the gene encoding neutrophil elastase. The uPMN CS harvested from these patients’ cells had reduced elastase activity (Figure 6A). Moreover, these uPMN CS were unable to increase DC TGF-β1 production (Figure 6B).
Figure 6.
Effect of PMN CS from cyclic neutropenic patients on elastase activity and TGF-β1 production. Elastase activity of uPMN CS and TGF-β production by uPMN CS were measured as described for Figure 5, A and C. In A, the data represent the CS from two cyclic neutropenic patients. In B, the TGF-β1 data represent the mean ± SD of one representative experiment of three. *P < 0.05 and **P < 0.01 compared with control group, using Dunnett’s multiple comparisons test. C: To examine the expression of IκBα by Western blot, DCs were untreated (column 1) or treated with LPS (columns 2 and 7), elastase (columns 3 and 8), uPMN CS (columns 4 and 9), SLPI (column 5), or uPMN CS + SLPI (column 6). Cells were treated for 10 and 20 minutes and processed as described in Materials and Methods. Data are representative of three independent experiments.
Mechanism of Action of Elastase and PMN CS
It has been shown that neutrophilic elastase induces NF-κB activation and nuclear translocation in bronchial epithelial cells. Furthermore, rhSLPI reverts the effect of elastase on DCs and inhibits the proinflammatory activity of bacterial products. This is achieved by the inhibition of the proteolytic degradation of IκB, the inhibitor of NF-κB. Therefore, we examined whether elastase activates NF-κB on DCs. On LPS or elastase stimulation, a decrease in IκBα was observed at 10 minutes (Figure 6C). Treating DCs with uPMN CS produced similar results. However, 20 minutes later, the effect of elastase and uPMN CS disappeared, but not so for LPS. Interestingly, rhSLPI was able to prevent the effect of uPMN CS at 10 minutes (Figure 6C). The present experiments suggest that elastase and uPMN CS induce NF-κB activation.
Discussion
Recent advances in the field of DC physiology have led to the finding that interaction of PMNs with DCs up-regulates the maturation and immunostimulatory ability of DCs and thus the T-cell response.7 Despite this development, certain questions related to the effect of PMNs on the adaptive immune response remain unresolved. PMNs have been reported to produce factors capable of potentiating or suppressing responses to T- or B-cell mitogens.15 In addition, PMN-derived taurine chloramines down-modulate murine DC functions in vitro.21 Furthermore, the uptake of apoptotic or necrotic PMNs inhibits CD40, CD80, and CD86 expression by DCs and reduces allogeneic T-cell responses.8 Recently, however, several authors have shown that PMNs are able to increase the maturation and immunostimulatory ability of murine and human DCs.7,14,22,23,24 In the present work, we demonstrate that PMNs are able to down-modulate DC function. This effect was associated with the amount of elastase released by PMNs, which in turn converted immature myeloid DCs into TGF-β1-secreting cells.
Neutrophil elastase is a serine proteinase stored in PMN azurophilic granules along with other serine proteinases.25 It is known that elastase can damage endothelial cells and cleave endothelial cell-associated adhesion molecules.26,27 Furthermore, elastase has been implicated in the pathogenesis of several lung inflammatory diseases, where proteinase-antiproteinase imbalance causes tissue damage.28 Under normal circumstances tissue damage by PMN elastase is prevented by systemic and locally produced serine proteinase inhibitors. During inflammation, the plasma concentration of these serine proteinase inhibitors increases three- to fourfold, and the inactivation of elastase is rapid and irreversible.29 However, this is incomplete in an inflammatory microenvironment with extensive PMN degranulation. It has been estimated that each PMN contains 399 ± 20 primary granules, and a single granule contains 1.1 ± 0.11 pg of elastase.30 This elastase concentration is more than two orders of magnitude higher than the physiological serpin concentration. Thus a proteolytically active zone around the PMNs can be generated with a perimeter of approximately 1.33 μm.28,31 In the human lung there is a pool size of 6.1 × 107 PMNs, and this increases by approximately two orders of magnitude during bacterial infection.28 Therefore, 7.21 mg of elastase can be released on stimulation, and only 30 μg/ml elastase is needed to cleave 90% of the complement receptor CR1.32 Moreover, cell surface elastase has been found on human neutrophils.33
In this work, we found similar elastase activity in PMN CS regardless of whether PMNs are primed. However, a longer exposure of DCs to uPMN CS than to pPMN CS is required to induce the same effect on DCs (data not shown). This could be due to the kinetics of degranulation, because PMN extracellular granule secretion occurs in the following order: secretory, gelatinase, specific, and azurophilic granules.34 In addition, it is important to point out that PMNs were thoroughly washed before the conditioning of PMN CS was initiated; thus, we excluded an effect on the DCs of the factors used to prime the PMNs.
The effect of PMN CS on DCs seems to be specific for immature DCs, because the treatment with PMN CS of LPS-matured DCs did not inhibit the MLR (Figure 2). Furthermore, uPMN-CS-treated DCs but not LPS-, IL-8-, or formyl-Met-Leu-Phe-primed PMN-CS-treated DCs increased the MLR. It is known that DC maturation involves a number of phenotypical and functional changes. Thus, maturation changes may also include the down-regulation and up-regulation of receptors involved in the inhibition of MLR induced by PMN-CS-treated DCs. Furthermore, the differences observed between uPMN CS and LPS, IL-8, or formyl-Met-Leu-Phe pPMN CS can be due to the interaction of factors that may up-regulate or down-regulate the production of TGF-β1 by DCs.
Differences in priming for inhibition of DC-induced lymphocyte proliferation was also observed when DCs and neutrophils were cocultured (Figure 1B). In those experiments, the uPMNs were better at reducing the MLR than LPS pPMNs. One reason for the more potent inhibition by uPMNs than LPS pPMNs could be that neutrophil priming with LPS induces neutrophil factors that partially block the inhibition. This putative factor could be membrane-associated or -soluble such as SLPI, which is present in neutrophil cytosol, and its secretion is enhanced when the cells are stimulated.
The effect of elastase on cells and receptors may impair the killing of pathogens. Herein, we demonstrate that another putative mechanism could be involved in this effect. We find that the increased TGF-β1 mRNA is translated into protein as determined by intracellular cytokine staining and ELISA. Furthermore, we demonstrate that functional TGF-β1 production by DCs is induced by PMN CS or elastase. This finding is supported by the experiments using PMNs from cyclic neutropenia patients. As we showed, one of the patients lacked elastase activity, and his PMN CS was not able to convert DCs into TGF-β1-secreting cells. The other patient had some elastase activity, but this activity was not enough to increase TGF-β1 production as much as the healthy donors. It is important to mention that most, but not all, mutations causing cyclic neutropenia reduce proteolytic activity.35
The TGF-β1 species detected in the PMN-CS-treated DCs appeared to be produced in an active form as suggested by the TGF-β1 bioassay. Furthermore, neutralizing TGF-β1 with mAb completely abrogated the inhibition of proliferation induced by TGF-β1 in the MLR. However, the same mAb partially reversed the inhibition observed in the MLR induced by PMN-CS-treated DCs. The reason for this result could be the presence of other mechanisms involved, in addition to TGF-β1-mediated inhibition, or incomplete neutralization by the TGF-β1 mAb of cytokine continuously produced by PMN-CS-treated DCs during culture, where it could have a rapid autocrine action.
It is known that neutrophil elastase is able to phosphorylate IκBα in human airway smooth muscle.36 A similar mechanism of action may function in DCs, since we presently report that elastase and even PMN CS decrease IκBα protein expression. The fact that pretreatment with SLPI prevented the PMN CS-induced IκBα degradation further supports the role of elastase. Thus, we propose that elastase and PMN CS, by activating NF-κB signaling in DCs and also TGF-β1 production, may promote or depress an ongoing immune response, respectively. Based on our results, it is possible to speculate that drugs that inhibit NF-κB activation, such as glucocorticoids and aspirin, may also block elastase-induced TGF-β1 secretion from DCs, thus favoring an enhanced immune response. However, TGF-β not only inhibits lymphocyte proliferation and stimulates apoptosis37 but also promotes overly reactive innate immune responses.38 Thus, TGF-β can act as a positive as well as a negative regulator of lymphocyte proliferation.38
TGF-β is secreted predominantly as a latent complex that has to be activated before being capable of eliciting biological effects.39 Because DC CS affected the proliferation of TGF-β1-sensitive cell lines, we can assume that the TGF-β1 produced and released by DCs is active. Interestingly, the production of TGF-β1 by PMN-CS-treated DCs is reversible with a mAb to CD40 that mimics engagement by CD40L present on T lymphocytes. Therefore, we suggest that a putative interaction between TGF-β1-producing DCs with an antigen-specific T cell might reverse the effect of PMN CS or elastase. Moreover, the effect of elastase on DCs may be reversible once elastase levels decrease below a threshold. Based on the lower inhibitory effect observed with LPS pPMNs, we propose that a balance between elastase and other factors released by neutrophils may determine the outcome of these interactions.
The mechanisms described herein involving neutrophils, elastase, NF-κB activation, and TGF-β1 production do not exist in isolation, and there are many mechanisms that integrate their activity with other cell-signaling networks. This cross talk constitutes a decision-making process that determines the consequences of PMN and DC interaction and, ultimately, the nature of the immune response and its regulation.
The mechanisms described herein may be important in all those pathologies that present an excessive or sustained PMN infiltration, such as bacterial invasion particularly by Staphylococcus aureus, Haemophilus influenzae, and Pseudomonas aeruginosa. However, it could be more relevant in the pathogenesis of several lung diseases, such as α1-antitrypsin deficiency, chronic obstructive pulmonary disease, and cystic fibrosis, where the inflammatory infiltrate contains high numbers of PMNs40 and high elastase concentrations.41 Interestingly, elastase has been shown to increase the release of TGF-β1 by airway smooth muscle.36 Furthermore, lung DCs have an immature phenotype.42 Recently, Roghanian et al43 showed that sputum from chronic obstructive pulmonary disease and patients with cystic fibrosis or elastase down-regulated the expression of CD40, CD80, CD86 (but not MHCII) on murine DCs, inhibited LPS-induced murine DC maturation and T-cell proliferation. Roghanian’s study 43 with murine DCs supports our work with human DCs, although we did not find changes in costimulatory molecule expression. A possible explanation for this difference could be due to a much lower concentration of elastase used in our experiments.
Overall these results establish a dual role for PMNs, either amplifying or restraining the immune response by acting on DCs. In addition, we demonstrate for the first time that PMN elastase can convert immature myeloid DCs into TGF-β1-secreting cells, and the PMNs exert their effect on DCs via this secreted product. We postulate that these mechanisms help control inappropriate or exaggerated immune response and are examples of physiological control characteristic of biological systems.
Supplementary Material
Acknowledgments
We thank Marcos Barbosa for technical support.
Footnotes
Address reprint requests to H. Eduardo Chuluyan, Laboratorio de Inmunogenética, Piso 3, Sala 4, Hospital de Clínicas “José de San Martín,” Facultad de Medicina, Universidad de Buenos Aires, Avenida Córdoba 2351, C.P. 1120, Buenos Aires, Argentina. E-mail: chulu@interar.com.ar; echuluyan@hospitaldeclinicas.uba.ar.
Supported by grants from the Agencia Nacional de Promocion Cientifica y Tecnologica, PICT11702 and PICT15069, UBACYT M019 (to H.E.C.), and National Institute of Dental and Cranial Research, National Institutes of Health grant DE 016063-01 (to H.E.R.). H.E.C. is grateful to Fundación Florencio Fiorini for financial support.
P.C.M. and S.E.Z. contributed equally to this work.
Supplemental material for this article can be found on http://ajp. amjpathol.org.
References
- Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- Hellewell PG, Williams TJ. The Neutrophils. San Diego: Academic Press,; 1994:pp 1–4. [Google Scholar]
- Henson P, Henson J, Fittschen C, Kimani G, Bratton D, Riches D. Phagocytic cells: degranulation and secretion. Gallin JI, Goldstein IM, Snyderman R, editors. New York: Raven Press,; InflammationBasic Principles and Clinical Correlates. 1988:pp 363–390. [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Kobayashi SD, Voyich JM, DeLeo FR. Regulation of the neutrophil-mediated inflammatory response to infection. Microbes Infect. 2003;5:1337–1344. doi: 10.1016/j.micinf.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Dallegri F, Ottonello L. Tissue injury in neutrophilic inflammation. Inflamm Res. 1997;46:382–391. doi: 10.1007/s000110050208. [DOI] [PubMed] [Google Scholar]
- Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052–6058. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- Clayton AR, Prue RL, Harper L, Drayson MT, Savage CO. Dendritic cell uptake of human apoptotic and necrotic neutrophils inhibits CD40, CD80, and CD86 expression and reduces allogeneic T cell responses: relevance to systemic vasculitis. Arthritis Rheum. 2003;48:2362–2374. doi: 10.1002/art.11130. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Granucci F, Ferrero E, Foti M, Aggujaro D, Vettoretto K, Ricciardi-Castagnoli P. Early events in dendritic cell maturation induced by LPS. Microbes Infect. 1999;1:1079–1084. doi: 10.1016/s1286-4579(99)00209-9. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Granucci F, Citterio S, Foti M, Ricciardi-Castagnoli P. Coordinated events during bacteria-induced DC maturation. Immunol Today. 1999;20:200–203. doi: 10.1016/s0167-5699(98)01427-3. [DOI] [PubMed] [Google Scholar]
- van Gisbergen KP, Sanchez-Hernandez M, Geijtenbeek TB, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med. 2005;201:1281–1292. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrick ML, Lamster IB, Sonis ST, Pender SG, Kolodkin AB, Fitzgerald JE, Wilson RE. Effects of supernatants of polymorphonuclear neutrophils recruited by different inflammatory substances on mitogen responses of lymphocytes. Inflammation. 1982;6:1–11. doi: 10.1007/BF00910714. [DOI] [PubMed] [Google Scholar]
- Burzyn D, Jancic CC, Zittermann S, Keller Sarmiento MI, Fainboim L, Rosenstein RE, Chuluyan HE. Decrease in cAMP levels modulates adhesion to fibronectin and immunostimulatory ability of human dendritic cells. J Leukoc Biol. 2002;72:93–100. [PubMed] [Google Scholar]
- Chuluyan HE, Issekutz AC. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Invest. 1993;92:2768–2777. doi: 10.1172/JCI116895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz AC, Chuluyan HE, Lopes N. CD11/CD18-independent transendothelial migration of human polymorphonuclear leukocytes and monocytes: involvement of distinct and unique mechanisms. J Leukoc Biol. 1995;57:553–561. doi: 10.1002/jlb.57.4.553. [DOI] [PubMed] [Google Scholar]
- Fukushima D, Butzow R, Hildebrand A, Ruoslahti E. Localization of transforming growth factor beta binding site in betaglycan: comparison with small extracellular matrix proteoglycans. J Biol Chem. 1993;268:22710–22715. [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz J, Nowak B, Grabowska A, Bobek M, Petrovska L, Chain B. Regulation of murine dendritic cell functions in vitro by taurine chloramine, a major product of the neutrophil myeloperoxidase-halide system. Immunology. 1999;98:371–378. doi: 10.1046/j.1365-2567.1999.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. Close encounters of neutrophils and DCs. Trends Immunol. 2005;26:626–631. doi: 10.1016/j.it.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Denkers EY, Butcher BA, Del Rio L, Bennouna S. Neutrophils, dendritic cells and Toxoplasma. Int J Parasitol. 2004;34:411–421. doi: 10.1016/j.ijpara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Megiovanni AM, Sanchez F, Robledo-Sarmiento M, Morel C, Gluckman JC, Boudaly S. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J Leukoc Biol. 2006;79:977–988. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Champagne B, Tremblay P, Cantin A, St. Pierre Y. Proteolytic cleavage of ICAM-1 by human neutrophil elastase. J Immunol. 1998;161:6398–6405. [PubMed] [Google Scholar]
- Lévesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- Döring G. The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med. 1994;150:S114–117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. [DOI] [PubMed] [Google Scholar]
- Morgan K, Kalsheker NA. Regulation of the serine proteinase inhibitor (SERPIN) gene alpha 1-antitrypsin: a paradigm for other SERPINs. Int J Biochem Cell Biol. 1997;29:1501–1511. doi: 10.1016/s1357-2725(97)00118-0. [DOI] [PubMed] [Google Scholar]
- Damiano VV, Kucich U, Murer E, Laudenslager N, Weinbaum G. Ultrastructural quantitation of peroxidase- and elastase-containing granules in human neutrophils. Am J Pathol. 1988;131:235–245. [PMC free article] [PubMed] [Google Scholar]
- Liou TG, Campbell EJ. Quantum proteolysis resulting from release of single granules by human neutrophils: a novel, nonoxidative mechanism of extracellular proteolytic activity. J Immunol. 1996;157:2624–2631. [PubMed] [Google Scholar]
- Tosi MF, Zakem H, Berger M. Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest. 1990;86:300–308. doi: 10.1172/JCI114699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol. 1995;131:775–789. doi: 10.1083/jcb.131.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengeløv H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995;154:4157–4165. [PubMed] [Google Scholar]
- Horwitz M, Benson KF, Duan Z, Li FQ, Person RE. Hereditary neutropenia: dogs explain human neutrophil elastase mutations. Trends Mol Med. 2004;10:163–170. doi: 10.1016/j.molmed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Lee KY, Ho SC, Lin HC, Lin SM, Liu CY, Huang CD, Wang CH, Chung KF, Kuo HP. Neutrophil-derived elastase induces TGF-β1 secretion in human airway smooth muscle via NF-κB pathway. Am J Respir Cell Mol Biol. 2006;35:407–414. doi: 10.1165/rcmb.2006-0012OC. [DOI] [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Wahl SM. Transforming growth factor-β: innately bipolar. Curr Opin Immunol. 2007;19:55–62. doi: 10.1016/j.coi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med. 2005;172:530–551. doi: 10.1164/rccm.200410-1384SO. [DOI] [PubMed] [Google Scholar]
- Roghanian A, Drost EM, Macnee W, Howie SE, Sallenave JM. Inflammatory lung secretions inhibit dendritic cell maturation and function via neutrophil elastase. Am J Respir Crit Care Med. 2006;174:1189–1198. doi: 10.1164/rccm.200605-632OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.