Abstract
Parkinsonism-dementia complex (PDC) remains a significant health burden to the Chamorro population. We tested the hypothesis that quantitative proteomics might provide fresh insight into this enigmatic illness by analyzing proteins resistant to surfactant extraction from patients with Alzheimer’s disease (AD) or PDC and their matched controls using isobaric tags for relative and absolute quantification. In addition to the expected increase in abnormal frontal cortical Aβ peptides, tau, ubiquitin, and apolipoprotein E in AD, and tau in PDC, we identified α-synuclein (SNCA) as a major abnormal protein in PDC but not AD. We confirmed our isobaric tags for relative and absolute quantification findings by enzyme-linked immunosorbent assay in frontal and temporal cortices. We extended our assays to include a limited number of cases of progressive supranuclear palsy (PSP) and dementia with Lewy bodies; we observed increased abnormal tau but not SNCA in PSP, and abnormal SNCA in dementia with Lewy bodies that was quantitatively similar to PDC. Finally, soluble Aβ oligomers were selectively increased in AD but not PDC or PSP. These results show that frontal and temporal cortex in PDC is distinguished from AD and PSP by its accumulation of abnormal SNCA and suggest that PDC be considered a synucleinopathy as well as a tauopathy.
In 1945, Dr. Harry Zimmerman.1 reported a neurodegenerative disease among the indigenous people of Guam, the Chamorro, that was virtually identical to amyotrophic lateral sclerosis (ALS) seen elsewhere in the world but was 50 to 100 times more prevalent than in the continental United States.1,2 Subsequent studies identified another often co-morbid neurodegenerative disease that was characterized by an akinetic-rigid syndrome with a progressive dementia that is now recognized as very similar to Alzheimer’s disease (AD)3,4; this was termed parkinsonism-dementia complex (PDC).3,4 By the mid-1960s, Chamorros had a lifetime risk of ∼20% for developing ALS, PDC, or some combination.2,5,6,7,8,9 Strikingly, the incidence of Guam ALS has been steadily decreasing throughout the last several decades to a level that now matches the incidence of ALS in the rest of world.10,11,12,13 In contrast, the same reports that showed profound reductions in the incidence of ALS observed less dramatic and sometimes no reduction in the incidence of PDC on Guam10,11,12,13 and an increase in the average age of onset by ∼10 years.12,14,15 Indeed, the most recent studies that used registry data as well as an extensive village public health network on Guam show that, unlike ALS, the incidence of PDC declined only slightly throughout the period of 1940 to 2000.16 Several environmental initiators, promoters, or suppressors have been proposed to explain these demographic changes (reviewed by Wiederholt12); however, none has adequately explained the shifting demographics and some,17,18,19,20,21 despite elegant anthropological and ethnological underpinning, have not withstood scrutiny.13,16,22,23
PDC is classified histopathologically as a tauopathy, a group of neurodegenerative diseases that share in common abnormal tau-immunoreactive structures in different brain regions. AD is the most common tauopathy, although there are several others, including PDC and a disease that shares several features with PDC, progressive supranuclear palsy (PSP).24 One group identified a polymorphism in the tau gene (MAPT) that seems to be a weak susceptibility factor for PDC but concluded that some other genetic or environmental factor was necessary to account for the majority of familial clustering of PDC.25 Recently, this same group identified single nucleotide polymorphism sites that modulate the risk for PDC.26 Moreover, a recent genome-wide analysis of patients with PDC failed to identify a single locus associated with this disease and again raised the possibility of gene environment or solely environmental factors to explain PDC clustering.27 Thus, discovery tools other than genomic methods may be especially useful in gaining insight into the pathogenesis of PDC.
Classic biochemical approaches in the 1980s and early 1990s established that increased accumulation of abnormally hydrophobic protein, defined by their insolubility when extracted with surfactants (often referred to as detergents) such as Triton X-100 (Triton-insoluble or TI) or N-lauroylsarcosine (sarkosyl-insoluble or SI), as a characteristic feature of several neurodegenerative diseases. Indeed, the proteins resistant to surfactant extraction were identified as the major components of hallmark lesions in AD: amyloid β (Aβ) peptides in senile plaques and abnormally phosphorylated tau in neurofibrillary tangles (NFTs).28,29,30,31 Subsequent genetic studies based on these discovered abnormal proteins ushered in the molecular era of investigation into neurodegenerative diseases.32 Given the apparent limitations of genomic screens to gain insight into the pathogenesis of PDC, here we pursued quantitative proteomics to discover those proteins resistant to surfactant extraction in PDC.
Materials and Methods
Patients
Tissue from frontal cortex and temporal cortex was investigated from six different groups of individuals from two different parts of the world (Table 1). One set of tissue was from patients and controls in the Seattle area; all were Caucasian and had been followed clinically by the Alzheimer’s Disease Research Center at the University of Washington. All patients from Seattle (AD, PSP, or dementia with Lewy bodies or DLB) had clinical and neuropathological diagnoses made according to established consensus criteria33,34,35; patients with AD were selected to be free of coexisting Lewy body disease or vascular damage, whereas all patients with DLB had coexisting AD. AD cases with long postmortem interval (PMI) were selected to match PMI for individuals from Guam. All controls had been seen by the center physicians within 2 years of death and had normal neurological examinations, and all psychometric tests were within normal ranges. The second set of tissue was from controls and patients with PDC from Guam; all were Chamorro and had been followed clinically by the National Institutes of Health-funded research consortium and were diagnosed either as having PDC or as neurologically normal.
Table 1.
Characteristics and Neuropathological Data of People Whose Tissue Was Used in This Study
| CS | AD | AD-LPMI | DLB | PSP | CG | PDC | |
|---|---|---|---|---|---|---|---|
| n | 6 | 7 | 3 | 5 | 3 | 6 | 7 |
| Ethnicity | All Caucasian from Seattle Area | All Chamorro from Guam | |||||
| Women (n) | 4 | 6 | 1 | 2 | 1 | 2 | 4 |
| Age (years) | 70 ± 15 | 84 ± 9 | 83 ± 6 | 86 ± 8 | 72 ± 10 | 66 ± 16 | 77 ± 10 |
| PMI (minutes)* | 421 ± 186 | 254 ± 72 | 2160 ± 720 | 285 ± 91 | 353 ± 70 | 1589 ± 624 | 1539 ± 797 |
| CERAD neuritic plaque score | None (none to sparse) | Frequent (moderate to frequent) | Frequent (moderate to frequent) | Frequent (moderate to frequent) | None | None (none to sparse) | None (none to sparse) |
| Braak stage | I (0 to III) | VI (V or VI) | VI (V or VI) | V (V or VI) | NA | 0 (0 to III) | NA† |
| Neocortical LBs or Lewy neurites | None | None | None | Five of five | None | None | None‡ |
Continuous data are presented as mean ± SD, whereas discontinuous data are presented as mode (range).
P < 0.0001 by analysis of variance for all six groups.
Although Braak staging is not applicable (NA) to PDC, entorhinal cortex from all PDCs but no CGs had stage 3 or 4 NFT density (>20 NFTs/mm2), whereas frontal cortex from all PDCs had stage 1 or 2 NFT density (1 to 20 NFTs/mm2) and no CGs had any neocortical NFTs.37
Two patients with PDC had Lewy bodies in the amygdala. Bonferroni-corrected post tests showed that all Seattle area groups had significantly shorter PMIs than groups from Guam (P < 0.01), except for AD-LPMI, whose PMI was not significantly different from CG or PDC.
Neuropathological Evaluation
Braak system for staging NFT pathology in AD,36 regional NFT density for characterizing PDC,37 and CERAD plaque score38 were accomplished with modified Bielschowsky-stained sections. All 37 cases underwent assessment for Lewy bodies and Lewy neurites in a single batch using SNCA immunohistochemistry (IHC) with antibody LB509 (1:50 to 1:400; Zymed, South San Francisco, CA).39 IHC for SNCA was performed on 10-μm sections that were pretreated with either 88% formic acid for 5 minutes or protease K for 1 minute, exposed to 3% hydrogen peroxide, blocked in 5% milk, incubated with primary antibody for 1 hour at room temperature, and then detected with avidin-biotin complex using diaminobenzidine as chromogen substrate.40 The positive control for each IHC run was a case of DLB. Negative control for each was elimination of primary antibody.
Tissue Preparation and Enzyme-Linked Immunosorbent Assay (ELISA)
Tissue was homogenized and sequentially extracted in buffer A [10 mmol/L Tris, 1 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1 mmol/L dithiothreitol, and 10% sucrose, pH 7.5], buffer B (buffer A + 1% Triton X-100), and buffer C (buffer A + 1% N-lauroylsarcosine). Phosphatase inhibitors (20 mmol/L NaF and 1 mmol/L sodium orthovanadate) and protease inhibitor cocktail (no. P2714; Sigma Chemical Co., St. Louis, MO) were added to buffers A, B, and C immediately before use. Surfactant-insoluble material insoluble in buffer B (Triton-insoluble or TI) or buffer C (sarkosyl-insoluble or SI) was extracted with 70% formic acid as previously described.41 Formic acid extracts of surfactant-insoluble proteins were dried by vacuum centrifugation and resolubilized by sonication in 20 vol of 5 mol/L guanidine HCl, 100 mmol/L Tris, pH 7.4, with 0.002% bromphenol blue added to confirm elimination of formic acid. These were further diluted 64-fold in 100 mmol/L Tris, pH 7.4, with 0.05% azide and 0.002% bromphenol blue, and 100 μl (representing 7.8 μg of starting brain tissue) were spotted onto 96-well plates and then incubated overnight at room temperature in a humidified chamber. Plates were subsequently washed twice with phosphate-buffered saline (PBS), blocked with 1% bovine serum albumin in PBS with 0.05% sodium azide, and washed again with PBS. ELISAs were performed as described by others and developed using tetramethylbenzidine with absorbances determined at 405 nm.42 Primary antibodies were directed against Aβ40 and Aβ42 (carboxy-terminal-specific antibodies; Signet Laboratories, Dedham, MA), tau, apoE, ubiquitin (all from DAKO, Carpinteria, CA), and SCNA (from Chemicon, Norcross, GA; or LabVision Neomarkers, Fremont, CA). Antibodies specific to tau phosphorylated at Ser199 and Ser396 were purchased from Invitrogen (Carlsbad, CA). Antibody specific to PHF tau phosphorylated at Ser212/Thr214 (AT100) was obtained from Pierce (Rockford, IL). Antibody specificity was confirmed by Western blot of brain extracts before use in capture assays. Secondary antibody/alkaline phosphatase conjugates were from Amersham (Piscataway, NJ). All antibodies were diluted 2000 times from the initial stock concentration before use in the detection assay.
Luminex for Soluble Aβ Oligomers
Luminex assays for soluble Aβ oligomers were performed on material solubilized by buffer A and buffer B using reagents from Biosource (Camarillo, CA) exactly according to the manufacturer’s instructions. This paired fluorescent bead-antibody conjugate assay uses a bead-A11 antibody conjugate that detects oligomers, but not monomers or fibrils, of several proteins that form amyloid including Aβ species,43 paired with a second bead-antibody conjugate that detects an epitope within the first 20 amino acids of the Aβ peptide. Standards were provided by the manufacturer, and standard curves were generated throughout 30 to 2000 pg/ml Aβ42 oligomers with a detection limit of 30 pg/ml.
Isobaric Tags for Relative and Absolute Quantification (iTRAQ) Labeling and Two-Dimensional Liquid Chromatography
SI protein was solubilized by sonication in 8 mol/L urea with 500 mmol/L triethylamine bicarbonate, pH 8.5, and equal amounts of SI protein was pooled for each group to yield 100 μg of SI protein from patients with PDC, AD, controls from Guam (CG), and controls from Seattle (CS); these were digested in parallel with trypsin and then labeled with one of the four-iTRAQ reagents following the manufacturer’s instructions. The four iTRAQ-labeled samples were combined (a total of 400 μg of proteins) and loaded onto a strong cation exchange column (0.5 × 200 mm) that had been equilibrated in 0.05% formic acid/20% acetonitrile (ACN), and pH 3.0, at a flow rate of 200 μl/minute. Peptides were eluted by applying a linear gradient from 0 to 100% of 500 mmol/L ammonium formiate/20% ACN, pH 3.0. Eleven fractions were collected from each sample and dried down in a SpeedVac (Thermo Savant, Holbrook, NY).
Strong cation exchange-fractionated peptides were then dissolved in 0.5% trifluoroacetic acid and separated using reverse phase chromatography. Nano-capillary liquid chromatography (LC) was performed using the UltiMate with Famos autosampler and Switchos automated switching valve (LC Packings, Sunnyvale, CA). Samples were loaded onto a capillary precolumn cartridge (Dionex, Sunnyvale, CA). The trap column was washed with mobile phase A containing 2% ACN and 0.1% trifluoroacetic acid in high-performance liquid chromatography water. The flow rate was set at 0.4 μl/minute. The sample was then loaded onto a 15 cm × 100-μm ID Magic C18 (3 μm), 100-angstrom packing capillary liquid chromatography column (Michrome BioResources Inc., Auburn, CA). The gradient run was from 5 to 90% mobile phase B (80% ACN, 20% high-performance liquid chromatography water, and 0.08% trifluoroacetic acid) for 85 minutes. The eluate was mixed with 7 mg/ml of recrystallized α-cyano-4-hydroxycinnamic acid (Sigma) in 60% ACN, 2.6% (5 mg/ml) ammonium citrate with internal standard (4700 mass standard kit; Applied Biosystems, Foster City, CA), and spotted onto a stainless steel matrix-assisted laser desorption ionization plate with the Probot (LC Packings). Samples were spotted at 5-second intervals using a 24 × 24 array pattern for a total of 576 spots per plate. In total, 11 liquid chromatography matrix-assisted laser desorption ionization plates were spotted and analyzed by a 4700 proteomic system (see below; Applied Biosystems).
Tandem Mass Spectrometry Analysis and Protein Identification
Quantitative mass spectrometry (MS) analysis was performed using the 4700 proteomics analyzer with time-of-flight (TOF)/TOF optics (Applied Biosystems). MS reflector-positive ion mode with automated acquisition of 800 to 4000 m/z range was used with 1000 shots per spectrum. A maximum of 15 peaks was selected per spot, with a minimum signal-noise (S/N) ratio of 75 and cluster area of 500. Greater than 36,000 precursors were selected and were submitted for tandem mass spectrometry in positive ion mode with CID cell on and 1 kV collision energy; 3000 shots were accumulated per spectrum. For each spotted plate, a total of 576 MS and more than 1200 tandem mass spectrometry spectra were acquired. Identification of proteins was achieved using Mascot (Matrix Science, Boston, MA) algorithm and searched against the International Protein Index Version 3.18 (http://www.ebi.ac.uk/IPI). Criteria for all protein identification were at least one peptide with individual composite score greater than the 95% confidence interval threshold and also the top-ranked matching sequence for that spectrum. For proteins identified by a single peptide, two additional criteria were applied: the Mascot ion score greater than 30 and all peptides had to contain tryptic digestion end. These criteria were applied because it has been estimated by several groups of investigators that the false-positive rate is typically less than 1% for protein identification when these criteria are used.44,45 Protein quantification was achieved by averaging ratios of all peptides of each identified protein. Normalization, which assumed a Gaussian distribution with median of 1 for all peptides among control and experimental groups, was performed before ratios were calculated.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism (San Diego, CA).
Results
Table 1 lists the characteristics and neuropathological data of individuals whose donated tissue was used in these studies. Because of the ethnic differences between individuals from the Seattle area and Guam, we included control groups from each site. The other significant difference between randomly selected tissue from the Seattle area and Guam was PMI (P < 0.0001), a reflection of the different logistical constraints on rapidly obtaining postmortem tissue in these two locations. In an effort to control for this systematic difference, we deliberately selected three additional AD cases from the Seattle area that had PMIs slightly longer (LPMI) than those from Guam but were not significantly different (P > 0.05). In this way, our AD cases bracketed all other groups’ PMIs either from the Seattle area or Guam. In some experiments, we used tissue from individuals who died of PSP as a comparison group because of its several similarities to PDC; however, only a few cases of PSP with frozen tissue were available for study.
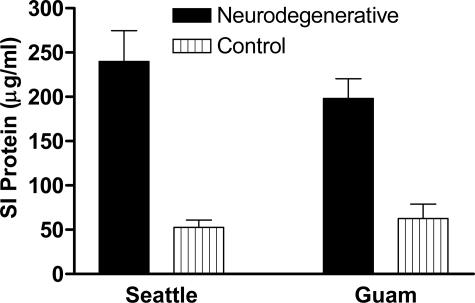
SI protein in PDC was increased similar to AD (Figure 1). We set out to discover the constituent SI proteins in PDC by using a quantitative proteomic technique called iTRAQ that can identify and relatively quantify three groups in comparison to a reference group. We used pooled SI fractions from temporal lobe of AD, PDC, CS, or CG groups, labeled these with iTRAQ reagents, and analyzed this combined sample by matrix-assisted laser desorption/ionization time-of-flight time-of-flight. We identified 106 SI proteins in all four groups (Table 2). We compared these results with a previous experiment in which we analyzed the SI protein fraction from a different group of AD patients analyzed by liquid chromatography-electrospray ionization-MS-MS in which we identified 125 proteins by Sequest with two or more unique peptides and confirmed 15 by Western blot.41 In the present study, we used a similar protocol to prepare SI protein fractions but analyzed these by an entirely different mass spectrometric approach, searching program, and database. Of these 106 proteins, 47 (44%) also were identified in our previous study, including 14 of those 15 validated by Western blot in our previous study.
Figure 1.
Data are average ± SEM protein. SI protein concentration determined by BCA assay. Neurodegenerative group for Seattle is AD and for Guam is PDC. One-way analysis of variance had P < 0.0001. Bonferroni-corrected repeated pair comparisons had P < 0.01 for PDC versus CG and P < 0.001 for AD versus CS. Two-way analysis of variance had P < 0.0001 for neurodegenerative disease versus control but not for location; there was no significant interaction between these two terms. Bonferroni-corrected post tests had P < 0.01 for both Seattle and Guam groups, but P > 0.05 for AD versus PDC or CS versus CG.
Table 2.
Temporal Cortex SI Proteins Identified in iTRAQ Experiment
| Protein name | Peptide count | Total ion score C.I. % | Previously ID’ed as SI in AD41 |
|---|---|---|---|
| 14-3-3 protein sigma* | 1 | 99.89 | + |
| 17-kd protein | 1 | 95.24 | |
| 2′,3′-Cyclic-nucleotide 3′-phosphodiesterase, isoform CNPII | 3 | 100.00 | + |
| 20-kd protein | 1 | 100.00 | |
| 33-kd protein | 1 | 96.70 | |
| 365-kd protein | 5 | 100.00 | |
| 40S ribosomal protein S30 | 1 | 100.00 | |
| 5′-3′ exoribonuclease 2 | 1 | 98.67 | |
| 60S ribosomal protein L7a | 1 | 100.00 | |
| 62-kd protein | 1 | 99.88 | |
| Actin, cytoplasmic 2* | 5 | 100.00 | + |
| ADP/ATP translocase 1 | 3 | 100.00 | |
| ADP/ATP translocase 2 | 3 | 100.00 | |
| Aldehyde dehydrogenase, mitochondrial | 1 | 97.69 | |
| α Crystallin B chain | 2 | 100.00 | + |
| α-Synuclein, isoform 2-4 | 1 | 99.85 | |
| Amyloid β A4* | 1 | 100.00 | + |
| Apolipoprotein D | 2 | 100.00 | + |
| Apolipoprotein E* | 8 | 100.00 | + |
| ATP synthase e chain, mitochondrial | 1 | 96.68 | |
| ATP synthase, H+ transporting, mitochondrial F0 complex, subunit F2 isoform 2a | 1 | 99.10 | |
| Brain acid-soluble protein 1 | 1 | 100.00 | |
| Calcium/calmodulin-dependent protein kinase type II α chain | 6 | 100.00 | + |
| Carbonyl reductase 1 | 1 | 96.81 | + |
| Cathepsin D | 4 | 100.00 | + |
| CDNA FLJ44161 fis, clone THYMU2033070 | 1 | 97.34 | |
| Clusterin isoform 1 | 1 | 99.86 | |
| Cofilin-2, isoform CFL2b | 1 | 99.53 | + |
| Collagen α-1(I) chain | 5 | 100.00 | |
| Collagen α-1(III) chain | 1 | 99.98 | |
| Collagen α-2 type IV* | 4 | 100.00 | + |
| Collagen α-2(I) chain | 4 | 100.00 | + |
| Collagen, type XXII, α-1 | 1 | 99.30 | |
| Contactin-1, isoform 2 | 1 | 99.37 | |
| Creatine kinase B-type | 3 | 100.00 | + |
| Creatine kinase, ubiquitous mitochondrial | 4 | 100.00 | + |
| Cytochrome c oxidase polypeptide Vb | 1 | 99.98 | + |
| Cytochrome c oxidase polypeptide VIc | 1 | 99.81 | + |
| Cytochrome c oxidase polypeptide VIIa | 1 | 99.92 | + |
| Cytochrome c oxidase subunit 4 isoform 1 | 3 | 100.00 | + |
| Desmuslin, isoform 2 | 1 | 98.71 | |
| Disheveled-associated activator of morphogenesis 2 | 1 | 97.80 | |
| F-box-like/WD-repeat protein TBL1XR1 | 1 | 96.01 | |
| Ferritin heavy chain | 3 | 100.00 | + |
| Fibrinogen β chain | 1 | 98.02 | + |
| FSCN1 protein | 1 | 99.98 | |
| FTL protein | 1 | 98.91 | |
| Glial fibrillary acidic protein* | 3 | 100.00 | + |
| Glutamate transporter variant EAAT1ex9skip | 1 | 96.16 | |
| Glyceraldehyde-3-phosphate dehydrogenase* | 2 | 100.00 | + |
| Guanine nucleotide-binding protein α-12 subunit | 1 | 100.00 | + |
| Guanine nucleotide-binding protein G(o), α subunit 2 | 2 | 100.00 | |
| HANP1 | 1 | 96.50 | |
| Heat shock-related 70 kd* | 2 | 100.00 | + |
| Heterogeneous nuclear ribonucleoprotein K, isoform 1 | 1 | 99.56 | |
| Heterogeneous nuclear ribonucleoprotein Q, isoform 4 | 1 | 98.60 | |
| Heterogeneous nuclear ribonucleoproteins A2/B1, isoform A2 | 3 | 100.00 | |
| Histone H1.0 | 3 | 100.00 | + |
| Histone H1.3 | 2 | 100.00 | + |
| Histone H2A type 3 | 2 | 100.00 | + |
| Histone H2B* | 4 | 100.00 | + |
| Histone H3.4 | 2 | 100.00 | + |
| Histone H4 | 3 | 100.00 | + |
| Tubulointerstitial nephritis antigen-like, isoform 1 | 4 | 100.00 | + |
| Cleavage and polyadenylation specificity factor 6, isoform 6 | 1 | 98.27 | |
| Laminin α-5 chain | 2 | 100.00 | |
| Laminin β-2 chain | 4 | 100.00 | |
| Laminin γ-1 chain | 2 | 100.00 | + |
| Methylcrotonoyl-CoA carboxylase β chain, isoform 1 | 2 | 100.00 | |
| Microtubule-associated protein tau, isoform A* | 4 | 100.00 | + |
| Microtubule-associated protein tau, isoform B* | 4 | 100.00 | + |
| Myelin basic protein* | 4 | 100.00 | + |
| NG22 protein isoform 1 | 1 | 96.03 | |
| Nidogen-2 | 1 | 99.98 | + |
| P2X7 isoform D | 1 | 98.24 | |
| Palmitoyl-protein thioesterase 1 | 1 | 100.00 | + |
| Pleckstrin homology-like domain family A member 3 | 1 | 97.99 | |
| Prion protein | 1 | 100.00 | |
| Prosaposin | 3 | 100.00 | |
| Proteasome β4 subunit | 1 | 100.00 | |
| Proteasome subunit α type 1 | 1 | 100.00 | |
| Proteasome subunit β type 2 | 1 | 99.00 | |
| Proteasome subunit β type 6 | 1 | 99.98 | |
| Protein KIAA0157 | 1 | 98.41 | |
| Proteolipid protein 1 isoform 1 | 1 | 100.00 | + |
| Ras-associated and pleckstrin homology domain-containing | 1 | 98.04 | |
| RNA binding motif protein, X-linked-like 1 | 1 | 98.80 | |
| Serine/threonine-protein kinase 38-like | 1 | 96.39 | |
| Serine/threonine-protein kinase RIO2 | 1 | 98.06 | |
| Similar to elongation factor Tu GTP binding domain | 1 | 99.18 | |
| Splicing factor 3A subunit 2 | 1 | 99.85 | |
| Synapsin-1, isoform IA | 5 | 100.00 | + |
| Syntaxin-binding protein 1, isoform 1 | 1 | 99.98 | + |
| Tripeptidyl-peptidase 1 precursor, isoform 3 | 1 | 99.99 | + |
| Tubulin α-1 chain | 3 | 100.00 | |
| Tubulin α-3 chain | 5 | 100.00 | |
| Tubulin α-ubiquitous chain | 5 | 100.00 | + |
| Tubulin β-2C chain* | 7 | 100.00 | |
| Tubulin, β polypeptide* | 6 | 100.00 | + |
| Tubulin, β, 4* | 5 | 100.00 | + |
| Ubiquitin* | 4 | 100.00 | + |
| Up-regulated in colorectal cancer gene 1 protein | 1 | 96.08 | |
| USP36 protein | 1 | 95.69 | |
| Vitamin K-dependent protein Z precursor | 1 | 96.15 | |
| Wolf-Hirschhorn syndrome candidate 1 protein isoform 4 | 1 | 98.26 | |
| Zinc finger, CW type with coiled-coil domain 2 | 1 | 97.07 |
C.I., confidence interval; CoA, coenzyme A.
SI proteins validated by Western blot in our previous study.41
We used CS as the reference group and computed the log2 transformation of the iTRAQ ratios for AD:CS, PDC:CS, and CG:CS; we calculated the PDC:CG ratio by dividing PDC:CS by CG:CS. We adopted cutoffs of 1.8 and −1.8. Those SI proteins increased above our cutoff for the three ratios are presented in Table 3. AD:CS had increased SI protein ratios for five proteins; this included several expected proteins, among which were tau isoforms A and B, the shortest isoforms of human tau.46 PDC:CG had increased tau (isoform A) and SNCA isoforms 2 to 4, the shortest isoform of SNCA.47,48 Our third comparison was between controls in both ethnic groups; there were no SI proteins increased above our cutoff for CG:CS. There were no proteins uniquely identified in either disease.
Table 3.
SI Protein in Temporal Cortex with Log2 (iTRAQ ratio) ≥1.8
| Groups | Protein | IPI | iTRAQ ratio |
|---|---|---|---|
| AD versus CS | Amyloid β A4 protein | IPI00219183 | 4.5 |
| Apolipoprotein E | IPI00021842 | 9.8 | |
| MAP tau, isoform A | IPI00293683 | 10.1 | |
| MAP tau, isoform B | IPI00220173 | 7.7 | |
| RNA binding motif protein, X-linked-like 1 | IPI00061178 | 4.6 | |
| Ubiquitin | IPI00456429 | 5.6 | |
| PDC versus CG | α-Synuclein | IPI00218467 | 6.8 |
| MAP tau, isoform A | IPI00293683 | 3.4 | |
| CG versus CS | None |
MAP, microtubule-associated protein.
We next sought to validate our iTRAQ findings by ELISA and extend them to a limited number of PSP cases as a means to assess specificity. We performed ELISAs for six of the seven SI proteins that were elevated in AD or PDC by our iTRAQ experiment: Aβ40, Aβ42, tau, apoE, ubiquitin, and SNCA. Antibodies to RNA-binding motif protein, X-linked-like 1 were not available. ELISAs were performed on frontal and temporal cortex for all cases listed in Table 1. Confirming our iTRAQ results, there was no significant difference in any of these ELISA data for the CS and CG groups (not shown); therefore, we collapsed them into a single control group. Moreover, because there was never a significant difference between any ELISA result for AD and LPMI-AD, we combined data from these two groups. Thus, the six groups in Table 1 were combined into four groups in the subsequent analyses.
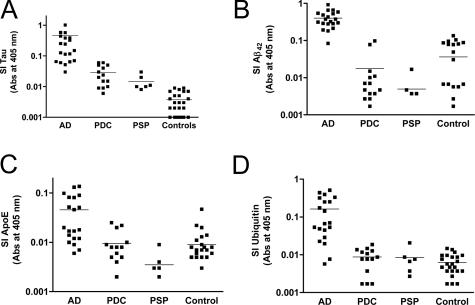
We quantified SI tau by ELISA among these three tauopathies and controls (Figure 2A). Nonparametric one-way analysis of variance for the four groups had P < 0.0001 with P < 0.05 for AD, PDC, or PSP versus control and for AD versus PDC. We also quantified selected phosphorylated tau (tau-P) epitopes in SI protein by ELISA, including tau-P199, tau-P212, tau-P214, and tau-P396. Although each of these followed a pattern similar to total tau, when normalized to the amount of total tau immunoreactivity in the same preparation, none of the four group ANOVAs was statistically significant (P > 0.05). We could not discriminate isoforms of tau by our ELISA so direct quantitative comparison to iTRAQ ratio is limited. Nevertheless, the AD:control for average SI tau by ELISA was 112.5, substantially higher than the iTRAQ ratios for tau isoforms presented in Table 3. In summary, SI tau was significantly increased in AD and PDC, confirming our iTRAQ result. We observed that SI tau was increased in PSP over control but less than in AD. Finally, SI tau in PDC was quantitatively similar to PSP (P > 0.05).
Figure 2.
Antibody capture assays for SI proteins in frontal and temporal cortex. Data are presented as log scatterplots (average marked with line) from patients with the three different tauopathies and controls (combined from Seattle and Guam). All data are from antibody capture assays with absorbance read at 405 nm and were analyzed by one-way nonparametric analysis of variance (Kruskal-Wallis test) followed by Dunn’s corrected multiple comparison test. A: SI tau analysis of variance had P < 0.0001, and Dunn’s tests had P < 0.05 for AD versus PDC and AD versus PSP, and P < 0.01 for AD versus control and PDC versus control. B: SI Aβ42 analysis of variance had P < 0.0001 and Dunn’s tests had P < 0.001 for AD versus PDC, AD versus PSP, and AD versus control, but P > 0.05 for all repeat comparisons among PDC, PSP, and controls. C: SI apoE analysis of variance had P < 0.0001, and Dunn’s tests had P < 0.05 for AD versus PDC, AD versus PSP, and AD versus control, but P > 0.05 for all repeat comparisons among PDC, PSP, and controls. D: SI ubiquitin analysis of variance had P < 0.0001, and Dunn’s tests had P < 0.01 for AD versus PDC, AD versus PSP, and AD versus control, but P > 0.05 for all repeat comparisons among PDC, PSP, and controls.
Next, we performed ELISAs for three other proteins with selectively increased AD:CS iTRAQ ratios: Aβ42, apoE, and ubiquitin (Figure 2, B–D). Nonparametric one-way analysis of variance for these three SI protein ELISAs had P < 0.001 and P < 0.05 for all three paired comparisons with AD but not for any other paired comparison among the four groups for apoE and ubiquitin. The AD:control for average SI Aβ42 and apoE by ELISA were 11.1 and 5.1, values in reasonably close agreement with the corresponding iTRAQ ratios of 4.5 and 9.8. The same average ratio for SI ubiquitin by ELISA was 26.7, a value severalfold higher than the corresponding iTRAQ ratio of 5.6. These ELISA data demonstrated selectively increased amounts of these SI proteins in AD but not PDC and showed that from this perspective, PDC and PSP were similar.
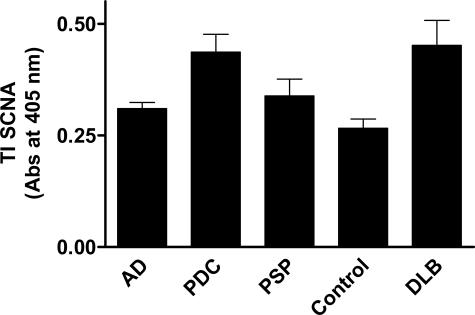
With strong validation of our iTRAQ results, we then turned our focus to the one SI protein selectively elevated in PDC but not AD. Our iTRAQ experiments showed substantially increased SI SNCA in PDC (iTRAQ PDC:CG ratio for SI SNCA = 6.8) but not AD (iTRAQ AD:CS ratio for SI SNCA = 1.2). We sought to confirm this in our SI fractions; however, neither of the two antibodies that we used could detect SNCA by ELISAs of SI protein. All of the SI proteins examined above by ELISA were more abundant in the TI fraction than the SI fraction (not shown), presumably a reflection of varying degrees of protein modification with the less extensively modified proteins (TI fraction) being more abundant than highly modified proteins (SI fraction). Therefore, we examined TI SNCA by ELISA (Figure 3). Increase in TI SNCA in PDC versus CG was 1.4-fold with Chemicon antibody (Figure 3). The ELISA signal using the Neomarkers antibody was on average 52% lower than the Chemicon antibody but still above background for most samples. Using the Neomarkers SNCA antibody, PDC:control for TI SNCA was 1.3, whereas for AD:control TI SNCA was 0.9. These ELISA results confirm the selective accumulation of abnormal hydrophobic forms of SNCA in PDC, but not AD, that was discovered in our iTRAQ experiment. We sought to establish some context for the increased TI SNCA in PDC by comparing against five cases of DLB, a neurodegenerative disease that shares the histopathological hallmarks of AD plus the accumulation of neocortical Lewy bodies (LBs). As shown in Figure 3, neocortical TI SNCA was virtually identical between cases of DLB and PDC despite the lack of LB accumulation in these regions of brain in PDC. It is noteworthy that ELISA for TI tau and Aβ species from DLB cases were not significantly different from AD (not shown).
Figure 3.
Antibody capture assay for TI SNCA in frontal and temporal cortex using Chemicon antibody. Data are average ± SEM for each group. One-way analysis of variance had P < 0.001. Bonferroni-corrected repeat paired comparisons had P < 0.01 for PDC versus control and P < 0.001 for DLB versus control, but P > 0.05 for AD or PSP versus control.
We also observed some SI proteins that were decreased below our lower cutoff of log2 (ratio) ≤−1.8. For AD versus CS, these were syntaxin 1 binding protein isoform 1 (IPI00084828), laminin α-5 (IPI00641693), and tubulin α-3 (IPI00180675). For PDC versus CG, there was a zinc finger protein (CW type with coiled domain 2, IPI00478161). For CG versus CS, there was contactin-1 isoform 2 (IPI00216641), syntaxin-binding protein 1 isoform 1, and tubulin α-1 (IPI00007750). We were skeptical of the significance of these findings because the iTRAQ experiment used the same amount of SI protein from each group; that could mean that some proteins that normally have a subset present in the SI fraction could be displaced by the accumulating pathological SI protein. Nevertheless, we performed Western blots for syntaxin-binding protein 1 isoform 1, and tubulin α and did not confirm a reduction of these proteins in the SI or soluble (buffer A and buffer B) fractions when normalized to the amount of wet weight tissue or normalized to protein (not shown).
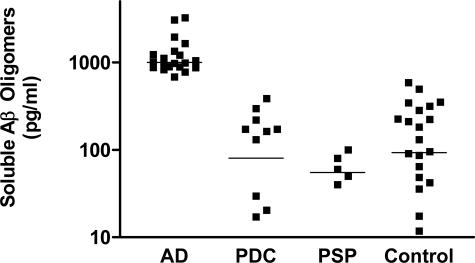
Our results showing increased TI and SI SNCA in PDC frontal and temporal cortex underscored the limitation of histochemical and IHC tools in evaluation of abnormal protein in neurodegenerative diseases. By extension, focus on only those proteins resistant to surfactant extraction also will give a limited perspective on abnormal protein formation in these diseases. Indeed, recent work in AD has highlighted an important role for soluble oligomers of Aβ in neuron dysfunction and perhaps death.49,50 Therefore, we determined tissue concentrations of soluble oligomeric species of Aβ in fractions A and B using Luminex reagents (Figure 4). Our results confirmed those of others by showing an average eightfold increase in soluble Aβ oligomers in cerebral cortex of patients with AD compared with controls50 but did not reveal any increase over controls in soluble Aβ oligomers in PDC or PSP.
Figure 4.
Luminex assay for soluble Aβ oligomers in frontal and temporal cortex. Data are presented as log scatterplot (average marked with line) from patients with three different tauopathies and controls (combined from Seattle and Guam). Note that the ordinate is a log scale and that PDC and AD groups do not overlap and PDC and controls completely overlap. One-way analysis of variance nonparametric analysis of variance (Kruskal-Wallis test) had P < 0.0001 with Dunn’s corrected repeat comparisons having P < 0.001 for AD versus all paired comparisons, but P > 0.05 for all other paired comparisons.
Discussion
In contrast to the precipitous decline in ALS among Chamorros throughout the last 2 decades, PDC remains a significant health burden to the indigenous people of Guam, especially the elderly. Recent studies have highlighted the limitations of genomic approaches to PDC,27 perhaps because major pathogenic factors lie outside of the genome. Given the partial clinical and pathological overlap between PDC and AD, as well as PDC and PSP, and given that accumulation of abnormal protein is thought to be a central element in several other neurodegenerative diseases including AD and PSP, we hypothesized that discovering the ensemble of proteins resistant to surfactant extraction in PDC might provide fresh insight into this enigmatic illness.
It is not clear exactly what underlies the modifications to protein that generate abnormal hydrophobic interactions that are no longer vulnerable to disruption by surfactants. Several hypothesized protein modifications exist, including misfolded forms and abnormal posttranslational modifications. We have shown recently that the repertoire of proteins in AD temporal cortex that is resistant to surfactant extraction contains ∼100 proteins, much larger than previously conceived, at least by us.41 We confirmed this fundamental finding here in completely independent samples and with different proteomic analysis and database searching; we observed ∼45% overlap in the DI proteins identified. Coincident with this original finding, another group reported that allelic variants in the gene that encoded GAPDH, one of the abnormal proteins we identified in both of our proteomic studies, is a risk factor for late-onset AD.51 In combination, these results highlight the potential value of proteomic investigation of proteins resistant to surfactant extraction to gain insight into neurodegenerative diseases.
Here we have applied the same logic to the investigation of PDC, using iTRAQ as a more sophisticated quantitative proteomic approach. We identified and confirmed SNCA as a major SI protein in frontal cortex of PDC at a level comparable with that in DLB. We further confirmed by ELISA many other findings from our iTRAQ experiment. These were expected given previous results from us and others and in many respects served as internal controls for our proteomic discovery approach: increased SI Aβ42, tau, ubiquitin, and apoE in AD and increased SI tau in PDC and PSP. One of the SI proteins identified was an RNA-binding motif protein, a feature shared with TAR-DNA-binding protein 43 (TDP-43), which recently was identified as a major component of ubiquitinated inclusions in some forms of frontotemporal lobar degeneration and ALS52; however, the significance of this shared functional motif is not clear. It is noteworthy that although always agreeing in the direction of change, the magnitude of change in SI proteins was sometimes quite different between iTRAQ and ELISA. There are many reasons for these quantitative discrepancies, which include differences in both the sensitivity and specificity of these two methods.
PDC has been characterized as a triple amyloidosis, meaning formation of brain amyloid from Aβ species, tau and SNCA, based on histopathological and IHC findings.53 Our data robustly establish some and challenge other aspects of this proposed description of PDC. Abnormal SNCA-immunoreactive structures have been identified in amygdala and cerebellum of approximately one-third of patients who died of PDC54,55,56; indeed, we also observed these in the amygdala of two of our PDC patients. One group used an extraction protocol similar to ours to show insoluble forms of SNCA in the amygdala only in those PDC patients with SNCA-immunoreactive inclusions.55 In contrast to these findings in regions of brain with unclear relevance to the clinical manifestations of PDC, our quantitative data demonstrated the accumulation of abnormally hydrophobic SNCA in frontal and temporal cortex of patients with PDC to levels comparable with DLB despite the lack of LB accumulation in these regions of brain that are clearly related to cognitive function. Others have presented elegant data demonstrating interactions between SNCA and tau57; however, we think this is an unlikely explanation for PDC because although AD, PSP, and PDC had increased SI and TI tau, only PDC had significantly increased TI SNCA.
In contrast to robustly establishing the presence of abnormal SNCA in relevant brain regions in PDC, our data do not support the characterization of PDC as an Aβ amyloidosis.53 Although it is true that various forms of senile plaques may be observed in patients with PDC, they also can be present in controls. We observed no quantitative differences in SI Aβ40, SI Aβ42, or soluble oligomers of Aβ in frontal or temporal cortex of PDC compared with controls despite large increases in the same regions of AD. Although the number of cases of PDC examined is substantial for this rare disease, it was still a small number of individuals; therefore, we cannot exclude the possibility that a subset of patients with PDC also may have cerebral Aβ amyloidosis.
There is an extensive literature on NFTs in patients with PDC and in older Chamorros apparently not affected by PDC. Some have reported greater than expected accumulation of NFTs in Chamorro controls that could suggest a preclinical state or ethnic vulnerability depending on the timeliness and rigor of clinical evaluation before death.37 Although we did not revisit NFT density here, our quantitative data both from iTRAQ and ELISA showed no difference between carefully characterized Chamorro controls from Guam and Caucasian controls from Seattle in amount of frontal or temporal cortex tau that was resistant to surfactant extraction.
We have shown that cerebral cortical regions of patients with PDC, but not AD or PSP, accumulated surfactant-resistant SNCA to a level comparable with DLB but did not form LBs. This finding parallels recent data by others showing that patients with PDC or PSP have high levels of surfactant-resistant tau in white matter with minimal formation of immunoreactive pathological structures.58 Despite limitations of this comparison, results from this study and ours indicate the presence of necessary steps between the generation of SI protein and formation of inclusions that are observable by IHC. What biochemical steps and interacting proteins regulate and modulate this process is not clear. Moreover, why PDC is different from other synucleinopathies in that SI SYCA in gray matter is not associated with IHC-observable inclusions is not apparent but may provide an opportunity to gain insight into these processes and their significance. Our discovery approach highlighted an entirely different pathogenic pathway that is present in PDC neocortex but absent in AD and PSP. These results suggest that PDC may be considered as much a synucleinopathy as a tauopathy. We propose that as new therapeutics are developed to treat other synucleinopathies they should at least be considered for patients with PDC.
Footnotes
Address reprint requests to Thomas J. Montine, M.D., Ph.D., Department of Pathology, University of Washington, Harborview Medical Center, Box 359791, Seattle, WA 98104. E-mail: tmontine@u.washington.edu.
Supported by the Nancy and Buster Alvord Endowment and the National Institutes of Health (grants AG24011 and NS48595).
References
- Zimmerman HM. Guam: Micronesian Area Research Center,; Monthly Report to Medical Officer in Command, U.S. Naval Medical Research Unit No. 2. 1945 [Google Scholar]
- Kurland LT, Mulder DW. Epidemiologic investigations of amyotrophic lateral sclerosis. I. Preliminary report on geographic distribution and special reference to the Mariana Islands, including clinical and pathologic observations. Neurology. 1954;4:438–448. doi: 10.1212/wnl.4.6.438. [DOI] [PubMed] [Google Scholar]
- Hirano A, Kurland LT, Krooth RS, Lessell S. Parkinsonism-dementia complex, an endemic disease on the island of Guam. I. Clinical features. Brain. 1961;84:642–661. doi: 10.1093/brain/84.4.642. [DOI] [PubMed] [Google Scholar]
- Hirano A, Malamud N, Kurland LT. Parkinsonism-dementia complex, an endemic disease on the island of Guam. II. Pathological features. Brain. 1961;84:662–679. doi: 10.1093/brain/84.4.662. [DOI] [PubMed] [Google Scholar]
- Kurland LT, Mulder DW, Sayre GP, Lambert E, Hutson W, Iriarte LL, Imus HA. Amyotrophic lateral sclerosis in the Mariana Islands. AMA Arch Neurol Psychiatry. 1956;75:435–441. doi: 10.1001/archneurpsyc.1956.02330220099008. [DOI] [PubMed] [Google Scholar]
- Reed D, Plato C, Elizan T, Kurland LT. The amyotrophic lateral sclerosis/parkinsonism-dementia complex: a ten-year follow-up on Guam. I. Epidemiologic studies. Am J Epidemiol. 1966;83:54–73. doi: 10.1093/oxfordjournals.aje.a120570. [DOI] [PubMed] [Google Scholar]
- Reed DM, Brody JA. Amyotrophic lateral sclerosis and parkinsonism-dementia on Guam, 1945–1972. I. Descriptive epidemiology. Am J Epidemiol. 1975;101:287–301. doi: 10.1093/oxfordjournals.aje.a112097. [DOI] [PubMed] [Google Scholar]
- Reed DM, Torres JM, Brody JA. Amyotrophic lateral sclerosis and parkinsonism-dementia on Guam, 1945–1972. II. Familial and genetic studies. Am J Epidemiol. 1975;101:302–310. doi: 10.1093/oxfordjournals.aje.a112098. [DOI] [PubMed] [Google Scholar]
- Reed D, Labarthe D, Chen KM, Stallones R. A cohort study of amyotrophic lateral sclerosis and parkinsonism-dementia on Guam and Rota. Am J Epidemiol. 1987;125:92–100. doi: 10.1093/oxfordjournals.aje.a114515. [DOI] [PubMed] [Google Scholar]
- Garruto RM, Yanagihara R, Gajdusek DC. Disappearance of high-incidence amyotrophic lateral sclerosis and parkinsonism-dementia on Guam. Neurology. 1985;35:193–198. doi: 10.1212/wnl.35.2.193. [DOI] [PubMed] [Google Scholar]
- Waring SC, Esteban-Santillan C, Reed DM, Craig UK, Labarthe DR, Petersen RC, Kurland LT. Incidence of amyotrophic lateral sclerosis and of the parkinsonism-dementia complex of Guam, 1950–1989. Neuroepidemiology. 2004;23:192–200. doi: 10.1159/000078505. [DOI] [PubMed] [Google Scholar]
- Wiederholt WC. Neuroepidemiologic research initiatives on Guam: past and present. Neuroepidemiology. 1999;18:279–291. doi: 10.1159/000026223. [DOI] [PubMed] [Google Scholar]
- Plato CC, Galasko D, Garruto RM, Plato M, Gamst A, Craig UK, Torres JM, Wiederholt W. ALS and PDC of Guam: forty-year follow-up. Neurology. 2002;58:765–773. doi: 10.1212/wnl.58.5.765. [DOI] [PubMed] [Google Scholar]
- Waring SC. Thesis: School of Public Health, University of Texas Health Science Center, Houston,; Amyotrophic Lateral Sclerosis and Parkinsonism-Dementia Complex of GuamDescriptive Epidemiology, Secular Trends, and Birth Cohort Effects on Incidence, 1950–1989, Ph.D. 1994 [Google Scholar]
- Galasko D, Salmon DP, Craig UK, Thal LJ, Schellenberg G, Wiederholt W. Clinical features and changing patterns of neurodegenerative disorders on Guam, 1997–2000. Neurology. 2002;58:90–97. doi: 10.1212/wnl.58.1.90. [DOI] [PubMed] [Google Scholar]
- Plato CC, Garruto RM, Galasko D, Craig UK, Plato M, Gamst A, Torres JM, Wiederholt W. Amyotrophic lateral sclerosis and parkinsonism-dementia complex of Guam: changing incidence rates during the past 60 years. Am J Epidemiol. 2003;157:149–157. doi: 10.1093/aje/kwf175. [DOI] [PubMed] [Google Scholar]
- Esclaire F, Kisby G, Spencer P, Milne J, Lesort M, Hugon J. The Guam cycad toxin methylazoxymethanol damages neuronal DNA and modulates tau mRNA expression and excitotoxicity. Exp Neurol. 1999;155:11–21. doi: 10.1006/exnr.1998.6962. [DOI] [PubMed] [Google Scholar]
- Garruto RM, Fukatsu R, Yanagihara R, Gajdusek DC, Hook G, Fiori CE. Imaging of calcium and aluminum in neurofibrillary tangle-bearing neurons in parkinsonism-dementia of Guam. Proc Natl Acad Sci USA. 1984;81:1875–1879. doi: 10.1073/pnas.81.6.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AJ, Rice GP. Similarities of Guamanian ALS/PD to post-encephalitic parkinsonism/ALS: possible viral cause. Can J Neurol Sci. 1990;17:427–433. doi: 10.1017/s0317167100031024. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, Robertson RC. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- Yanagihara R, Garruto RM, Gajdusek DC, Tomita A, Uchikawa T, Konagaya Y, Chen KM, Sobue I, Plato CC, Gibbs CJ., Jr Calcium and vitamin D metabolism in Guamanian Chamorros with amyotrophic lateral sclerosis and parkinsonism-dementia. Ann Neurol. 1984;15:42–48. doi: 10.1002/ana.410150108. [DOI] [PubMed] [Google Scholar]
- Duncan MW. β-Methylamino-l-alanine (BMAA) and amyotrophic lateral sclerosis-parkinsonism dementia of the western Pacific. Ann NY Acad Sci. 1992;648:161–168. doi: 10.1111/j.1749-6632.1992.tb24534.x. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Li K, Perl DP, Galasko D. Lack of β-methylamino-l-alanine in brain from controls, AD, or Chamorros with PDC. Neurology. 2005;65:768–769. doi: 10.1212/01.wnl.0000174523.62022.52. [DOI] [PubMed] [Google Scholar]
- Steele JC. Parkinsonism-dementia complex of Guam. Mov Disord. 2005;20(Suppl 12):S99–S107. doi: 10.1002/mds.20547. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Tsuang D, Wijsman E, Steinbart E, Garruto RM, Craig UK, Chapman NH, Anderson L, Bird TD, Plato CC, Perl DP, Weiderholt W, Galasko D, Schellenberg GD. TAU as a susceptibility gene for amyotropic lateral sclerosis-parkinsonism dementia complex of Guam. Arch Neurol. 2001;58:1871–1878. doi: 10.1001/archneur.58.11.1871. [DOI] [PubMed] [Google Scholar]
- Sundar PD, Yu CE, Sieh W, Steinbart E, Garruto RM, Oyanagi K, Craig UK, Bird TD, Wijsman E, Galasko DR, Schellenberg GD. Two sites in the MAPT region confer genetic risk for Guam ALS/PDC and dementia. Hum Mol Genet. 2007;16:295–306. doi: 10.1093/hmg/ddl463. [DOI] [PubMed] [Google Scholar]
- Morris HR, Steele JC, Crook R, Wavrant-De Vrieze F, Onstead- Cardinale L, Gwinn-Hardy K, Wood NW, Farrer M, Lees AJ, McGeer PL, Siddique T, Hardy J, Perez-Tur J. Genome-wide analysis of the parkinsonism-dementia complex of Guam. Arch Neurol. 2004;61:1889–1897. doi: 10.1001/archneur.61.12.1889. [DOI] [PubMed] [Google Scholar]
- Wong CW, Quaranta V, Glenner GG. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci USA. 1985;82:8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Lee VM, Wang J, Trojanowski JQ. Purification of paired helical filament tau and normal tau from human brain tissue. Methods Enzymol. 1999;309:81–89. doi: 10.1016/s0076-6879(99)09008-4. [DOI] [PubMed] [Google Scholar]
- Tsuang DW, Bird TD. Genetics of dementia. Med Clin North Am. 2002;86:591–614. doi: 10.1016/s0025-7125(02)00003-2. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- McKeith I, Galasko D, Kosaka K, Perry E, Dickson D, Hansen L, Salmon D, Lowe J, Mirra S, Byrne E, Lennox G, Quinn N, Edwardson J, Ince P, Bergeron C, Burns A, Miller B, Lovestone S, Collerton D, Jansen E, Ballard C, de Vos R, Wilcock G, Jellinger K, Perry R. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Perl DP, Hof PR, Purohit DP, Loerzel AJ, Kakulas BA. Hippocampal and entorhinal cortex neurofibrillary tangle formation in Guamanian Chamorros free of overt neurologic dysfunction. J Neuropathol Exp Neurol. 2003;62:381–388. doi: 10.1093/jnen/62.4.381. [DOI] [PubMed] [Google Scholar]
- Mirra S, Heyman A, McKeel D, Sumi S, Crain B, Brownlee L, Vogel F, Hughes J, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Jakes R, Crowther RA, Lee VM, Trojanowski JQ, Iwatsubo T, Goedert M. Epitope mapping of LB509, a monoclonal antibody directed against human α-synuclein. Neurosci Lett. 1999;269:13–16. doi: 10.1016/s0304-3940(99)00411-5. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Fishel MA, Peskind ER, Montine TJ, Nochlin D, Steinbart E, Raskind MA, Schellenberg GD, Bird TD, Tsuang D. Lewy body pathology in familial Alzheimer disease—evidence for disease- and mutation-specific pathologic phenotype. Arch Neurol. 2006;63:370–376. doi: 10.1001/archneur.63.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjer RL, Cimino PJ, Boutte AM, Schantz AM, Montine KS, Larson EB, Bird T, Quinn JF, Zhang J, Montine TJ. Proteomic determination of widespread detergent-insolubility including Aβ but not tau early in the pathogenesis of Alzheimer’s disease. FASEB J. 2005;19:1923–1925. doi: 10.1096/fj.05-4263fje. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies. Cold Spring Harbor: Cold Spring Harbor Laboratory Press,; A Laboratory Manual. 1988 [Google Scholar]
- Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- Weatherly DB, Astwood JA, III, Minning TA, Cavola C, Tarleton RL, Orlando R. A heuristic method for assigning a false-discovery rate for protein identifications from mascot database search results. Mol Cell Proteomics. 2005;4:762–772. doi: 10.1074/mcp.M400215-MCP200. [DOI] [PubMed] [Google Scholar]
- Kapp EA, Schutz F, Connolly LM, Chakel JA, Meza JE, Miller CA, Fenyo D, Eng JK, Adkins JN, Omenn GS, Simpson RJ. An evaluation, comparison, and accurate benchmarking of several publicly available MS/MS search algorithms: sensitivity and specificity analysis. Proteomics. 2005;5:3475–3490. doi: 10.1002/pmic.200500126. [DOI] [PubMed] [Google Scholar]
- Goedert M, Hasegawa M. The tauopathies: toward an experimental animal model. Am J Pathol. 1999;154:1–6. doi: 10.1016/S0002-9440(10)65242-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion D, Martin C, Heilig R, Charbonnier F, Moreau V, Flaman JM, Petit JL, Hannequin D, Brice A, Frebourg T. The NACP/synuclein gene—chromosomal assignment and screening for alterations in Alzheimer’s disease. Genomics. 1995;26:254–257. doi: 10.1016/0888-7543(95)80208-4. [DOI] [PubMed] [Google Scholar]
- Uéda K, Saitoh T, Mori H. Tissue-dependent alternative splicing of messenger-RNA for NACP, the precursor of non-A-β component of Alzheimer’s disease amyloid. Biochem Biophys Res Commun. 1994;205:1366–1372. doi: 10.1006/bbrc.1994.2816. [DOI] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer’s disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Nowotny P, Holmans P, Smemo S, Kauwe JS, Hinrichs AL, Tacey K, Doil L, van Luchene R, Garcia V, Rowland C, Schrodi S, Leong D, Gogic G, Chan J, Cravchik A, Ross D, Lau K, Kwok S, Chang SY, Catanese J, Sninsky J, White TJ, Hardy J, Powell J, Lovestone S, Morris JC, Thal L, Owen M, Williams J, Goate A, Grupe A. Association of late-onset Alzheimer’s disease with genetic variation in multiple members of the GAPD gene family. Proc Natl Acad Sci USA. 2004;101:15688–15693. doi: 10.1073/pnas.0403535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Lee VM, Trojanowski JQ. Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol Mech Dis. 2006;1:151–170. doi: 10.1146/annurev.pathol.1.110304.100113. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Arai Y, Baba M, Iwatsubo T, Mori O, Katayama Y, Oyanagi K. α-Synuclein inclusions in amygdala in the brains of patients with the parkinsonism-dementia complex of Guam. J Neuropathol Exp Neurol. 2000;59:585–591. doi: 10.1093/jnen/59.7.585. [DOI] [PubMed] [Google Scholar]
- Forman MS, Schmidt ML, Kasturi S, Perl DP, Lee VM, Trojanowski JQ. Tau and α-synuclein pathology in amygdala of parkinsonism-dementia complex patients of Guam. Am J Pathol. 2002;160:1725–1731. doi: 10.1016/s0002-9440(10)61119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebeo J, Hof PR, Perl DP. Occurrence of α-synuclein pathology in the cerebellum of Guamanian patients with parkinsonism-dementia complex. Acta Neuropathol (Berl) 2004;107:497–503. doi: 10.1007/s00401-004-0840-4. [DOI] [PubMed] [Google Scholar]
- Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R. α-Synuclein binds to tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem. 1999;274:25481–25489. doi: 10.1074/jbc.274.36.25481. [DOI] [PubMed] [Google Scholar]
- Zhukareva V, Joyce S, Schuck T, Van Deerlin V, Hurtig H, Albin R, Gilman S, Chin S, Miller B, Trojanowski JQ, Lee VM. Unexpected abundance of pathological tau in progressive supranuclear palsy white matter. Ann Neurol. 2006;60:335–345. doi: 10.1002/ana.20916. [DOI] [PubMed] [Google Scholar]