Abstract
Our understanding of the biology of the complement system has undergone a drastic metamorphosis since its original discovery. This system, which was traditionally primarily described as a “complement” to humoral immunity, is now perceived as a central constituent of innate immunity, defending the host against pathogens, coordinating various events during inflammation, and bridging innate and adaptive immune responses. Complement is an assembly of proteins found in the blood and body fluids and on cell surfaces. Soluble complement components form the proteolytic cascade, whose activation leads to the generation of complement effectors that target various cells involved in the immune response. Membrane-bound receptors and regulators transmit signals from complement effectors to target cells and limit complement activation to the surfaces of pathogens and damaged or activated host cells. The multiple interconnections among complement proteins, immune cells, and mediators provide an excellent mechanism to protect the organism against infections and support the repair of damaged tissues. However, disturbances in this “defense machinery” contribute to the pathogenesis of various diseases. The role of complement in various inflammatory disorders is multifaceted; for example, the activation of complement can significantly contribute to inflammation-mediated tissue damage, whereas inherited or acquired complement deficiencies highly favor the development of autoimmunity.
Complement was discovered approximately 100 years ago as a heat-sensitive component of plasma that enhances the opsonization of bacteria by antibodies and facilitates antibody-dependent killing of bacteria.1 The original report assigned the name “complement” to this component to reflect the idea that its function is complementary to that of humoral immunity. Thus, its very name reflects our traditional view that the complement system plays a supporting role “behind the scenes.” This terminology also corresponds to the historical concept that complement components exert their functions at the “periphery” of the defense reaction, acting exclusively as a supplement to the action of other components of the immune system. The inadequacy of this “complementary” concept becomes apparent in light of recent studies demonstrating functions of complement that are essential and “central” to the innate immune response, as well as functions that bridge innate with adaptive immunity.2 Today, complement should rather be viewed as a system that orchestrates and connects various responses during immune and inflammatory reactions and not merely as a killer of bacteria.3
The complement system is composed of membrane-bound regulators and receptors as well as numerous plasma proteins that interact with various cells and mediators of the immune system.4 These interactions vary according to the pathophysiologic context, and they occur at different steps of an immune reaction. This wide network of interactions, together with the broad range of mechanisms of complement activation, make the components of the complement system ideal candidates for coordinating events that occur as a consequence of the induction of innate immunity, as well as those that occur later during the adaptive immune reaction. This review describes the role of complement proteins in the regulation and synchronization of an acute inflammatory reaction, which aims to eliminate pathogens and clear damaged host cells and proceeds with obvious benefit for the host. In addition, we discuss the contribution of complement to the pathophysiology of selected diseases. Unfortunately, several associations of complement with other immune mediators or cells that contribute to the successful battle against microbes or facilitate the clearance and repair of damaged host tissues can paradoxically become responsible for various pathologies when control mechanisms fail or the immune system encounters unusual stimulatory factors. In some pathophysiologic situations, an excess of complement is deleterious to the host, whereas under other circumstances a total lack or deficiency of complement contributes to the development of disease. These two aspects of complement pathophysiology will also be discussed.
An Acute Inflammatory Response
The complement system actively regulates various steps of an inflammatory response. Inflammation is currently viewed as a complex pathophysiologic process that engages literally hundreds of mediators and different cell types and tissues and can be initiated by any stimulus causing cell injury. Often, inflammation is a response to infection. However, chemical or physical injury alone can also induce this reaction.5 The ultimate goal of this process is to eliminate the causative agent with minimal destruction to host tissues and to repair the damage caused by the initiating factor.
The duration of an inflammatory response depends in part on whether a successful resolution and neutralization of the initiating agent has occurred. Acute inflammation is a relatively short process, lasting from minutes to a few days, and its main characteristics are an exudation of fluid and plasma proteins and an emigration of leukocytes into an extravascular compartment. These vascular and cellular responses are mediated by chemical factors derived from plasma or cells and are responsible for the classic clinical signs of inflammation, originally described by Aulus Cornelius Celsus and later modified by Rudolph Virchow: tumor (swelling), rubor (redness), dolor (pain), calor (warmth), and functio laesa (loss of function). If the acute response is unable to remove the causative factor or repair the damage at the site of inflammation, a chronic process, in which tissue destruction and repair occur while the inflammatory reaction continues, may develop. Chronic inflammation can also result from stimuli that initiate a low-grade and asymptomatic response.6
Although an inflammatory reaction can occur in any tissue exposed to an injurious stimulus, the hallmark of this process is the response of vascularized connective tissue.6 The first changes observed during inflammation are alterations in the vascular flow and changes in the caliber of small blood vessels. Vasodilation of arterioles, resulting in the opening of new capillary beds in the area of injury, follows transient vasoconstriction, which lasts only seconds. Larger arterioles and newly opened capillary vessels increase the blood flow to this area. Gradually, progressive changes in the endothelium enhance the vascular permeability of the microvasculature, leading to the escape of the fluid into an extravascular compartment. The decrease in the amount of the fluid in the lumen of blood vessels enhances the viscosity of the blood and slows down the flow rate. As a result of these alterations in blood flow, the margination of leukocytes begins. Over time, leukocytes stick to the endothelium, at first transiently (rolling), then more avidly (adhesion), and soon afterward, they migrate through the vascular wall (transmigration) into the interstitial tissue. At this point, the essential goal of acute inflammation, to bring leukocytes and plasma mediators to site of injury, is achieved.6
Initiation of the Complement Cascade
Efficient, rapid, and occurring in synchrony with the initiation of an inflammatory reaction, the activation of complement is a prerequisite for its involvement in regulation of inflammatory processes. The mechanisms that govern this activation are well defined and understood mainly in terms of the reactions occurring in the intravascular space. However, the soluble components of complement are present not only in the circulation but also in body fluids and tissues, ready to engage in defense reactions triggered by exogenous (eg, infectious agents) or endogenous (eg, ischemia, autoimmunity) stimuli that could cause cell injury.7 In addition to the specific activation induced by antigen-antibody complexes, complement is activated through the pattern recognition receptors, which have the ability to discriminate between self and non-self antigens based on repeating patterns of molecular structure (pathogen-associated molecular patterns) present on the surface of pathogens.8 Complement-activating pattern recognition receptors include mannose-binding lectin (MBL), ficolins, C-reactive protein, C1q, and natural IgM (IgM). These molecules share many common properties with Toll-like receptors, which often initiate an innate immune response and also belong to the category of pattern recognition receptors.8 The contribution of the pattern recognition system to complement activation assures the rapid initiation of the complement cascade as a part of an early immune response and inflammation. In fact, the activation of complement occurs immediately with high efficiency when the system encounters appropriate stimuli. For example, in infection induced by Leishmania, approximately 90% of promastigotes are lysed by complement in just 2.5 minutes after contact with human blood.9 Therefore, complement effectors generated as a result of this activation can participate in the earliest events of an inflammatory reaction.7
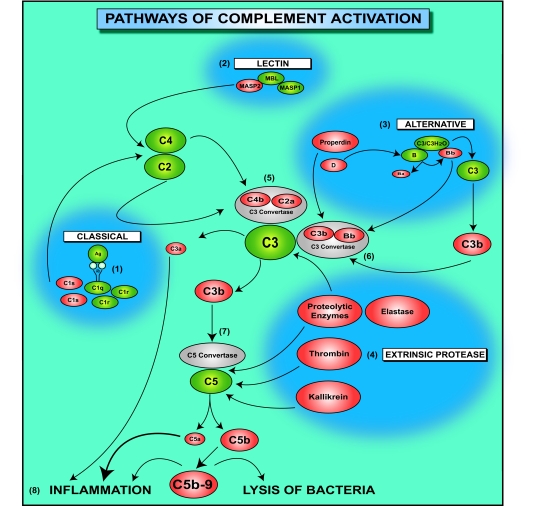
Complement is activated through the classical, lectin, or alternative pathways (Figure 1). The classical pathway is initiated by antibodies produced during the humoral response, by natural antibodies, and by other molecules that are generated as a result of an inflammatory reaction such as C-reactive protein or serum amyloid protein. The binding of C1q to antigen-antibody complexes initiates the proteolytic cleavage of complement components in the classical pathway. The lectin pathway begins with the recognition and binding of pathogen-associated molecular patterns by lectin proteins, including MBL. C1q and MBL are structurally similar molecules, and both the classical and lectin pathways require C2 and C4 complement components for the generation of the C3 convertase. The alternative pathway differs significantly from the classical and lectin pathways because it is initiated by the spontaneous hydrolysis of C3, leading to the formation of C3(H2O) and the binding of a small amount of C3b to hydroxyl groups on cell surface carbohydrates and proteins. C3(H2O) forms a complex with factor B, followed by the cleavage of factor B within this complex by factor D. The final product of these enzymatic reactions is the C3(H2O)Bb complex. Once deposited on the surface of cells or pathogens, C3b binds more factor B, and this binding gradually amplifies the activation cascade.
Figure 1.
Pathways of complement activation. The complement cascade is composed of proteins that are activated through the partial cleavage by an upstream enzyme. Complement is activated through the classical (1), lectin (2), or alternative (3) pathways that converge at the central molecule of the complement system, C3. In addition, C3 and C5 can be cleaved, independent of conventional convertases, by proteolytic enzymes that are released from leukocytes or by thrombin or kallikrein. In this diagram, enzymes that cleave complement proteins, independently of conventional convertases, are grouped in the “extrinsic protease pathway” (4). The binding of the C1 complex to antigen-antibody complexes initiates proteolytic cleavage of complement components by C1s in the classical pathway (1). C1s first cleaves C4, which binds to the bacterial surface, then cleaves C2, leading to the formation of a C4b2a enzyme complex, the C3 convertase of the classical pathway (5). The lectin pathway begins with binding of the complex MBL and mannose-binding lectin-associated proteases 1 and 2 (MASP1 and MASP2, respectively) to a bacterial cell wall (2). MASP2, similarly to C1s, leads to the formation of the C3 convertase C4b2a (5). The alternative pathway is initiated by the spontaneous hydrolysis of C3, leading to the formation of C3(H2O) (3). C3(H2O) forms a complex with factor B, followed by the cleavage of factor B within this complex by factor D. The final product of these enzymatic reactions is the C3(H2O)Bb complex. The C3bBb complex has the capacity to cleave C3 to C3b and C3a. Once deposited on the surface of cells or pathogens, C3b binds more factor B, and this binding gradually amplifies the activation cascade. The binding of properdin stabilizes the C3bBb complex, the C3 convertase of the alternative pathway (6). C3 convertases generated through various pathways cleave C3 to C3a and C3b. C3b contributes to the formation of the C5 convertase (7), which cleaves C5 to C5a and C5b. C3a, C5a, and the C5b-9 complex are complement effectors that contribute to inflammation (8). In addition, the C5b-9 complex induces lysis of gram-negative bacteria of the Neisseria genus.
All three pathways of complement activation lead to the formation of C3 convertases that cleave C3 to C3a and C3b. C3b contributes to the formation of the C5 convertase, which in turn cleaves C5 to C5a and C5b. Complement anaphylatoxins C3a, C4a, and C5a are potent inflammatory mediators, and C5b initiates the formation of C5b-9 terminal complement complex, which is incorporated into bacterial cell walls and induces the lysis of pathogens, in particular gram-negative Neisseria strains.10
The activity of the anaphylatoxins is regulated by carboxypeptidases, including carboxypeptidase N, which circulate in the plasma or are present in the tissues. Carboxypeptidase N hydrolyzes the carboxy-terminal peptide bond of anaphylatoxins, releasing the carboxy-terminal arginine. These derivatives of anaphylatoxins are known as C3a-desArg and C5a-desArg. C3a, C3a-desArg, and C4a exhibit direct antibacterial properties;11 however, some of the biological activities of the desArg forms of C3a and C5a are significantly reduced (by 10- to 100-fold) when compared with those of their full-length forms.12
C3 and C5 can also be cleaved locally at sites of ongoing inflammatory reactions without the participation of other complement components (Figure 1). Several proteolytic enzymes present within inflammatory exudates, such as lysosomal enzymes or elastase released from neutrophils,13 have been shown to activate C3 and C5. Kallikrein, a component of the kinin and fibrinolysis systems, has the ability to cleave C5 and release C5a.14 Recent work has shown that C5a can be generated in mice lacking C3 through the proteolytic cleavage of C5 by thrombin.15 These cleavage reactions result in the generation of active complement components that have proinflammatory properties such as anaphylatoxins as well as C3b or C5b. C3b might contribute to the formation of the alternative pathway convertase. Therefore, complement activation initiated by noncomplement proteins might be amplified through the alternative pathway. We have categorized the mechanisms, initiating complement activation by noncomplement proteins into a separate pathway, and named it here as the extrinsic protease pathway of complement activation (Figure 1). Thus, a large variety of mechanisms and pathways involved in complement activation ensure that active complement components can potentially be generated at almost every step of both innate and adaptive immune responses.
Complement in Acute Inflammation
Complement components that are activated in plasma and body fluids are engaged in the regulation of virtually all phases of an acute inflammatory reaction, including changes in vascular flow and caliber, the increase in vascular permeability, extravasation of leukocytes, and chemotaxis. Several regulatory functions of complement affect other inflammatory mediators, whereas other complement activities are associated with the direct action of complement proteins on target cells. Because of its variety of activating mechanisms, complement can independently participate in the regulation of inflammation, in either the presence or absence of an infection.
Anaphylatoxins and Changes in Vascular Flow and Blood Vessel Caliber
Tissue injury, the primary signal for launching the innate immune response and inflammation, initiates several signaling cascades that regulate changes in the microvasculature, including the activation of complement. Damaged cells release a number of constitutively expressed proteins, such as heat shock proteins,16 the transcription factor HMGB1 (for the high mobility group),17 and mitochondrial peptides bearing the N-formyl group that are characteristic of prokaryotic proteins.17 It is interesting that heat shock proteins can activate the complement system in both an antibody-dependent and -independent manner.18 The complement activation initiated by heat shock proteins can occur in the absence of pathogens. Therefore, even aseptic tissue injury activates the complement cascade. In addition, microbes and their shed or secreted products activate complement through binding to C1q or MBL.4 Anaphylatoxins generated through complement activation interact with their receptors expressed on various cells, thereby modulating their inflammatory properties.
Mast cells are widely distributed in the connective tissue around blood vessels and are among the first responders during inflammation.19 During their response to activation by anaphylatoxins, mast cells release histamine, preformed tumor necrosis factor (TNF)-α, newly synthesized cytokines, tryptases, other proteases, and chemokines. In addition, C5a activates the lipoxygenase pathway of arachidonic acid metabolism in neutrophils and monocytes, leading to an acceleration of eicosanoid production by these cells.20 Mast cells and neutrophils that are activated by complement anaphylatoxins release mediators that cause vasodilation and extravasation of fluid.19
Another cell population that is activated at the very beginning of the inflammatory reaction are the macrophages, which are found in large numbers in connective tissue, the submucosal layer of the gastrointestinal tract, the lung, the liver, and the spleen.7 Although the migration of neutrophils to tissue is viewed as a hallmark of acute inflammation, macrophages are among the first cells to recognize pathogens that cross the epithelial barrier and begin to replicate in the host tissue.7 The recognition of foreign antigens occurs through the pattern recognition receptors expressed by these cells; therefore, macrophages can immediately respond to infection. Macrophages also express receptors for anaphylatoxins, which deliver additional activation signals to these cells. This activation leads to the secretion of cytokines and chemokines by macrophages.
C5a has the capacity to induce gene expression and protein synthesis of TNF-α and interleukin (IL)-1β in monocytes and macrophages.21 Although the role of C3a in the modulation of TNF-α and IL-1β production and release is not as well characterized as that for C5a, it has been shown that C3a and C3a-desArg may stimulate the production of these cytokines.22 C3a signaling seems to be costimulatory with lipopolysaccharide signaling and, depending on the pathophysiologic background and target cell population, it has a stimulatory or an inhibitory effect.22 C3a also increases the expression of IL-6 when acting as a costimulator of lipopolysaccharide signaling.23
The signals controlling TNF-α and IL-1β secretion seem to be especially important for the course of the acute inflammatory response, because these cytokines have a significant influence on local (ie, at the site of injury) and peripheral homeostasis. These two cytokines share many properties, such as the capacity to activate the endothelium, leukocytes, and fibroblasts.24,25 In the endothelium, TNF-α and IL-1β induce a spectrum of changes, mostly regulated at the transcription level, that are referred to as endothelial activation. These changes include the synthesis of endothelial adhesion molecules, other cytokines, chemokines, growth factors, eicosanoids, nitric oxide, and enzymes associated with matrix remodeling.26 In addition, TNF-α and Il-1β increase the surface thrombogenicity of the endothelium.27 TNF-α causes aggregation and priming of neutrophils, leading to augmented responses of these cells to other mediators. TNF-α also induces the release of proteolytic enzymes from mesenchymal cells, thus contributing to tissue damage.24,25
The interactions between the complement system and proinflammatory cytokines are reciprocal. Several reports have suggested that proinflammatory cytokines enhance the expression of anaphylatoxin receptors in inflammatory cells.28,29 It also seems that the effect of anaphylatoxins on cytokine expression depends strictly on the pathophysiological context of the ongoing inflammatory response. For example, in sepsis, excessive C5a generation leads to up-regulation of IκBα (nuclear factor κB inhibitor) in neutrophils, consequently decreasing nuclear factor κB transcriptional activity and inhibiting lipopolysaccharide-mediated stimulation of TNF-α production.30
Increased Vascular Permeability
Changes in the caliber of the arterioles and blood flow alterations are associated with the increased vascular permeability induced by changes in the endothelium. The hemodynamic changes and the increased permeability of the endothelial barrier lead to the formation of an inflammatory exudate, which contains protein-rich fluid and inflammatory cells. Formation of inflammatory exudates permits inflammatory mediators and cells to accumulate at the sites of injury and to efficiently fight invading pathogens or eliminate other inflammation-causing factors. Increased vascular permeability is caused by functional and structural changes in the endothelial barrier. These include the formation of endothelial gaps in venules, reorganization of the endothelial cytoskeleton, increased transcytosis across the endothelial cytoplasm, direct and leukocyte-mediated endothelial injury, delayed prolonged leakage (illustrated by sunburn), and leakage from blood vessels newly formed during angiogenesis.31 Many of these endothelial alterations are mediated by factors already discussed here, including histamine, kinins, leukotrienes, and TNF-α.31 The expression and release of these mediators is controlled, at least in part, by anaphylatoxins. Intriguingly, C5a can also be involved in angiogenesis, which is an essential process involved in tissue repair after injury. C5a-stimulated human umbilical vein endothelial cells exhibit an increased expression of genes involved in endothelial adhesion, migration, and angiogenesis.32 In addition, it has recently been shown that C3a and C5a contribute to the angiogenesis associated with age-related macular degeneration (AMD).33
Leukocyte Extravasation
Increased vascular permeability and slower blood flow also facilitate the process of extravasation, in which leukocytes leave the blood stream and migrate into the interstitial space. The most important molecular event associated with leukocyte extravasation is an up-regulation of adhesion molecules expressed by leukocytes and endothelial cells, which permit interactions to occur between these cells. These interactions cause leukocyte rolling, adhesion, and finally transmigration through the vascular barrier.
Anaphylatoxins, mainly C5a, participate in endothelial activation. C5a-stimulated human umbilical vein endothelial cells show up-regulation of genes for E-selectin, intracellular adhesion molecule-1, vascular cell adhesion molecule-1, and IL-6.32 Anaphylatoxins also influence the expression of adhesion molecules on leukocytes. It has been postulated that C5a is involved in eosinophil adhesion to bronchial epithelial cells during allergic inflammation in the airways.34 Another study has shown that C5a is an activator of integrin-dependent adhesion and transmigration of eosinophils and neutrophils.35 These examples indicate that anaphylatoxins can play a role in the process of extravasation, directly influencing the expression of adhesion molecules on endothelial cells as well as leukocytes. In addition, anaphylatoxins also participate indirectly in this process by regulating TNF-α and IL-1 expression. These two cytokines seem to be major regulators of adhesion molecule expression on both leukocytes and endothelium during inflammation.
Chemotaxis
Leukocytes that cross the walls of blood vessels migrate toward the site of injury, directed by chemotactic mediators along a chemical gradient. Anaphylatoxins, and particularly C5a, are well-established chemotactic factors that affect leukocyte migration directly through binding to related receptors expressed on inflammatory cells.10 An additional property of anaphylatoxins is their capacity to induce the synthesis of other chemotactic factors, including eicosanoids and chemokines. C5a stimulates mouse dermal microvascular endothelial cells, primed with IL-6, to produce monocyte chemoattractant protein-1 and macrophage inflammatory protein-2.36 C3a induces the synthesis of monocyte chemoattractant protein-1 and regulated on activation, normal T cells expressed and secreted (RANTES) in human mast cells. 37 C5a also stimulates the release of IL-8 from human bronchial epithelial cells38 as well as peripheral blood mononuclear cells and mouse macrophages.39 IL-8 production requires nuclear factor κB activation through C5a signaling.39
“Waste Disposal”
Inflammation is usually associated with host tissue damage of various degrees of severity. Restitutio ad integrum (the return of the organism, organ, or tissue to its original status after the cessation of a diseased process) requires the efficient clearance of necrotic or apoptotic cells. Apoptotic and necrotic cells are known to activate complement, and several complement components significantly facilitate the clearance of dead cells by a system involving phagocytes. Complement also plays a role in maintaining the solubility of and removing circulating immune complexes generated during the adaptive immune response. These complement functions prevent the deposition of antigen-antibody complexes within tissues and the subsequent injury induced by activation of an inflammatory reaction by these complexes.40 A detailed discussion of the role of complement in the clearance of dying cells and immune complexes is included in the section on autoimmunity (see below).
Our discussion thus far has indicated that complement proteins contribute significantly to various steps of an acute inflammatory reaction (Figures 2 and 3), which is triggered to protect the host from injury and to eliminate pathogens. The contribution of the complement system to host defense against infections has been confirmed by an increased susceptibility of complement-deficient individuals to recurrent pyogenic infections.41 For reasons that are not yet completely understood, this increased susceptibility is restricted to a limited number of bacteria. For example, deficiencies in late complement components (C5 to C9) and properdin are associated with recurrent infections caused by Neisseria meningitides and Neisseria gonorrhoeae. An increased frequency of bacterial infections is also characteristic of deficiencies in classical pathway proteins and C3.41 Despite the protective role of complement against microbial invasion, associations of complement with an inflammatory network can have pathogenic consequences when certain regulatory mechanisms fail.
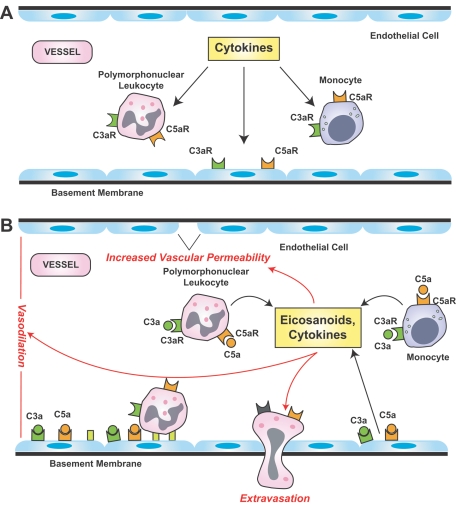
Figure 2.
Contribution of complement anaphylatoxins to vascular events of acute inflammation. A: Cytokine signaling contributes to an up-regulation of anaphylatoxin receptors (C3aR, C5aR) by endothelial cells in arterioles and on circulating leukocytes. B: Binding of C3a and C5a to the reciprocal receptors on these cells enhances the release of cytokines and eicosanoids that contribute to an increase in vascular permeability, vasodilation, and leukocyte extravasation.
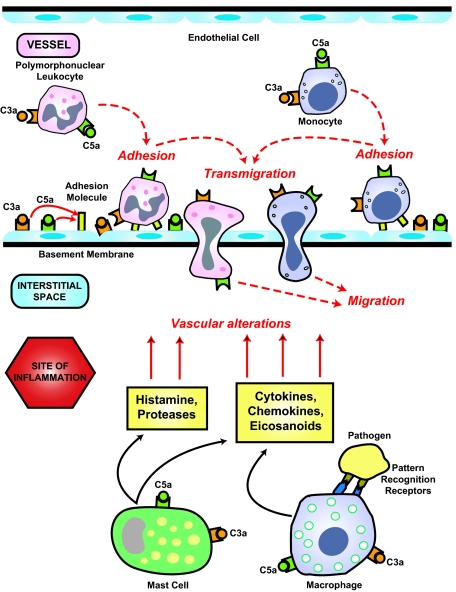
Figure 3.
Anaphylatoxin-mediated extravasation of leukocytes. Anaphylatoxins up-regulate adhesion molecules on endothelial cells and leukocytes, facilitating the adhesion of leukocytes to the vascular wall and their subsequent transmigration into the interstitial tissue at sites of inflammation. C3a and C5a stimulate mast cells to release histamine and proteases that also contribute to vascular alterations. Monocyte-derived macrophages are among the first cells encountering non-self-antigens, and through the pattern recognition system they initiate an innate immunity response.
Complement in Inflammatory Disorders
Although the purpose of inflammation is to protect the host against microbes, to repair injuries, and to contribute to the removal of apoptotic cells or immune complexes, when it becomes excessive, deregulated, or chronic, this process can initiate or contribute to several pathologies including the development of cancer.5 The integral participation of complement components in various steps of an inflammatory reaction explains in part its involvement in the pathogenesis of variety of inflammatory disorders. The contribution of complement to the pathogenesis of these diseases can be viewed from various perspectives.
First, the activation of complement during an inflammatory reaction contributes to inflammation-driven tissue injury, which occurs in the ischemia/reperfusion (I/R) setting, vasculitides of various etiologies, nephritis, and arthritis. Second, a deficiency in complement components, even those necessary for efficient complement activation, may also result in tissue injury, as observed in autoimmune reactions. Finally, alterations in the expression of complement regulatory proteins, which lead to the excessive complement activation, can also contribute to tissue injury.
I/R Injury
I/R injury is defined as cellular damage occurring after the reperfusion of previously viable ischemic tissue. It is a consequence of pathophysiologic conditions that lead initially to a deprivation of tissue blood supply, followed by the return of tissue perfusion, which paradoxically may induce more damage than the ischemia itself. The augmentation of tissue injury after reperfusion is a result of an intense inflammation developing simultaneously with the return of tissue perfusion.42 Several diseases, syndromes, and clinical conditions can lead to I/R injury, including myocardial infarction, stroke, hemorrhagic shock, severe trauma, and organ transplantation.42 The return of the blood supply to tissues damaged by ischemia results in increased microvascular permeability and the attraction, activation, and migration of inflammatory cells (including neutrophils) to the site of injury; aggregation of platelets and leukocytes; and decreased endothelium-dependent relaxation. Activated inflammatory cells produce several mediators, including cytokines, oxygen radical species, and eicosanoids, which enhance the injury initially induced by ischemia.42 In addition, ischemic tissue activates the complement system, which contributes to the development of tissue damage directly or indirectly by enhancing inflammation.43 Severe inflammation, developing as a consequence of reperfusion of large areas of ischemic tissue, may even lead to systemic inflammatory response syndrome or multiple organ dysfunction syndrome, which accounts for up to 30 to 40% of intensive care unit mortality.44
The complement system seems to be a major contributor to the tissue damage that occurs after ischemia and reperfusion. Complement activation following ischemia/reperfusion occurs during myocardial infarction45,46; ischemia of the intestine,47 hind limb,48 and kidney49; hemorrhagic shock50; sepsis51; and pulmonary injury.52 As yet there is no definitive answer to the question of how complement is activated after I/R.43 It seems that all three pathways of complement activation are involved in the initiation of the proteolytic cleavage of complement cascade components. However, the specific contribution of the classical, alternative, and lectin pathways varies, depending on pathophysiologic setting. Results of several studies have postulated the involvement of the lectin pathway in myocardial46 and gastrointestinal I/R-induced complement activation.43 However, the amplification of complement activation occurs through the alternative pathway in gastrointestinal I/R.43 Skeletal muscle injury resulting from I/R is likely associated with complement activation occurring via the lectin and classical pathways.53 In contrast, several experiments using various complement-deficient mouse strains have suggested that the alternative pathway contributes to kidney injury.49,54
Whatever the mechanism of activation, the components of complement are often deposited on the surface of endothelial cells. The endothelium, together with the underlying basement membrane, builds a vascular barrier, which has particular significance for the pathogenesis of I/R injury.45 It has been postulated that natural antibodies reacting with antigen epitopes that are exposed after damage to endothelial cells play an important role in the activation of complement during I/R injury.55 iC3b, formed by the proteolytic cleavage of C3b deposited on the endothelial surface, acts as a ligand for β2 integrin CD11b/CD18 (CR3). This adhesion molecule is up-regulated in activated leukocytes and contributes to the accumulation of these cells in the extravascular compartment. The deposition of several complement proteins on the surface of endothelium ultimately leads to the formation of terminal complement complex, which, when incorporated into the cellular membrane, activates endothelial nuclear factor κB. Thereby, it increases the transcription and expression of several adhesion molecules, including vascular cell adhesion molecule-1, intracellular adhesion molecule-1, and E- and P-selectins.56 Terminal complement complex also stimulates the endothelium to produce IL-8 and monocyte chemoattractant protein-1 and directly affects vascular tone by inhibiting endothelium-dependent relaxation and decreasing endothelial cyclic guanosine monophosphate.57 Terminal complement complex also induces the activation of platelets, contributing to platelet-leukocyte aggregation.58 Anaphylatoxins C3a and C5a activate endothelial cells and leukocytes. C5a, more potent as an inflammatory mediator than C3a, up-regulates the expression of adhesion molecules on endothelium and induces the release of various cytokines, including IL-1, IL-6, monocyte chemoattractant protein-1, and TNF-α.21,32 C5a is also a strong chemoattractant, contributing to the accumulation of inflammatory cells at the site of injury.59 Thus, several mechanisms used by complement to orchestrate the events of acute inflammation are also important for the pathogenesis of I/R injury.
Further confirmation of the importance of complement for the pathogenesis of injury after ischemia and reperfusion is the successful implementation of anticomplement therapies in clinical trials for the treatment of patients suffering from the consequences of I/R-related diseases. Inhibition of complement activation has been shown to reduce I/R injury in many experimental models.60 Administration of soluble complement receptor 1 (CR1), a C3 convertase inhibitor, significantly reduces the size of an experimental myocardial infarct. Similarly, a recombinant single-chain antibody against human C5 (pexelizumab) attenuates leukocyte activation, myocardial injury, and acute postoperative mortality in patients undergoing cardiopulmonary bypass surgery.60 Both of these compounds, as well as many others, are currently undergoing clinical trials, and anticomplement therapies seem to be a novel and promising approach to minimizing the consequences of I/R-associated diseases.
Autoimmune Disorders
Autoimmune diseases occur when a specific adaptive immune response is directed against self-antigens. In contrast to an adaptive immune response against foreign antigens, the elimination of self-antigens is usually impossible for the effector mechanisms of an immune system to achieve. Therefore, a sustained immune response occurs and leads to a chronic inflammatory injury to the host tissues. The archetypical autoimmune disease is systemic lupus erythematosus (SLE), a disease of unknown etiology, in which deposition of pathogenic antibodies and immune complexes triggers damage to cells and tissues. Studies on the pathogenesis of SLE have highlighted several mechanisms in this disease that apply to various other autoimmune disorders as well.
The role of the complement system in the pathogenesis of SLE is diverse. The first evidence of a link between complement and the pathogenesis of this disease originated from the observation that the levels of several complement components are decreased in patients with SLE. This “decomplementation” is often a result of deposition of complement components in host tissues, associated with the deposition of immune complexes that activate complement through the classical pathway.61 Complement effectors that are generated as a result of this activation enhance the inflammatory reaction in affected host tissue, thereby exacerbating the tissue injury. The magnitude of the complement activation and the levels of complement proteins correlate to some extent with the disease activity, at least during the initial phase of SLE.61 This harmful role of complement in the pathogenesis of inflammation-mediated tissue injury seems to contradict the observation that inherited and acquired complement deficiencies are associated with a higher prevalence of SLE in humans. In fact, the homozygous deficiencies of early components of the classical pathway, including C1q, C1r, C1s, C4, and C2, are the strongest susceptibility factors for the development of SLE that have been identified in humans thus far.61 The prevalence and the severity of disease are associated with the position of the particular deficient protein in the complement cascade. The highest frequency and the most severe course of disease is observed in patients deficient in one of the C1 complex proteins or C4, whereas most of the individuals with homozygous C2 deficiency remain asymptomatic.62 The results of analyses of heterozygous individuals and people with complement gene polymorphisms are less conclusive. However, some interesting associations have been observed. For example, gene polymorphisms associated with lower levels of MBL are found in higher frequencies in patients with SLE, and, conversely, polymorphisms that result in high levels of MBL expression are underrepresented in patients with SLE.63
Although homozygous complement deficiencies are extremely rare, they have provided a significant insight into the mechanism of development of SLE and autoimmunity. It seems that complement proteins involved in the activation of the classical pathway, independent of C3 cleavage, protect against the development of autoimmune reactions. This hypothesis is also supported by the higher prevalence of autoimmune reactions in patients with acquired complement deficiencies.61 SLE is observed more often in patients with hereditary angioedema, which is characterized by an autosomal dominant functional deficiency of C1 inhibitor that results in the failure to regulate the classical pathway of complement activation. As a consequence, the levels of C4 and C2 are chronically low in these individuals. There is a predominance of females in this group, as well as a high incidence of antinuclear antibodies, skin lesions, and photosensitivity, which are all common clinical features of SLE.64 SLE ultimately develops in a significant number of patients with C3 nephritic factors, autoantibodies that stabilize the C3 convertase and thereby cause uncontrolled complement activation and hypocomplementemia. In many cases, the development of SLE occurs years after the initial appearance of the nephritic factor.65
The initial observations suggesting a link between the complement system and SLE originated from studies on humans. However, recent investigations using various complement-deficient mouse strains and experimental disease models have provided deeper insight into the mechanism behind complement’s protective role in preventing the development of autoimmunity. Studies performed using C1q-, C4-, C3-, and CD35/CD21 (CR1/2)-deficient mice have confirmed that an absence of proteins participating in the activation of complement through the classical pathway is associated with the development of autoimmunity, and these associations are independent of C3 activation.66,67,68 It is interesting that the expression of the disease phenotype was background-related, suggesting the contribution of other genes to the higher susceptibility of complement-deficient mice to autoimmune reactions.66
Mechanism of Complement-Dependent Development of Autoimmunity
Several, not necessarily exclusive, hypotheses have been proposed to explain the link between complement deficiencies and the development of autoimmunity. One generally accepted hypothesis is that the high prevalence of autoimmune reactions in complement-deficient patients and animals is associated with an impairment of the clearance of dying cells and immune complexes, which occurs when complement proteins are missing. An alternative hypothesis is that complement determines the thresholds for B- and T-cell activation, and the absence of complement proteins disturbs the tolerance for self-antigens.
Clearance of Apoptotic and Necrotic Cells
Under physiological conditions, complement fulfills several functions related to the removal of waste material originating from necrotic or apoptotic cells, as well as the clearance of circulating immune complexes. Activation of complement by necrotic cells is well established. Apoptotic cells have also been reported to initiate the classical pathway of complement activation through the binding of the globular head of C1q to the blebs present on apoptotic cells, and the intensity of C1q binding has been found to increase along with bleb maturation.69 The role of C1q, however, is not restricted to the initiation of the classical pathway. A defect in the uptake of apoptotic cells by monocyte-derived macrophages has been shown in patients carrying hereditary C1q deficiencies, as well as in patients with SLE.70,71 These observations have been confirmed by studies using C1q-deficient mice, which develop a proliferative glomerulonephritis characterized by the presence of multiple apoptotic bodies in affected glomeruli, suggesting an inability of these mice to clear cellular debris.72 In addition, the opsonization of apoptotic cells by C3 cleavage products facilitates the engulfment of apoptotic cells or apoptotic bodies by phagocytes bearing CR3 and CR4 receptors.73
The phagocytosis of apoptotic cells is an extremely complex process involving the interactions of multiple receptors with various ligands. In addition to C1q, other collectins such as MBL also contribute to this process through their binding to calreticulin and CD91 on phagocytes. A number of other molecules that bind to C1q and activate the classical pathway recognize the modified cell membranes of apoptotic cells, including C-reactive protein and IgM. The targets for these proteins are membrane lysophospholipids containing phosphorylcholine.61
Impaired clearance of dying cells leads to the prolonged presence of self-antigens, which are normally hidden within cells from immune recognition mechanisms. This extended exposure is thought to contribute to the development of an immune response directed against host tissues. This hypothesis has been confirmed by the restriction of the specificity of autoantibodies present in patients with SLE. The repertoire of target autoantigens for SLE autoantibodies is surprisingly limited, mainly to the components of the cell nucleus. In particular, several of these antigens are found in high concentration on the surface of apoptotic cell blebs,74 a localization that strongly suggests that apoptotic cells are indeed the source of the self-antigens that induce autoimmune reactions in these patients.
The attractiveness of this hypothesis relies mainly on the fact that it provides an explanation of the origin of autoantigens, which can potentially initiate an autoimmune reaction. However, the steps that follow the defective clearance of apoptotic cells and the development of autoimmunity remain unclear. In addition, the dependence of the disease phenotype on the genetic background of C1q-deficient mice suggests that other genes contribute to the autoimmune phenotype, at least in mice, further demonstrating the complex and still unresolved nature of autoimmunity in SLE.
Clearance of Immune Complexes
Complement maintains the solubility of immune complexes and promotes their recognition and clearance by phagocytes. It preserves solubility by preventing an increase in the size of immune complexes through the binding of the C1 complex, C4, and C3 to the antigen and the heavy chain of IgG. This binding reduces the effective valency of the antigen and antibody and also interferes with Fc-Fc interactions.75 Complement fragments bound to immune complexes also provide ligands that facilitate the clearance of these complexes by phagocytes. For example, in primates, erythrocytes expressing CR1 are responsible for the transfer of immune complexes to the liver and spleen, where tissue macrophages remove them from circulation.61
Another example of cooperation between phagocytes and complement is the up-regulation of Fcγ receptors (FcγRs) on Kupffer cells by C5a. This cross-talk has been shown to play an important role in the development of autoimmune hemolytic anemia in mice.76 Given that patients with SLE often develop hematological abnormalities, including hemolytic anemia, we can speculate that C5a-FcγR interactions also link complement to autoimmunity in SLE. Thus, genetic complement deficiencies or acquired hypocomplementemia in patients with SLE leads to increased levels and decreased solubility of circulating immune complexes, facilitating their deposition within host tissues. However, inhibition of C5a signaling attenuates antibody-dependent hemolytic anemia.76
Immunity or Tolerance
Unlike necrosis, apoptosis does not induce an inflammatory reaction and immune response. This tolerance is a result of down-regulation of the immune response by dendritic cells (DCs), which avidly take up apoptotic cells. This uptake by immature DCs, in contrast to the uptake of necrotic cells, prevents the maturation of immature DCs that is required to activate the immune response.77 The phagocytosis of apoptotic cells by immature DCs suppresses inflammation and promotes tolerance by increasing the production of anti-inflammatory mediators such as transforming growth factor-β and IL-10 and inhibiting proinflammatory cytokines such as TNF-α.77 The phagocytosis of apoptotic cells also reduces the capacity of DCs to stimulate T cells directly. In addition, cells undergoing apoptosis secrete immunosuppressive cytokines.78
The impaired clearance of apoptotic cells that results from complement deficiency, together with their prolonged presence in the tissues of patients with autoimmune reactions, may result in the development of secondary necrosis. This process in turn induces the maturation of DCs and activation of proinflammatory signaling, leading to a break in tolerance to self-antigens. Several studies using tumor cell lines have confirmed that necrotic cells can induce the maturation of DCs in vitro. However, this maturation does not occur when DCs phagocytose necrotic cells originating from primary cultures. Furthermore, recent investigations have shown that the ability of necrotic cells to induce DC maturation was limited to cells that originated from cell lines contaminated with mycoplasma.61 Therefore, the role of secondary necrosis in the development of autoimmunity still requires further clarification. The disruption of tolerance to self-antigens could also be induced by the large dose of autoantigen present as a result of impaired clearance of apoptotic cells.
Deficiencies in complement proteins can also be associated with a breakdown in B-cell tolerance, which results in the escape of autoreactive B cells from deletion or anergy. These autoreactive B cells are subsequently activated to produce the autoreactive antibodies responsible for immune complex formation and tissue injury in autoimmune disorders. It has also been suggested that the attachment of C4b to self-antigens and localization of these complexes to CD35 on stromal cells within the bone marrow regulate the selection of potentially autoreactive B cells.79
CD4+ T cells play a critical role in the pathogenesis of SLE. Effector T cells directly induce tissue damage and costimulate autoreactive B cells to produce high-affinity autoantibodies. Certain subpopulations of T cells express C1q receptors, and the proliferation of these cells is significantly inhibited by the addition of soluble C1q to mitogen-activated T-cell cultures.80 Although the pathophysiological role of these in vitro observations is not clear, they may suggest an important link between complement and the regulation of T-cell activity. This hypothesis is further supported by the finding that in patients with SLE, the levels of C1q can be decreased as a consequence of the presence of autoantibodies against this protein.81 The insufficient amount of C1q available might contribute to the activation of autoreactive T cells and the breakdown of tolerance for self-antigens.
Dysfunction of Complement Regulators
Alterations in the expression of the complement regulators that limit the activation of complement in the healthy individual can lead to unregulated and excessive complement activation, resulting in tissue injury. Examples of such conditions include atypical hemolytic-uremic syndrome (aHUS) and AMD.
Atypical aHUS
This rare disorder is characterized by thrombocytopenia, Coombs test-negative microangiopathic hemolytic anemia, and acute renal failure. It occurs in adults and in very young children and is associated with mutations or polymorphism in the genes encoding complement regulatory proteins, including factor H, membrane cofactor protein, and factor I.82,83 Recently, it has been also reported that mutations in the gene encoding factor B are present in a subgroup of patients with aHUS.84 Several studies have suggested that mutations in the factor H gene result in inappropriate regulation of C3 convertase activity.85 In addition, mutant forms of factor H exhibit weaker binding to C3b and C3d, heparin, and the surface of endothelial cells than is exhibited by their normal counterparts. These reduced binding properties of the mutated factor H likely contribute to the progressive damage of the endothelium and microvasculature that occurs in factor H-associated genetic forms of aHUS.86 An inhibition of factor H function by anti-factor H antibodies has been also reported in some forms of aHUS.87 The genetic alterations in the factor B gene that have been identified in a subgroup of patients with aHUS have been associated with a persistent activation of the alternative pathway of complement, further confirming its importance for the pathogenesis of this disease.84
AMD
AMD is the leading cause of irreversible vision loss in Western societies. The connection between this disease and the complement system has been suggested by studies demonstrating that a tyrosine-histidine polymorphism at amino acid 402 of factor H is associated with the development of AMD.88 The retinal lesions of AMD, called drusen, contain deposited components of terminal complement complex, C3a, C5a, and serum amyloid P, suggesting that impaired inhibition of the alternative complement pathway by altered factor H contributes to the development of AMD.88 In addition, recent studies have pointed to a possible role for C3a and C5a in AMD-associated choroidal neovascularization, which is directly responsible for the loss of vision in patients with AMD.33
Concluding Remarks
In recent years, the simplicity of the original description of complement has been replaced by the complexity of modern biology. Although such a transition can be attributed to many biological systems, it is particularly fitting in the case of complement, which has advanced from a role as an adjunct to the humoral response to being the central player in innate immunity. In addition, several recent studies have pointed to an important role for complement components in adaptive immune responses. Therefore, it is not surprising that there is an increased interest in the exploration of complement biology, especially in the context of the involvement of complement in the development and progression of numerous diseases. However, the role of complement in various pathologies is not straightforward. For example, common sense would dictate that complement activation is harmful in many clinical conditions, because it leads to inflammation-mediated tissue injury. However, several observations have indicated that the opposite is true as well. Inherited or acquired deficiencies in components of the classical pathway of complement activation are strongly associated with an increased risk of developing autoimmunity. These complex associations between complement and pathophysiology raise essential questions regarding therapeutic approaches to patients with inflammatory disorders. It seems that “Hamlet’s question”—to inhibit or not to inhibit complement—becomes more valid for current therapy that it was in the past. If the answer to this question is yes, then our previously acquired knowledge of the complement system supports the design of therapeutics that could specifically inhibit complement components at various activation steps, block reciprocal complement receptors, or prevent the generation of complement effectors.
Acknowledgments
We thank Dr. Robert A. DeAngelis, Dr. Daniel Ricklin, and Dr. Michael Schuster for critical review of our manuscript and for their invaluable comments and suggestions. We also thank Dr. Deborah McClellan for excellent editorial assistance. We thank Benjamin E. Weston for assistance with drawing Figures 2 and 3.
Footnotes
Address reprint requests to John D. Lambris, 422 Curie Blvd., 401 Stellar-Chance Bldg., Philadelphia, PA 19104. E-mail: lambris@mail.med.upenn.edu.
Supported by National Institutes of Health grants AI-30040, GM-55698, GM-62134, EB-003968, DK-059422, and CA-112162 (to J.D.L.).
References
- Bordet J, Gengou O. Sur l’existences de substance sensibilisatrices dans la plupart des serum antimicrobiens. French. Ann Inst Pasteur. 1901;15:289–302. [Google Scholar]
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Mastellos D, Lambris JD. Cross-disciplinary research stirs new challenges into the study of the structure, function and systems biology of complement. Lambris JD, editor. New York: Springer Science and Business Media, LLC,; Current Topics in Complement. 2006:pp 1–16. doi: 10.1007/0-387-34134-X_1. [DOI] [PubMed] [Google Scholar]
- Mastellos D, Morikis D, Isaacs SN, Holland MC, Strey CW, Lambris JD. Complement: structure, functions, evolution, and viral molecular mimicry. Immunol Res. 2003;27:367–385. doi: 10.1385/IR:27:2-3:367. [DOI] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Collins T. Acute and chronic inflammation. Cotran RS, Kumar V, Collins T, editors. Philadelphia: W.B. Saunders,; Pathologic Basis of Disease. 1999:pp 50–88. [Google Scholar]
- Janeway CA Jr, Travers P, Walport M, Shlomchik MJ, editors. New York: Garland Publishing,; Innate immunity. Immunobiology. 2005:pp 37–100. [Google Scholar]
- Kohl J. Self, non-self, and danger: a complementary view. Lambris JD, editor. New York: Springer Science and Business Media, LLC,; Current Topics in Complement. 2006:pp 71–94. [Google Scholar]
- Domínguez M, Moreno I, Aizpurua C, Torano A. Early mechanisms of Leishmania infection in human blood. Microbes Infect. 2003;5:507–513. doi: 10.1016/s1286-4579(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement: first of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Pasupuleti M, Walse B, Nordahl EA, Morgelin M, Malmsten M, Schmidtchen A. Preservation of antimicrobial properties of complement peptide C3a, from invertebrates to humans. J Biol Chem. 2007;282:2520–2528. doi: 10.1074/jbc.M607848200. [DOI] [PubMed] [Google Scholar]
- Matthews KW, Mueller-Ortiz SL, Wetsel RA. Carboxypeptidase N: a pleiotropic regulator of inflammation. Mol Immunol. 2004;40:785–793. doi: 10.1016/j.molimm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Vogt W, Damerau B, Luhmann B, Hesse D, Haller Y. Complement activation in human lymph: modulation by the contact activation system and by leukocytes. Int Arch Allergy Appl Immunol. 1986;79:423–433. doi: 10.1159/000234013. [DOI] [PubMed] [Google Scholar]
- Wiggins RC, Giclas PC, Henson PM. Chemotactic activity generated from the fifth component of complement by plasma kallikrein of the rabbit. J Exp Med. 1981;153:1391–1404. doi: 10.1084/jem.153.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Prohászka Z, Singh M, Nagy K, Kiss E, Lakos G, Duba J, Fust G. Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones. 2002;7:17–22. doi: 10.1379/1466-1268(2002)007<0017:hspiap>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- Clancy RM, Dahinden CA, Hugli TE. Complement-mediated arachidonate metabolism. Prog Biochem Pharmacol. 1985;20:120–131. [PubMed] [Google Scholar]
- Schindler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Blood. 1990;76:1631–1638. [PubMed] [Google Scholar]
- Takabayashi T, Vannier E, Clark BD, Margolis NH, Dinarello CA, Burke JF, Gelfand JA. A new biologic role for C3a and C3a desArg: regulation of TNF-α and IL-1β synthesis. J Immunol. 1996;156:3455–3460. [PubMed] [Google Scholar]
- Fischer WH, Jagels MA, Hugli TE. Regulation of IL-6 synthesis in human peripheral blood mononuclear cells by C3a and C3a(desArg). J Immunol. 1999;162:453–459. [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Beutler B. TNF, immunity and inflammatory disease: lessons of the past decade. J Investig Med. 1995;43:227–235. [PubMed] [Google Scholar]
- Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Burg M, Martin U, Rheinheimer C, Kohl J, Bautsch W, Bottger EC, Klos A. IFN-γ up-regulates the human C5a receptor (CD88) in myeloblastic U937 cells and related cell lines. J Immunol. 1995;155:4419–4426. [PubMed] [Google Scholar]
- Mäck C, Jungermann K, Gotze O, Schieferdecker HL. Anaphylatoxin C5a actions in rat liver: synergistic enhancement by C5a of lipopolysaccharide-dependent α(2)-macroglobulin gene expression in hepatocytes via IL-6 release from Kupffer cells. J Immunol. 2001;167:3972–3979. doi: 10.4049/jimmunol.167.7.3972. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Bernacki KD, Reuben JS, Laudes IJ, Neff TA, Gao H, Speyer C, Sarma VJ, Zetoune FS, Ward PA. Regulation by C5a of neutrophil activation during sepsis. Immunity. 2003;19:193–202. doi: 10.1016/s1074-7613(03)00206-1. [DOI] [PubMed] [Google Scholar]
- Bacon PA. Endothelial cell dysfunction in systemic vasculitis: new developments and therapeutic prospects. Curr Opin Rheumatol. 2005;17:49–55. doi: 10.1097/01.bor.0000149084.16639.b0. [DOI] [PubMed] [Google Scholar]
- Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA. C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol. 2004;164:849–859. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke-Gaffney A, Blease K, Hartnell A, Hellewell PG. TNF-α potentiates C5a-stimulated eosinophil adhesion to human bronchial epithelial cells: a role for α5β1 integrin. J Immunol. 2002;168:1380–1388. doi: 10.4049/jimmunol.168.3.1380. [DOI] [PubMed] [Google Scholar]
- DiScipio RG, Daffern PJ, Jagels MA, Broide DH, Sriramarao P. A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transendothelial migration in vitro and in vivo. J Immunol. 1999;162:1127–1136. [PubMed] [Google Scholar]
- Laudes IJ, Chu JC, Huber-Lang M, Guo RF, Riedemann NC, Sarma JV, Mahdi F, Murphy HS, Speyer C, Lu KT, Lambris JD, Zetoune FS, Ward PA. Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol. 2002;169:5962–5970. doi: 10.4049/jimmunol.169.10.5962. [DOI] [PubMed] [Google Scholar]
- Venkatesha RT, Berla TE, Zaidi AK, Ali H. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol. 2005;42:581–587. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Heires AJ, Sanderson SD, Floreani AA. Protein kinase C activation is required for cigarette smoke-enhanced C5a-mediated release of interleukin-8 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;21:283–288. doi: 10.1165/ajrcmb.21.2.3636. [DOI] [PubMed] [Google Scholar]
- Hsu MH, Wang M, Browning DD, Mukaida N, Ye RD. NF-κB activation is required for C5a-induced interleukin-8 gene expression in mononuclear cells. Blood. 1999;93:3241–3249. [PubMed] [Google Scholar]
- Walport MJ. Complement: second of two parts. N Engl J Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- Sjöholm AG, Jonsson G, Braconier JH, Sturfelt G, Truedsson L. Complement deficiency and disease: an update. Mol Immunol. 2006;43:78–85. doi: 10.1016/j.molimm.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- Hart ML, Walsh MC, Stahl GL. Initiation of complement activation following oxidative stress: in vitro and in vivo observations. Mol Immunol. 2004;41:165–171. doi: 10.1016/j.molimm.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104:1413–1418. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- Zhao H, Montalto MC, Pfeiffer KJ, Hao L, Stahl GL. Murine model of gastrointestinal ischemia associated with complement-dependent injury. J Appl Physiol. 2002;93:338–345. doi: 10.1152/japplphysiol.00159.2002. [DOI] [PubMed] [Google Scholar]
- Kyriakides C, Austen W, Jr, Wang Y, Favuzza J, Kobzik L, Moore FD, Jr, Hechtman HB. Skeletal muscle reperfusion injury is mediated by neutrophils and the complement membrane attack complex. Am J Physiol. 1999;277:C1263–C1268. doi: 10.1152/ajpcell.1999.277.6.C1263. [DOI] [PubMed] [Google Scholar]
- Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170:1517–1523. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- Spain DA, Fruchterman TM, Matheson PJ, Wilson MA, Martin AW, Garrison RN. Complement activation mediates intestinal injury after resuscitation from hemorrhagic shock. J Trauma. 1999;46:224–233. doi: 10.1097/00005373-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- Mulligan MS, Schmid E, Beck-Schimmer B, Till GO, Friedl HP, Brauer RB, Hugli TE, Miyasaka M, Warner RL, Johnson KJ, Ward PA. Requirement and role of C5a in acute lung inflammatory injury in rats. J Clin Invest. 1996;98:503–512. doi: 10.1172/JCI118818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105:1363–1371. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, Jensenius JC, Ezekowitz RA, Moore FD, Carroll MC. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177:4727–4734. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- Collard CD, Lekowski R, Jordan JE, Agah A, Stahl GL. Complement activation following oxidative stress. Mol Immunol. 1999;36:941–948. doi: 10.1016/s0161-5890(99)00116-9. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A, Halperin JA. Membrane signaling by complement C5b-9, the membrane attack complex. Immunol Res. 1993;12:244–257. doi: 10.1007/BF02918256. [DOI] [PubMed] [Google Scholar]
- Sims PJ, Wiedmer T. The response of human platelets to activated components of the complement system. Immunol Today. 1991;12:338–342. doi: 10.1016/0167-5699(91)90012-I. [DOI] [PubMed] [Google Scholar]
- Dreyer WJ, Michael LH, West MS, Smith CW, Rothlein R, Rossen RD, Anderson DC, Entman ML. Neutrophil accumulation in ischemic canine myocardium: insights into time course, distribution, and mechanism of localization during early reperfusion. Circulation. 1991;84:400–411. doi: 10.1161/01.cir.84.1.400. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–409. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–456. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- Davies EJ, Snowden N, Hillarby MC, Carthy D, Grennan DM, Thomson W, Ollier WE. Mannose-binding protein gene polymorphism in systemic lupus erythematosus. Arthritis Rheum. 1995;38:110–114. doi: 10.1002/art.1780380117. [DOI] [PubMed] [Google Scholar]
- Koide M, Shirahama S, Tokura Y, Takigawa M, Hayakawa M, Furukawa F. Lupus erythematosus associated with C1 inhibitor deficiency. J Dermatol. 2002;29:503–507. doi: 10.1111/j.1346-8138.2002.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Walport MJ, Davies KA, Botto M, Naughton MA, Isenberg DA, Biasi D, Powell RJ, Cheung NT, Struthers GR. C3 nephritic factor and SLE: report of four cases and review of the literature. Q J Med. 1994;87:609–615. [PubMed] [Google Scholar]
- Mitchell DA, Pickering MC, Warren J, Fossati-Jimack L, Cortes-Hernandez J, Cook HT, Botto M, Walport MJ. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J Immunol. 2002;168:2538–2543. doi: 10.4049/jimmunol.168.5.2538. [DOI] [PubMed] [Google Scholar]
- Chen Z, Koralov SB, Kelsoe G. Complement C4 inhibits systemic autoimmunity through a mechanism independent of complement receptors CR1 and CR2. J Exp Med. 2000;192:1339–1352. doi: 10.1084/jem.192.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einav S, Pozdnyakova OO, Ma M, Carroll MC. Complement C4 is protective for lupus disease independent of C3. J Immunol. 2002;168:1036–1041. doi: 10.4049/jimmunol.168.3.1036. [DOI] [PubMed] [Google Scholar]
- Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- Hong K, Takata Y, Sayama K, Kozono H, Takeda J, Nakano Y, Kinoshita T, Inoue K. Inhibition of immune precipitation by complement. J Immunol. 1984;133:1464–1470. [PubMed] [Google Scholar]
- Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, Gessner JE. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116:512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Chen W, Frank ME, Jin W, Wahl SM. TGF-β released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- Prodeus AP, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot EM, Goodnow CC, Carroll MC. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721–731. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- Chen A, Gaddipati S, Hong Y, Volkman DJ, Peerschke EI, Ghebrehiwet B. Human T cells express specific binding sites for C1q. Role in T cell activation and proliferation. J Immunol. 1994;153:1430–1440. [PubMed] [Google Scholar]
- Trendelenburg M. Antibodies against C1q in patients with systemic lupus erythematosus. Springer Semin Immunopathol. 2005;27:276–285. doi: 10.1007/s00281-005-0007-y. [DOI] [PubMed] [Google Scholar]
- Rougier N, Kazatchkine MD, Rougier JP, Fremeaux-Bacchi V, Blouin J, Deschenes G, Soto B, Baudouin V, Pautard B, Proesmans W, Weiss E, Weiss L. Human complement factor H deficiency associated with hemolytic uremic syndrome. J Am Soc Nephrol. 1998;9:2318–2326. doi: 10.1681/ASN.V9122318. [DOI] [PubMed] [Google Scholar]
- Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi G, International Registry of Recurrent and Familial HUS/TTP Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, Garrido CA, López-Trascasa M, Sánchez-Corral P, Morgan BP, Ródriguez de Córdoba S. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2007;104:240–245. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokiranta TS, Jaakola VP, Lehtinen MJ, Parepalo M, Meri S, Goldman A. Structure of complement factor H carboxyl-terminus reveals molecular basis of atypical haemolytic uremic syndrome. EMBO J. 2006;25:1784–1794. doi: 10.1038/sj.emboj.7601052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelian T, Hellwage J, Meri S, Caprioli J, Noris M, Heinen S, Jozsi M, Neumann HP, Remuzzi G, Zipfel PF. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003;111:1181–1190. doi: 10.1172/JCI16651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Fremeaux-Bacchi V. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:555–563. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]