Abstract
Chronic obstructive pulmonary disease (COPD) is a progressive disease associated with a cellular inflammatory response. CD8+ T cells are implicated in COPD pathogenesis, and their numbers significantly correlate with the degree of airflow limitation. Dendritic cells (DCs) are important sentinel immune cells, but little is known about their role in initiating and maintaining the CD8 T-cell response in COPD. To investigate the mechanisms for CD8+ T-cell recruitment to the lung, we used resected human lung tissue to analyze chemokine receptor expression by CD8+ T cells and chemokine production by CD1a+ DCs. Among 11 surveyed chemokine receptors, only CC chemokine receptor (CCR5), CXC chemokine receptor (CXCR) 3, and CXCR6 correlated with COPD severity as defined by criteria from the Global Initiative for Chronic Obstructive Lung Disease. The CD8+ T cells displayed a Tc1, CD45RA+ effector memory phenotype. CD1a+ DCs produced the respective ligands for CCR5 and CXCR3, CCL3 and CXCL9, and levels correlated with disease severity. CD1a+ DCs also constitutively expressed the CXCR6 ligand, CXCL16. In conclusion, we have identified major chemokine elements that potentially mediate CD8+ T-cell infiltration during COPD progression and demonstrated that CD1a+ mucosal-associated DCs may sustain CD8+ T-cell recruitment/retention. Chemokine targeting may prove to be a viable treatment approach.
Chronic obstructive pulmonary disease (COPD) is a diagnostic umbrella that encompasses emphysema and chronic bronchitis. COPD is characterized by progressive airflow limitation associated with an abnormal inflammatory response. Neutrophils, macrophages, and CD8+ T cells have been implicated in COPD pathogenesis in a number of studies.1,2,3,4,5,6 More recently, airway infiltration by CD4 T cells and B cells has also been shown to associate with the progression of COPD.7,8 This cellular inflammation is associated with airway remodeling and destruction, but it is not clear which cell types are responsible for the damage. Neutrophils and macrophages are sources of reactive oxygen metabolites, inflammatory cytokines, metalloproteinases, and other tissue-damaging enzymes.9 By contrast, it is less clear how CD8+ T cells could destroy lung parenchyma, although in response to antigen, CD8+ T cells are able to lyse target cells, either through the release of cytotoxic proteins, such as perforin or granzyme, or by inducing apoptosis via the Fas ligand-Fas pathway.10 However, the nature of the antigen that could trigger this CD8+ T-cell response in COPD is unknown. One hypothesis suggests that intracellular pathogens, such as adenovirus or rhinovirus, may provide a foreign antigenic stimulus; and in fact, viral infections are a frequent occurrence in patients with COPD.11,12,13 Autoimmunity has also been postulated, thus far without supporting evidence.14
To initiate antigen-specific CD8+ T-cell immune responses, it is necessary for the CD8 T-cell receptor to recognize foreign antigen in combination with a major histocompatibility class I molecule presented by an antigen-presenting cell (APC). It seems that dendritic cells (DCs) are the predominant APCs for the priming of naïve CD8 T cells15,16,17 and for initiating CD8 memory T-cell proliferative responses.15,16,17,18 Surprisingly, the role of dendritic cells has not been well studied in COPD. In murine models of cigarette smoke exposure, there are conflicting results as to whether DC numbers are increased or decreased in the lung in response to cigarette smoke.19,20 In the human lung, four subsets of pulmonary DCs have been identified: myeloid DC 1, myeloid DC 2, plasmacytoid DC, and CD1a+ DCs.21 In chronic asthmatics, CD1a+ DCs were present in greater numbers in the bronchial mucosa compared with healthy controls and were shown to play an important role in modulating the immune response.22 In view of the role of DCs in initiating and maintaining antigen-specific CD8 T-cell responses, their role in COPD deserves study.
Because CD8+ T cells potentially contribute to the pathophysiology of COPD, understanding how these cells are recruited to the lung may lead to novel treatment. The families of proteins known as chemokines and chemokine receptors are considered key mediators of recruitment. Chemokine receptors play an important role in the trafficking of immune cells to sites of injury, inflammation, and antigen encounter. Approximately 50 chemokines and 20 chemokine receptors have been identified. They are classified into four subgroups based on the position of critical cysteine residues: CXC, CC, C, and CX3C. Besides the ability of chemokines to drive leukocyte migration, they are also involved in proliferation, differentiation, retention, and survival.23 CC chemokine receptor 5 (CCR5) and CXC chemokine receptor 3 (CXCR3) have already been implicated in COPD because T cells infiltrating the lungs of COPD patients have been shown to express these chemokine receptors.24,25,26 However, many of these studies used immunoperoxidase or immunofluorescent analyses, which have limited sensitivity to detect the characteristically low surface expression of chemokine receptors and, moreover, may not reflect activity in the interstitium. In addition, previous studies did not correlate chemokine receptor expression with specific cell sources of chemokine ligands or disease severity.
In the present study, we performed a comprehensive chemokine receptor analysis of interstitial CD8+ T cells and DCs derived from clinically indicated lung resections from patients with COPD. We found that three chemokine receptors, CCR5, CXCR3, and CXCR6, demonstrated increased expression on lung CD8+ T cells that correlated with severity of COPD. These CD8+ lung T cells displayed a type-1 (Tc1) effector memory phenotype. Further analysis of CD1a+ DC chemokine transcripts revealed that CCL3 and CXCL9, respective ligands for CCR5 and CXCR3, likewise displayed a correlation with disease severity, whereas the ligand for CXCR6, CXCL16, was constitutively produced. Our findings suggest that CCR5 and CXCR3 may be involved in the recruitment of CD8+ T cells into the lungs of COPD patients, whereas CXCR6 may be more important in adhesion or retention events. These findings provide a potential rationale for the design of therapeutic agents to impact chronic CD8+ T cell-mediated inflammation in COPD patients.
Materials and Methods
Specimens and Patient Population
Specimens were obtained from patients undergoing clinically indicated resection procedures for tumor nodules, lung volume reduction surgery, or lung transplant surgery at the VA Ann Arbor Healthcare and the University of Michigan Healthcare Systems. Studies and consent procedures were approved by Institutional Review Boards. Tissues and clinical data were de-identified before analysis. Only non-neoplastic lung tissue remote from tumor nodules and lacking postobstruction changes was collected. Histological sections were reviewed to confirm absence of pneumonia, lymphangitic neoplasm, or other unrelated interstitial diseases. The study population was composed of 30 subjects, all with a history of cigarette smoking. All subjects underwent two or more preoperative spirometric tests and full evaluation by a pulmonologist. A five-stage classification system derived from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) was used to categorize patients.27,28 Stage 0 represents patients at risk, whereas stage 4 represents patients with the most severe cases of COPD. Classification is based on the forced expiratory volume in 1 second (FEV1) of the predicted value in combination with the ratio of FEV1 to forced vital capacity. Table 1 shows the FEV1 range for each GOLD stage and the number of patients in each stage along with age and smoking history ranges.
Table 1.
Summary of Patient Age, Smoking History, and Disease Severity
| COPD severity | GOLD guidelines classification | Patients (n) | Age range (years) | Smoking history (pack years) |
|---|---|---|---|---|
| 0 | Normal spirometry with symptoms | 8 | 44 to 79 | 35 to 60 |
| 1 | FEV1/FVC < 70% FEV1 ≥ 80%* | 7 | 61 to 72 | 16 to 50 |
| 2 | 50% ≤ FEV1 < 80% | 6 | 51 to 71 | 38 to 80 |
| 3 | 30% ≤ FEV1 < 50% | 5 | 48 to 66 | 30 to 50 |
| 4 | FEV1 < 30% | 4 | 53 to 70 | 50 to 64 |
According to the GOLD guidelines, to be diagnosed with COPD, the ratio between FEV1 and forced vital capacity (FVC) should be <70%.
Immunohistochemistry
For histological evaluation, samples of lung tissue from each patient were embedded in paraffin and sectioned for standard hematoxylin and eosin staining and immunohistochemical staining. Immunohistochemical staining for CD8 and CD1a was performed by the University of Michigan Health Systems Laboratory using an automated immunostainer (Ventana Medical Systems, Tucson, AZ).
Flow Cytometry
Lung sections weighing an average of 2.5 g were rinsed in RPMI 1640 medium (JRH Biosciences, Lenexa, KS) to remove excess blood and were homogenized in a Waring blender at low speed for 45 seconds. Cells were passed through a 70-μm nylon filter to remove debris and resuspended at 10 × 106 cells per ml of flow buffer (2% fetal bovine serum in phosphate-buffered saline) along with 20 μl/ml Human FC block (Miltenyi Biotec, Auburn, CA). Cells were incubated at 4°C for 10 minutes and then added in a volume of 100 μl to each flow tube. Monoclonal antibodies against CD8, CD1a, CCR5, CD45RA, CXCR3, CXCR1, CCR7, CD83 (BD Biosciences, San Jose, CA), CXCR6, CCR2, CCR3, CCR8, and CCR6 (R&D Systems, Minneapolis, MN) were used. Antibodies were conjugated to fluorescein isothiocyanate, phycoerythrin, or phycoerythrin-cyanine 5. Appropriate isotype-matched controls were used in all experiments. Cells were incubated with antibodies for 25 minutes at 4°C. After incubation, cells were washed twice and analyzed using a FACScan cytometer running CellQuest software (BD Biosciences).
CD8+ T-Cell and CD1a+ Cell Isolation
Lung samples were homogenized as described above, and cells were resuspended in 80 μl of phosphate-buffered saline containing 0.5% fetal bovine serum and 2 mmol/L ethylenediamine tetraacetic acid per 10 × 106 cells. Cells were divided into two samples and incubated with either human-specific CD8 or CD1a microbeads (Miltenyi Biotec) according to manufacturer’s instructions. MACS LS columns (Miltenyi Biotec) were used to separate cells, and the positively labeled CD8+ or CD1a+ cells were collected. Cells were lysed for RNA isolation and real-time reverse transcriptase-polymerase chain reaction (RT-PCR) analyses.
Real-Time RT-PCR
Micro Poly(A) Pure kits (Ambion, Austin, TX) were used to isolate RNA from lysed cells or from whole tissues. DNA-free (Ambion) was used to remove any contaminating genomic DNA. Each RNA sample was reverse-transcribed in a 20-μl reaction using SuperScript II RNase H− Reverse Transcriptase (Invitrogen Corporation, Carlsbad, CA). Analysis of the transcripts was performed by real-time PCR using the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Human glyceraldehyde-3-phosphate dehydrogenase, which acted as the endogenous reference, and primer-probe sets for target genes were purchased commercially (Applied Biosystems). Transcript levels were expressed as arbitrary units and were calculated using the comparative threshold cycle method, as recommended by the manufacturer.
Protein Analysis
Lung sections were snap-frozen in dry ice and stored at −80°C before use. For protein extraction, lobes were resuspended in 2 ml of phosphate-buffered saline and homogenized using a tissue homogenizer. Samples were centrifuged at 300 × g for 20 minutes. Supernatants were collected and stored at −80°C. Using the Luminex 200 (Luminex Corporation, Austin, TX), protein levels for CCL3 and CXCL9 were determined using Biosource Multiplex Assays (Invitrogen) according to manufacturer’s instructions. Total lung protein concentration was determined using a Micro BCA Protein Assay kit (Pierce Biotechnology, Rockford, IL), and chemokine levels were normalized to milligrams of lung protein.
Statistics
Nonparametric (Spearman) correlation analysis was used to determine the correlation coefficient r. A two-tailed P value of <0.05 was considered to indicate significance.
Results
Distribution of CD1a+ and CD8+ Cells in the Lungs of COPD Patients
Initially, we determined the anatomical tissue compartment distribution of target cell populations in subject lung specimens. Paraffin-embedded tissues taken from COPD patients were sectioned and immunohistochemically stained for CD1a and CD8 (Figure 1). Lung sections from individuals with a GOLD stage of 0, 1, 2, or 3 are depicted and are representative of the staining patterns seen for other individuals. The majority of CD1a+ cells were found within bronchial mucosal epithelium, whereas the CD8+ cells localized to peribronchial cuffs and epithelium. In general, these populations appeared to be increased in GOLD stages 2 and 3. These studies confirmed a close proximity of CD8+ and CD1a+ cells in a bronchocentric process. Similar findings for the localization of CD1a+ DCs21,29 and CD8+ T cells30 have been reported.
Figure 1.
Immunohistochemical localization of CD1a+ (left) and CD8+ (right) cells in the lungs of smokers with GOLD stage 0 (A and B), stage 1 (C and D), stage 2 (E and F), and stage 3 (G and H) COPD. Stain is immunoperoxidase based with diaminobenzidine as the chromagen substrate. Arrows point to CD1a+ cells. Magnification: ×200 (A, B, D, F, and H); ×400 (C, E, and G).
CD8+ Cells Isolated from Lungs of COPD Patients Have an Effector Tc1 Phenotype
Previous studies have suggested that peripheral blood CD4+ T cells from COPD patients display a Th1 phenotype, evidenced in part by their production of interferon γ (IFNγ).31 However, recent studies have shown that CD8+ T lymphocytes from bronchoalveolar lavage fluid show a Tc2 profile.32,33 To address this issue in our study population, we analyzed transcript expression of IFNγ and interleukin (IL)-4 from isolated lung CD8+ T cells. We found that transcripts for IFNγ displayed a significant correlation to COPD severity, with an r value of 0.81 (Figure 2A). However, there was little to no transcript expression for IL-4 (data not shown). To characterize these cells further, we examined CD45RA and CCR7 expression by flow cytometry. Human CD8+ cells can be classified as either naïve (CCR7+ CD45RA+), central memory (CCR7+ CD45RA−), or effector memory (CCR7− CD45RA− or CD45RA+) cells.34 Figure 2B demonstrates that the percentage of CD8 cells positively expressing CD45RA increased with COPD severity. These cells did not express CCR7 (data not shown), consistent with CD45RA+ effector memory (TEMRA) cells that apparently become more prevalent as COPD progresses. Our findings suggest that there is induction of an activated subpopulation of TEMRA cells in the lungs of COPD patients with a Tc1 effector phenotype that correlates with disease severity.
Figure 2.
Lung CD8+ T cells from COPD patients express a Tc1 effector memory phenotype. CD8+ T cells isolated from human lung samples by immunomagnetic beads were used for RNA analysis. CD8+ T cells expressed transcripts for IFNγ (A). Transcripts are expressed as arbitrary units and were measured by quantitative real-time RT-PCR. Surface expression of CD45RA was determined by flow cytometry (B) and is shown as the percentage of CD8+ cells among total leukocytes. Correlation statistical analysis is shown.
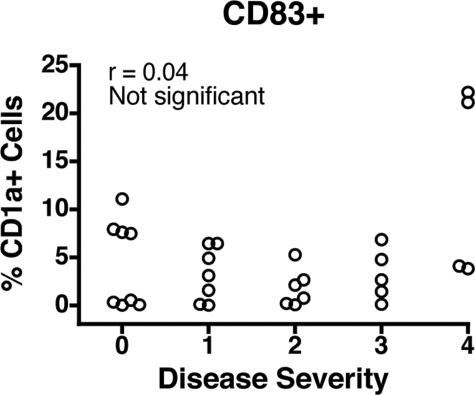
CD1a+ DCs in the Human Lung Display Low CD83 Costimulatory Expression at All COPD Stages
CD1a has been shown to be a reliable marker for a subset of intramucosal DCs identified in the human lung.21 They have been implicated in modulating the immune response during chronic asthma,22 but little is known regarding their role in COPD. Because little is known regarding the maturation state of CD1a+ DCs in human lungs affected by COPD, we attempted to correlate DC costimulatory molecule expression to COPD stage. We analyzed expression of CD83, reportedly a marker of DC maturity.35 Figure 3 shows that on average, only 5% of total CD1a+ DCs were expressing CD83. Others have shown that lung DCs possess an immature phenotype, yet like mature DCs, they have the capacity to stimulate T-cell proliferation.36 Our findings are in accord with this notion and further suggest that disease severity is not associated with changes in the expression of CD83 by CD1a+ DCs.
Figure 3.
Lung CD1a+ DCs do not express CD83. Surface expression of CD83 was determined by flow cytometry and is expressed as the percentage of CD1a+ cells among total leukocytes. Correlation statistical analysis is shown.
CCR5, CXCR3, and CXCR6 Expression Among Lung CD8+ T Cells Positively Correlates with Disease Severity
To determine the potential role of chemokine receptors in the infiltration of CD8+ T cells into the lungs of COPD patients, we analyzed the expression of 11 different chemokine receptors by flow cytometry and real-time PCR. For flow cytometric analyses, chemokine receptor-positive CD8+ T cells were expressed as the percentage of total CD8+ cells. To analyze transcript expression by real-time PCR, RNA was isolated from purified interstitial lung CD8+ cells. Of the examined receptors, CCR5, CXCR3, and CXCR6 were expressed by CD8+ T cells and demonstrated a positive correlation with disease severity using flow cytometric (Figure 4, A–C) and transcript (Figure 4, D–F) analyses. Representative histograms show the increased expression of CCR5, CXCR3, and CXCR6 in GOLD stage 4 versus GOLD stage 0 patients (Figure 5). Our findings regarding CXCR3 expression by CD8+ T cells in COPD patients are in accord with the immunohistochemical study of Saetta et al.24 Table 2 lists the Spearman correlation coefficient r and the P value for all 11 chemokine receptors. Statistics were determined using flow cytometry data, except for CCR1, which was determined by RNA transcript levels. After observing the correlation between disease severity and CCR5 expression, CCR1 was included in the analysis because these chemokine receptors share some of the same ligands. However, CCR1 showed no correlation to COPD severity. In summary, we identified three chemokine receptors with the potential to mediate CD8+ T-cell infiltration into the lungs of COPD patients.
Figure 4.
Chemokine receptors CCR5, CXCR3, and CXCR6 are expressed by lung CD8+ T cells and correlate with COPD severity. Chemokine receptor expression was profiled in dispersed lungs by flow cytometry (A, B, and C) and in preparations of enriched CD8+ T cells by real-time RT-PCR (D, E, and F). CCR5 (A and D), CXCR3 (B and E), and CXCR6 (C and F) expression was correlated to COPD severity, as determined by GOLD stage. Correlation analysis is shown.
Figure 5.
Flow cytometric histograms of CCR5, CXCR3, and CXCR6 in GOLD stage 0 and GOLD stage 4 patients. Representative histograms show expression by gated CD8+ cells. The white profiles show receptor-specific antibody staining, and the shaded profiles show staining with isotype-matched control antibodies.
Table 2.
Summary of Chemokine Receptor Expression on Lung CD8+ T Cells and Correlation with COPD Severity
| Chemokine receptor | r value | P value |
|---|---|---|
| CCR1* | −0.07 | 0.79 |
| CCR2 | 0.14 | 0.61 |
| CCR3 | 0.21 | 0.39 |
| CCR4 | 0.33 | 0.17 |
| CCR5 | 0.66 | 0.0001 |
| CCR6 | 0.14 | 0.71 |
| CCR7 | 0.28 | 0.24 |
| CCR8 | 0.08 | 0.84 |
| CXCR1 | 0.20 | 0.37 |
| CXCR3 | 0.64 | 0.0004 |
| CXCR6 | 0.67 | 0.0001 |
Value determined by RNA transcript levels.
Chemokine Ligand Expression in COPD Lung Tissues
To investigate further the potential chemotactic factors involved in CD8+ T-cell recruitment, we analyzed chemokine expression in the lung. Fresh lung specimens were homogenized, and RNA was isolated for real-time RT-PCR analysis. The expression of CCL3 (macrophage inflammatory protein 1α), a CCR5 ligand; CXCL9 (monokine induced by interferon-γ), a CXCR3 ligand; and CXCL16, a CXCR6 ligand, are shown in Figure 6, A–C. Transcript expression of CCL3 and CXCL9 in the whole lung showed significant correlations to disease severity, with r values of 0.58 and 0.63, respectively. Although the transcripts for CXCL16 were highly expressed in the majority of samples, there was no correlation to COPD severity. To confirm the RNA data, we extracted protein from the whole lung. Using Luminex xMAP microsphere technology, we measured chemokine levels for CCL3 and CXCL9 (Figure 6, D and E). Again, CCL3 and CXCL9 protein levels significantly correlated with COPD disease severity. Additional cytokine transcripts measured in the whole lung included CX3CL1, CCL17, CCL22, CXCL10, CXCL11, and granulocyte macrophage–colony-stimulating factor (data not shown). These genes displayed either low transcription or no association with disease severity. Taken together, our results suggest that there is a coordinated expression of chemokine receptors and corresponding ligands in COPD patients that could mediate CD8+ T-cell recruitment or retention.
Figure 6.
Chemokine expression in lungs of COPD patients. Real-time PCR was used to measure chemokine transcripts for CCL3 (A), CXCL9 (B), and CXCL16 (C) among mRNA isolated from whole lung samples. Results are expressed in arbitrary units. CCL3 (D) and CXCL9 (E) protein levels were measured using Luminex xMAP microsphere technology. Results are expressed as picograms of chemokine per milligram of total protein. Levels were correlated to COPD severity, as determined by GOLD stage. Correlation analysis is shown.
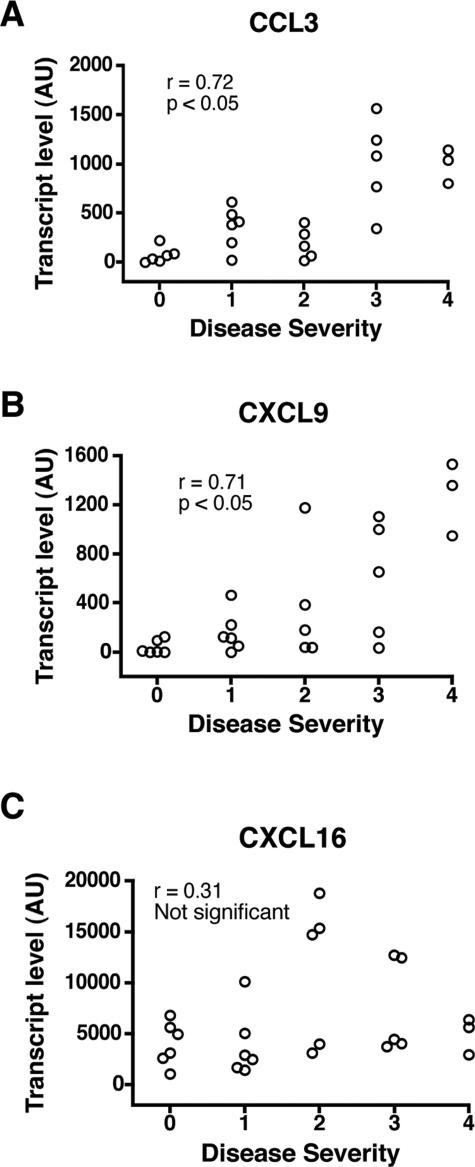
CCL3 and CXCL9 Are Produced by CD1a+ DCs
DCs are known to attract immune effector cells through chemokines.37,38,39 In view of their mucosal location, we determined whether CD1a+ DCs were a source of chemokines in COPD. To this end, CD1a+ DCs were isolated from lung tissue using immunomagnetic beads, and chemokine transcripts were measured by real-time PCR. As shown in Figure 7, CCL3 and CXCL9 expression by CD1a+ DCs significantly correlated to COPD severity with respective r values of 0.72 and 0.71. CXCL16, although demonstrating high transcript levels in DCs, again showed no correlation to disease severity. Other chemokine transcript levels measured included CCL17, CCL22, CXCL8, CXCL10, CXCL11, and CX3CL1. Other than CXCL8, these chemokines were expressed by CD1a+ DCs at very low levels. CXCL8 was more strongly expressed but displayed no correlation to disease severity (data not shown). In our analysis, we also used flow cytometry to measure chemokine receptor expression by CD1a+ DCs. None of the receptors analyzed (CCR3, CCR4, CCR5, CCR6, CCCR7, CCR8, CXCR1, CXCR3, and CXCR6) displayed an association with disease severity (data not shown). Our results imply that CD1a+ DCs are active in COPD and may promote CD8+ T-cell recruitment to the lungs through the production of chemokines.
Figure 7.
CCL3, CXCL9, and CXCL16 transcript expression by purified CD1a+ DCs. CD1a+ DCs were isolated from human lungs by immunomagnetic beads and used for RNA analysis. Chemokine transcripts for CCL3 (A), CXCL9 (B), and CXCL16 (C) are shown. Transcripts were measured by real-time PCR, and results are expressed as arbitrary units. Levels were correlated to COPD severity, as determined by GOLD stage. Correlation statistical analysis is shown.
Discussion
This study is the first comprehensive and quantitative analysis of chemokine receptor expression by lung CD8+ T cells in COPD and the first study to examine the role of CD1a+ DCs in COPD. Our analysis of CD8+ T cells revealed that among 11 examined chemokine receptors, only CCR5, CXCR3, and CXCR6 correlated with COPD severity as measured by GOLD staging. The validity of our results was supported by concordant findings using two independent methods: flow cytometry and direct cell isolation with gene expression analysis. The chemokine receptors were likely expressed by a subpopulation of CD45RA+ effector CD8+ T cells. Moreover, our study further demonstrated that CD1a+ DCs might contribute to CD8+ T-cell recruitment through coordinated production of CCL3, CXCL9, and CXCL16, respective ligands for CCR5, CXCR3, and CXCR6. Production of CCL3 and CXCL9 correlated with COPD severity, whereas CXCL16 was constitutively expressed by CD1a+ DCs in all stages of COPD severity, possibly reflecting different functionalities.
Our study implicates CCR5 and CXCR3, along with their respective ligands, in mediating recruitment of CD8+ T cells to the lung. CCR5 could have an important role in CD8 activity because CCR5 stimulation is known to induce production of IL-2 and IFNγ by T cells.40 Persistent production of IFNγ could provide an amplification loop to continuously drive T-cell accumulation into the lungs by promoting additional chemokine synthesis. In addition, CCR5 has been shown to be important for selective leukocyte migration in response to chemotactic stimuli.41 CCR5 is the receptor not only for CCL3 but also for CCL4 (macrophage inflammatory protein 1β) and CCL5 (regulated on activation normal T cell expressed and secreted). We have not yet investigated whether these other CCR5 agonists are expressed in the lungs of COPD patients. However, CCL4 is reportedly increased in the bronchoalveolar lavage fluid of patients with chronic bronchitis and mild to moderate airflow limitation.42 Our finding that CCR5 expression correlated with disease severity did not agree with a previous study reporting decreases in CD3+ CCR5+ cells in severe COPD.43 However, that study was based on immunohistochemical observations in small biopsy fragments and did not determine expression specifically by CD8+ T cells, nor were patients stratified by GOLD stage. This apparent discrepancy could also be due to the fact that severe COPD may represent a more complicated disease state.44 We cannot exclude that end-stage COPD may differ from mild to moderate stages. Patients with end-stage COPD (stage 4) are seldom candidates for lobectomies, from which we most readily recover sufficient cells for analysis. Although these patients are candidates for lung volume reduction surgery or lung transplants, the tissues removed in these operations have proven far less amenable to isolation of adequate cell number. Hence, we obtained fewer specimens; therefore further evaluation may reveal more subtle differences. Nevertheless, for therapeutic purposes, it is more important to identify the chemotactic factors involved at earlier stages of COPD. In addition, it is important to recognize that in human studies, a high degree of experimental variation is not uncommon. Thus, our ability to reveal underlying correlations gains in significance. In the current GOLD classification of disease severity based on spirometric tests, there is an imperfect relationship between the degree of airflow limitation and the presence of symptoms. More precise classification using parameters such as chemokine receptor expression may help better define prognostic groups.
Our analysis of transcripts for all known CXCR3 ligands extends a previous observation that lung CXCR3+ T cells, most coexpressing CD8, are increased in smokers with COPD.24 That study also detected immunoreactivity for CXCL10 in the bronchiolar epithelium of COPD patients. Using a quantitative approach, we found that CXCL9 (monokine induced by interferon-γ), and not the other known CXCR3 ligands, CXCL10 (interferon-inducible protein 10) and CXCL11 (interferon-inducible T-cell α-chemokine), correlated with disease status. Transcripts for CXCL10 were detected in the lungs, in agreement with the above study, but showed no correlation to disease severity. However, CXCL11 displayed low transcript levels, even though it reportedly has the highest affinity for CXCR3.45
Our finding that CXCL16 transcripts were constitutively expressed by CD1a+ DCs suggests that CXCR6 and CXCL16 could be involved in homeostatic and inflammatory cell recruitment and retention events. CXCL16 and its receptor CXCR6 are relatively new additions to the chemokine family. Importantly, CXCL16 exists in membrane-bound and in secreted forms. Although studies have suggested that CXCR6 facilitates recruitment of activated CD8+ T cells to sites of inflammation,46,47,48 our results imply that this is not its role in COPD. Because CXCL16 has been shown to function as a membrane-bound adhesion molecule for cells expressing CXCR6,49 one possibility is that CXCR6 might allow binding of CD8+ T cells to CD1a+ DCs to promote more efficient stimulation of cell-activating chemokine receptors such as CCR5. Coexpression of CXCR6 and CCR5 has been demonstrated on peripheral blood T cells.50 This would allow DCs and CD8+ T cells to interact, facilitating antigen presentation and allowing the time for T cells to activate or up-regulate additional genes. Our immunohistochemical study indicated that CD1a+ and CD8+ cells are in proximity to each other with the potential for contact. Another possibility is that CXCL16 may be expressed constitutively in its membrane-bound form but that under inflammatory conditions is released in soluble form. Such a functional change would not be detected by transcript analysis and merits further study.
Our analysis of lung CD8+ T cells in COPD supports a previous report of Grumelli et al26 in favor of a Tc1 phenotype. Like them, we found that lung CD8+ T cells expressed IFNγ but little IL-4. Furthermore, the receptors that we identified, CCR5, CXCR3, and CXCR6, have been reported to be associated with Tc1 cells.46,51 This area remains controversial, for although CD4+ cells were shown to display a Th1 phenotype in COPD,31 two recent studies found CD8+ T cells in COPD to be Tc2-like, IL-4 producers.32,33 We did not identify such a population, possibly because those investigators examined bronchoalveolar lavage rather than interstitial cells.
The detection of CCR7− CD45RA+ CD8+ lung T cells revealed the presence of an effector memory (TEMRA) population. This population was probably expressing chemokine receptors and IFNγ and, as such, is a candidate for COPD-related effector cells. CCR7− CD45RA+ TEMRA cells are associated with respiratory viral infections.52,53 Viruses have been implicated in promoting COPD by inducing CD8+ T cells to differentiate into IFNγ-producing effector cells and establishing a cycle of chronic inflammation. It should be noted that not all CD8+ T cells had an effector phenotype. Although not detected by flow cytometry, RNA transcript analysis revealed that there was low expression of CCR7 among CD8+ T cells, suggesting that a population of naïve or central memory CD8+ T cells was present in the lung (data not shown).
Together, our findings suggest a means by which the CD1a+ DC and CD8+ TEMRA interaction could contribute to lung pathology as COPD progresses. Although the absence of CD83 indicates that most CD1a+ DCs in the lungs are immature, more work is necessary to confirm the activation status of these DCs. However, some studies have demonstrated that immature DCs stimulate T cells.36,54 Dumortier et al54 showed in vivo that immature antigen-presenting DCs stimulate naïve CD8+ T cells to acquire cytotoxic T-cell function and take on a central memory phenotype. Activated CD8+ T cells might cause the lung damage observed in COPD via their high levels of perforin and Fas ligand.55 We did not examine perforin, but its expression would be predicted. Sputum CD8+ T cells from COPD patients have been shown to secrete perforin.56 CXCR6/CXCL16 interactions could also contribute to lung damage. Interestingly, CXCR6+ Tc1 cells reportedly contain preformed granzyme A and are cytotoxic. Constitutive CXCL16 expression might contribute to the creation of an environment that promotes apoptosis of structural cells.57
In conclusion, we have demonstrated the potential participation of the chemokine receptors CCR5, CXCR3, and CXCR6 in the infiltration of CD8+ Tc1 effector memory cells into the lungs of COPD patients. We further show a role for CD1a+ DCs in the production of complimentary chemokines. The identified receptors represent possible therapeutic targets to ameliorate CD8+ T cell-mediated inflammation during COPD.
Footnotes
Address reprint requests to Dr. Stephen W. Chensue, Pathology and Laboratory Medicine 113, VA Ann Arbor Healthcare System, 2215 Fuller Rd., Ann Arbor, MI 48105. E-mail: schensue@med.umich.edu.
Supported by a Research Enhancement Award Program and Merit Review awards from the Biomedical Laboratory Research and Development Service, Department of Veterans Affairs; by grants A143460, HL082480, and T32 HL07749 from the United States Public Health Service; and by the Tissue Procurement Core of the University of Michigan Comprehensive Cancer Center (grant CA46952).
References
- Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-α in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- Saetta M, Turato G, Facchini FM, Corbino L, Lucchini RE, Casoni G, Maestrelli P, Mapp CE, Ciaccia A, Fabbri LM. Inflammatory cells in the bronchial glands of smokers with chronic bronchitis. Am J Respir Crit Care Med. 1997;156:1633–1639. doi: 10.1164/ajrccm.156.5.9701081. [DOI] [PubMed] [Google Scholar]
- Di Stefano A, Turato G, Maestrelli P, Mapp CE, Ruggieri MP, Roggeri A, Boschetto P, Fabbri LM, Saetta M. Airflow limitation in chronic bronchitis is associated with T-lymphocyte and macrophage infiltration of the bronchial mucosa. Am J Respir Crit Care Med. 1996;153:629–632. doi: 10.1164/ajrccm.153.2.8564109. [DOI] [PubMed] [Google Scholar]
- Grashoff WF, Sont JK, Sterk PJ, Hiemstra PS, de Boer WI, Stolk J, Han J, van Krieken JM. Chronic obstructive pulmonary disease: role of bronchiolar mast cells and macrophages. Am J Pathol. 1997;151:1785–1790. [PMC free article] [PubMed] [Google Scholar]
- Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:822–826. doi: 10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155:852–857. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Turato G, Zuin R, Miniati M, Baraldo S, Rea F, Beghe B, Monti S, Formichi B, Boschetto P, Harari S, Papi A, Maestrelli P, Fabbri LM, Saetta M. Airway inflammation in severe chronic obstructive pulmonary disease: relationship with lung function and radiologic emphysema. Am J Respir Crit Care Med. 2002;166:105–110. doi: 10.1164/rccm.2111084. [DOI] [PubMed] [Google Scholar]
- O’Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006;61:448–454. doi: 10.1136/thx.2004.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1304–1309. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164:469–473. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- Seemungal TA, Harper-Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J. 2000;16:677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio MG, Majo J. Inflammation of the airways and lung parenchyma in COPD: role of T cells. Chest. 2002;121:160S–165S. doi: 10.1378/chest.121.5_suppl.160s. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst HC, van den Broek M. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J Immunol. 2005;174:3920–3924. doi: 10.4049/jimmunol.174.7.3920. [DOI] [PubMed] [Google Scholar]
- Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol. 2005;175:3268–3272. doi: 10.4049/jimmunol.175.5.3268. [DOI] [PubMed] [Google Scholar]
- Zammit DJ, Lefrancois L. Dendritic cell-T cell interactions in the generation and maintenance of CD8 T cell memory. Microbes Infect. 2006;8:1108–1115. doi: 10.1016/j.micinf.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Robbins CS, Dawe DE, Goncharova SI, Pouladi MA, Drannik AG, Swirski FK, Cox G, Stampfli MR. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Respir Cell Mol Biol. 2004;30:202–211. doi: 10.1165/rcmb.2003-0259OC. [DOI] [PubMed] [Google Scholar]
- D’hulst AI, Maes T, Bracke KR, Demedts IK, Tournoy KG, Joos GF, Brusselle GG. Cigarette smoke-induced pulmonary emphysema in scid-mice: is the acquired immune system required? Respir Res. 2005;6:147. doi: 10.1186/1465-9921-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demedts IK, Brusselle GG, Vermaelen KY, Pauwels RA. Identification and characterization of human pulmonary dendritic cells. Am J Respir Cell Mol Biol. 2005;32:177–184. doi: 10.1165/rcmb.2004-0279OC. [DOI] [PubMed] [Google Scholar]
- Hoogsteden HC, Verhoeven GT, Lambrecht BN, Prins JB. Airway inflammation in asthma and chronic obstructive pulmonary disease with special emphasis on the antigen-presenting dendritic cell: influence of treatment with fluticasone propionate. Clin Exp Allergy. 1999;29(Suppl 2):116–124. doi: 10.1046/j.1365-2222.1999.00020.x. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P, D’Ambrosio D. Chemokines and their receptors in asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2003;9:104–110. doi: 10.1097/00063198-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, Bellettato CM, Papi A, Corbetta L, Zuin R, Sinigaglia F, Fabbri LM. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, Arrigoni G, Fabbri LM, Sinigaglia F. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, Kheradmand F. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS, GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46:798–825. [PubMed] [Google Scholar]
- Fabbri L, Pauwels RA, Hurd SS. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary updated 2003. COPD. 2004;1:105–141. doi: 10.1081/COPD-120030163. discussion 103–104. [DOI] [PubMed] [Google Scholar]
- van Haarst JM, de Wit HJ, Drexhage HA, Hoogsteden HC. Distribution and immunophenotype of mononuclear phagocytes and dendritic cells in the human lung. Am J Respir Cell Mol Biol. 1994;10:487–492. doi: 10.1165/ajrcmb.10.5.8179911. [DOI] [PubMed] [Google Scholar]
- Maestrelli P, Saetta M, Mapp CE, Fabbri LM. Remodeling in response to infection and injury: airway inflammation and hypersecretion of mucus in smoking subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:S76–S80. doi: 10.1164/ajrccm.164.supplement_2.2106067. [DOI] [PubMed] [Google Scholar]
- Majori M, Corradi M, Caminati A, Cacciani G, Bertacco S, Pesci A. Predominant TH1 cytokine pattern in peripheral blood from subjects with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 1999;103:458–462. doi: 10.1016/s0091-6749(99)70471-9. [DOI] [PubMed] [Google Scholar]
- Barceló B, Pons J, Fuster A, Sauleda J, Noguera A, Ferrer JM, Agusti AG. Intracellular cytokine profile of T lymphocytes in patients with chronic obstructive pulmonary disease. Clin Exp Immunol. 2006;145:474–479. doi: 10.1111/j.1365-2249.2006.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk A, Pierzchala W, Kon OM, Cosio B, Adcock IM, Barnes PJ. Cytokine production by bronchoalveolar lavage T lymphocytes in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2006;117:1484–1492. doi: 10.1016/j.jaci.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochand L, Isler P, Songeon F, Nicod LP. Human lung dendritic cells have an immature phenotype with efficient mannose receptors. Am J Respir Cell Mol Biol. 1999;21:547–554. doi: 10.1165/ajrcmb.21.5.3785. [DOI] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Vicari A, Lebecque S, Caux C. Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. J Leukoc Biol. 1999;66:252–262. doi: 10.1002/jlb.66.2.252. [DOI] [PubMed] [Google Scholar]
- Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- Weninger W, von Andrian UH. Chemokine regulation of naive T cell traffic in health and disease. Semin Immunol. 2003;15:257–270. doi: 10.1016/j.smim.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes [letter]. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Capelli A, Di Stefano A, Gnemmi I, Balbo P, Cerutti CG, Balbi B, Lusuardi M, Donner CF. Increased MCP-1 and MIP-1β in bronchoalveolar lavage fluid of chronic bronchitis. Eur Respir J. 1999;14:160–165. doi: 10.1034/j.1399-3003.1999.14a27.x. [DOI] [PubMed] [Google Scholar]
- Di Stefano A, Capelli A, Lusuardi M, Caramori G, Balbo P, Ioli F, Sacco S, Gnemmi I, Brun P, Adcock IM, Balbi B, Barnes PJ, Chung KF, Donner CF. Decreased T lymphocyte infiltration in bronchial biopsies of subjects with severe chronic obstructive pulmonary disease. Clin Exp Allergy. 2001;31:893–902. doi: 10.1046/j.1365-2222.2001.01098.x. [DOI] [PubMed] [Google Scholar]
- Di Stefano A, Donner CF. Severe vs. mild COPD: a different disease? Monaldi Arch Chest Dis. 2003;59:238–239. [PubMed] [Google Scholar]
- Clark-Lewis I, Mattioli I, Gong JH, Loetscher P. Structure-function relationship between the human chemokine receptor CXCR3 and its ligands. J Biol Chem. 2003;278:289–295. doi: 10.1074/jbc.M209470200. [DOI] [PubMed] [Google Scholar]
- Kim CH, Kunkel EJ, Boisvert J, Johnston B, Campbell JJ, Genovese MC, Greenberg HB, Butcher EC. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J Clin Invest. 2001;107:595–601. doi: 10.1172/JCI11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenkamp A, Nagata K, Murphy K, Wu L, Lanzavecchia A, Sallusto F. Kinetics and expression patterns of chemokine receptors in human CD4+ T lymphocytes primed by myeloid or plasmacytoid dendritic cells. Eur J Immunol. 2003;33:474–482. doi: 10.1002/immu.200310023. [DOI] [PubMed] [Google Scholar]
- Sato T, Thorlacius H, Johnston B, Staton TL, Xiang W, Littman DR, Butcher EC. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol. 2005;174:277–283. doi: 10.4049/jimmunol.174.1.277. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Nakayama T, Fukumoto N, Kume N, Takahashi S, Yamaguchi J, Minami M, Hayashida K, Kita T, Ohsumi J, Yoshie O, Yonehara S. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J Leukoc Biol. 2004;75:267–274. doi: 10.1189/jlb.1003465. [DOI] [PubMed] [Google Scholar]
- Unutmaz D, Xiang W, Sunshine MJ, Campbell J, Butcher E, Littman DR. The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J Immunol. 2000;165:3284–3292. doi: 10.4049/jimmunol.165.6.3284. [DOI] [PubMed] [Google Scholar]
- Agace WW, Roberts AI, Wu L, Greineder C, Ebert EC, Parker CM. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur J Immunol. 2000;30:819–826. doi: 10.1002/1521-4141(200003)30:3<819::AID-IMMU819>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8+ T cell responses from primary to persistent phases of Epstein-Barr virus infection. J Exp Med. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne PJ, Faint JM, Gudgeon NH, Fletcher JM, Plunkett FJ, Soares MV, Hislop AD, Annels NE, Rickinson AB, Salmon M, Akbar AN. Epstein-Barr virus-specific CD8+ T cells that re-express CD45RA are apoptosis-resistant memory cells that retain replicative potential. Blood. 2002;100:933–940. doi: 10.1182/blood-2002-01-0160. [DOI] [PubMed] [Google Scholar]
- Dumortier H, van Mierlo GJ, Egan D, van Ewijk W, Toes RE, Offringa R, Melief CJ. Antigen presentation by an immature myeloid dendritic cell line does not cause CTL deletion in vivo, but generates CD8+ central memory-like T cells that can be rescued for full effector function. J Immunol. 2005;175:855–863. doi: 10.4049/jimmunol.175.2.855. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Chrysofakis G, Tzanakis N, Kyriakoy D, Tsoumakidou M, Tsiligianni I, Klimathianaki M, Siafakas NM. Perforin expression and cytotoxic activity of sputum CD8+ lymphocytes in patients with COPD. Chest. 2004;125:71–76. doi: 10.1378/chest.125.1.71. [DOI] [PubMed] [Google Scholar]
- Agostini C, Cabrelle A, Calabrese F, Bortoli M, Scquizzato E, Carraro S, Miorin M, Beghe B, Trentin L, Zambello R, Facco M, Semenzato G. Role for CXCR6 and its ligand CXCL16 in the pathogenesis of T-cell alveolitis in sarcoidosis. Am J Respir Crit Care Med. 2005;172:1290–1298. doi: 10.1164/rccm.200501-142OC. [DOI] [PubMed] [Google Scholar]