Abstract
Protease-activated receptor-2 (PAR-2) is a cellular receptor expressed prominently on epithelial, mesangial, and endothelial cells in the kidney and on macrophages. PAR-2 is activated by serine proteases such as trypsin, tryptase, and coagulation factors VIIa and Xa. It induces pleiotropic effects including vasodilatation, increasing plasminogen activator inhibitor (PAI-1) expression, mesangial cell proliferation, and cytokine production by macrophages. The role of PAR-2 in renal inflammation was studied in antiglomerular basement membrane antibody-induced crescentic glomerulonephritis (CGN) using PAR-2-deficient (PAR-2−/−) mice and wild-type littermate controls. PAR-2−/− mice had reduced crescent formation, proteinuria, and serum creatinine compared with wild-type mice 21 days after initiation of CGN. Glomerular accumulation of CD4+ T cells and macrophages and the number of proliferating cells in glomeruli were similar in both groups. Glomerular fibrin deposition was significantly reduced in PAR-2−/− mice, and this was associated with reduced renal plasminogen activator inhibitor expression and increased renal matrix-metalloprotinase-9 activity. These results demonstrate a proinflammatory role for PAR-2 in CGN that is independent of effects on glomerular leukocyte recruitment and mesangial cell proliferation. PAR-2-mediated augmentation of renal plasminogen activator inhibitor expression and inhibition of matrix-metalloprotinase-9 activity may contribute to increased glomerular fibrin accumulation and glomerular injury in CGN.
Protease-activated receptor 2 (PAR-2) is a member of a unique family of seven transmembrane domain G protein-coupled receptors that is activated by proteolytic cleavage of its extracellular N-terminal domain. PAR-2 is activated by serine proteases such as trypsin, tryptase,1,2 and coagulation factors VIIa and Xa.3 Proteolytic cleavage results in exposure of a new N terminus that acts as a tethered ligand that binds and activates the receptor. PAR-2 is abundantly expressed in various organs including the respiratory tract,4 gastrointestinal tract, brain, epidermis,5 pancreas, liver, and kidney6 and on a variety of cell types including endothelial cells,7 epithelial cells, smooth muscle cells,6 neurons, astrocytes,5 T cells,8 neutrophils in humans,9 and macrophages.10 PAR-2 gene expression can be induced on cultured human umbilical vein endothelial cells in response to stimulation by interleukin-1α, tumor necrosis factor-α, or lipopolysaccharide.11
A number of physiological and pathological roles have been demonstrated for PAR-2 in the cardiovascular system, nervous system, gastrointestinal tract, respiratory system, and the skin.12 PAR-2 induces endothelium-dependent vasodilatation in various vascular beds.13,14,15,16 In the kidney, PAR-2 induces both endothelium-dependent and -independent vasodilatation of afferent renal arteries.7,17 Activation of PAR-2 has been shown to induce proliferation of vascular and airway smooth muscle cells18,19 and of renal mesangial cells in vitro.20
In addition to its physiological roles, PAR-2 expression is up-regulated during inflammation and modulates inflammatory responses. Both proinflammatory and protective roles for PAR-2 have been demonstrated using agonist peptides in various models of respiratory12 and colonic inflammation.21,22 PAR-2-deficient mice are protected from allergic airway inflammation,23 tryptase and PAR-2 agonist peptide-induced colonic inflammation,24 and adjuvant-induced arthritis,25 indicating proinflammatory effects of PAR-2. However, PAR-2-deficient mice are more susceptible to acute pancreatitis,26 and administration of PAR-2 inhibitory antibody reduces acute lung injury in a combined pancreatitis/endotoxemia model of systemic inflammation,27 suggesting a protective role for PAR-2 in these models. In systemic inflammation induced by endotoxin challenge, PAR-2-deficient mice do not show any survival advantage,28,29,30 although PAR-2-activating peptides aggravate hypotension following endotoxin challenge.31
The contribution of PAR-2 to renal inflammation is largely unexplored. In IgA nephropathy, elevated expression of PAR-2 mRNA and protein has been demonstrated in proximal tubular cells, infiltrating interstitial mononuclear cells, and glomerular parietal epithelial cells. PAR-2 expression correlated directly with the severity of the interstitial infiltrate and serum creatinine.32 In addition, PAR-2 induces plasminogen activator inhibitor-1 (PAI-1) and transforming growth factor (TGF)-β expression by proximal tubular cells and mesangial cells in vitro.32 To examine the contribution of PAR-2 to renal inflammation in vivo, we used a model of crescentic glomerulonephritis (CGN) in mice in which injury is mediated through a Th1-dependent delayed-type hypersensitivity-like mechanism.33 CD4+ T cell-directed macrophage recruitment and prominent local deposition of fibrin34 due to augmented tissue factor (TF) activity are characteristic of Th1 cell-mediated effector responses in CGN. Our studies show that PAR-2-deficient (PAR-2−/−) mice demonstrate significant protection from renal inflammation associated with reduced glomerular fibrin deposition and renal PAI-1 expression and augmented matrix metalloproteinase-9 (MMP-9) activity.
Materials and Methods
Induction of Glomerulonephritis
PAR-2−/− mice derived on a mixed 129/SvJ and C57BL/6 background were obtained from Dr. Shaun Coughlin35 (University of California, San Francisco, CA) and were bred with C57BL/6 mice to obtain heterozygotes (PAR-2+/−). PAR-2−/− mice and PAR-2+/+ (wild type; WT) littermate controls for experiments were bred from heterozygote parents and genotyped by polymerase chain reaction (PCR). The experimental groups consisted of 13 PAR-2−/− and 13 WT male and female mice. Glomerulonephritis (GN) was induced in mice 9 to 10 weeks of age by intravenous injection of 18 mg of sheep anti-mouse glomerular basement membrane globulin as previously described36 and tissues were collected for analysis 21 days later.
Assessment of Functional Injury
Mice were housed in metabolic cages for 24-hour periods to collect urine before initiation of disease and on days 1, 14, and 21 of the model. Urinary protein levels were measured using a modified Bradford’s assay as previously described.37 At the end of the experiment blood was allowed to clot overnight at 4°C to collect serum. Creatinine levels were measured using an enzymatic creatininase assay.
Histological Assessment of Glomerular Injury
Glomerular Crescent Formation
Kidney tissue was fixed in Bouin’s fixative and embedded in paraffin, 3-μm sections were stained with periodic acid-Schiff’s reagent, and crescent formation was assessed by light microscopy in a blinded protocol. Glomeruli were considered to exhibit crescent formation when two or more layers of cells were observed in Bowman’s space. A minimum of 50 glomeruli was assessed to determine the crescent score for each animal.
Assessment of Glomerular Basement Membranes and Mesangial Matrix
Bouin’s-fixed, paraffin-embedded (1 μm) tissue sections were stained by the silver methenamine technique. Slides were incubated in 1% periodic acid for 15 minutes followed by 5% silver nitrate/3% methenamine/5% sodium borate solution for 1 hour at 60°C and then counterstained with hematoxylin and eosin. Sections were assessed for basement membrane integrity and amount of mesangial matrix by light microscopy by an experienced pathologist, using a blinded protocol.
Glomerular T Cell and Macrophage Accumulation
Kidney tissue was fixed in periodate-lysine paraformaldehyde, sectioned, and stained to demonstrate CD4+ T cells and macrophages using monoclonal antibodies GK1.5 and FA/11, respectively, and a three-layer immunoperoxidase technique as previously described.38 Positively stained cells in glomeruli were counted using a blinded protocol in a minimum of 20 equatorially sectioned glomeruli per animal. Results are expressed as cells per glomerular cross section.
Detection of Proliferating Cells in Glomeruli
Snap-frozen kidneys were sectioned, and endogenous avidin and biotin were blocked using a blocking kit (DAKO, Carpinteria, CA) followed by incubation with phosphate-buffered saline plus 10% rabbit serum and 5% bovine serum albumin. The Ki-67 antibody (DAKO) was incubated overnight at 4°C and then with a biotinylated rabbit anti-rat secondary antibody for 1 hour. Endogenous peroxidases were blocked using 0.5% H2O2 in methanol, avidin-biotinylated enzyme complex solution (DAKO) was applied for 30 minutes, and staining was developed using diaminobenzidine brown (Sigma, St. Louis, MO). The numbers of proliferating cells in glomeruli were assessed in a blinded protocol as described above, and results were expressed as cells per glomerular cross section.
Fibrinogen Staining
Bouin’s-fixed, paraffin-embedded kidney tissues were stained for fibrinogen using a rabbit anti-mouse fibrinogen antibody (a gift of Dr. J. Degen, Childrens Research Foundation, Cincinnati, OH), a biotinylated secondary antibody, and incubation with avidin-biotinylated enzyme complex solution as previously described.34 Fibrinogen staining was scored on a 0 to 3 scale using a blinded protocol on a minimum of 50 glomeruli. A score of 0 was given to glomeruli with little or no staining, a score of 1 was given if fibrin was deposited in at least 25% of the glomerulus, a score of 2 was given if fibrin was deposited in at least 50% of the glomerulus, and a score of 3 was given when more than 75% of the glomerulus was positive for fibrin.
Measurement of Mouse Anti-Sheep Globulin IgG Levels
Circulating antibodies to the nephritogenic antigen (mouse anti-sheep globulin IgG) were measured by enzyme-linked immunosorbent assay (ELISA). Microtiter plates (Greiner, Longwood, FL) were coated overnight with 10 μg/ml normal sheep globulin, and mouse serum was added in serial dilutions of 1:50 to 1:1600. After incubation for 1 hour at 37°C, bound mouse IgG was detected by incubation with peroxidase-conjugated sheep anti-mouse IgG (1 in 2000), followed by tetramethylbenzadine solution (Sigma) as a chromogenic substrate. The reaction was stopped using 0.1 mol/L H2SO4, and absorbances were measured at 450 nm on a microplate reader (Bio-Rad, Hercules, CA).
Determination of TF mRNA Levels
Isolation of RNA
One milliliter of TRIzol reagent (Invitrogen, Carlsbad, CA) was added to 50- to 100-μg pieces of frozen kidney and homogenized using an electric homogenizer (Kinematica, Littan/Lucerne, Switzerland). Samples were incubated at room temperature for 5 minutes, and 0.2 ml of chloroform was added, mixed for 15 seconds, and incubated for 2 to 3 minutes at room temperature. Samples were spun at 12,000 × g for 15 minutes at 4°C, and 0.5 ml of isopropanol was added, incubated for 10 minutes at room temperature, and spun at 12,000 × g for 10 minutes. The RNA was washed with 1 ml of 75% ethanol in RNase/DNase-free water, spun at 7500 × g for 5 minutes, air-dried, resuspended in water, and stored at −70°C for later use.
Reverse Transcription-PCR
Five micrograms of RNA, 150 ng of hexamer, and 1 mmol/L deoxynucleoside-5′-triphosphate were incubated at 65°C for 5 minutes and cooled on ice for 1 minute. Reverse transcriptase buffer, 12 mmol/L MgCl2, 0.044 mol/L dithiothreitol, and 1 μl of RNase-out (Invitrogen) was added and kept at room temperature for 2 minutes. Superscript III (50 units) was added and incubated for 10 minutes at room temperature. The tubes were then incubated for 50 minutes at 42°C, then for 15 minutes at 70°C, and then chilled on ice.
Real-Time PCR
Primers to mouse TF (forward: 5′-GAAACTGGAAAAACAAGTGCTTCT-3′; reverse: 5′-CCAGGTCACATCCTTCACGAT-3′) were used to amplify an 80-bp fragment coding the extracellular domain of TF. The optimal temperature ramp cycle was 95°C for 5 seconds, 60°C for 5 seconds, and 72°C for 15 seconds for 40 cycles using a Rotor Gene 3000 light cycler (Corbett, Sydney, NSW, Australia). The final PCR reactions contained 0.5 μmol/L primers, 1 μl of SYBR Green Mastermix (Roche, Indianapolis, IN), and made to a volume of 10 μl with water. A standard curve using plasmid cDNA of the TF gene allowed quantitation, and values were normalized using β-actin as a housekeeping gene.
Determination of Nitric Oxide Production
Renal and systemic NO production was determined by measuring nitrite (a stable product of NO) in 24-hour urine collections and serum using the Griess assay. The Griess reagent was made by mixing equal quantities of 1.5% sulfanilamide in 1 mol/L HCl and 0.15% naphthyl ethylenediamine. One hundred microliters of Griess reagent was combined with 100 μl of sample or sodium nitrite standards, and the chromogenic reaction product was measured by its absorbance at 540 nm. Urinary nitrite production was expressed as micromoles/24 hours by adjustment according to the volume of the 24 hours’ urine collection.
Renal PAI-1 Determination
Mouse PAI-1 was measured by ELISA as previously described.39 Kidney tissue stored at −70°C was homogenized in 1 ml of lysis buffer (0.1 mol/L Tris-HCl, pH 7.5, 0.15 mol/L NaCl, and 1% Triton X-100). Samples were spun at 13,000 × g for 10 minutes at 4°C and the supernatant kept for analysis by ELISA. Plates were coated with a monoclonal anti-murine PAI-1 antibody (clone H34G6). After washing, samples and recombinant mouse PAI-1 standards (a generous gift of Dr. Paul Declerk, Katholieke Universiteit, Leuven, Belgium) were incubated overnight at 4°C. Bound antigen was detected with a biotinylated anti-murine PAI-1 monoclonal antibody (clone H14H7), and color was developed using tetramethylbenzadine solution. The reaction was stopped using 0.1 mol/L H2SO4, absorbances were measured at 450 nm on a microplate reader (Bio-Rad), and unknowns were calculated using the inbuilt software.
Renal MMP Activity
Renal MMP activity was determined by gelatin zymography as described previously.40 In brief, kidney protein extracts were prepared as described above, and 20 μg of protein from each sample was diluted in 4× sodium dodecyl sulfate loading buffer (200 mmol/L Tris, pH 6.8, 2% sodium dodecyl sulfate, 0.1% bromphenol blue, and 40% glycerol) and loaded onto 10% polyacrylamide gels containing 0.01% gelatin. Gels were run for approximately 1 hour at 180 V (constant voltage), developed overnight at 37°C in developing buffer (50 mmol/L Tris, pH 7.4, 5 mM CaCl2, 200 mmol/L NaCl2, and 0.02% NaN3), fixed for 15 minutes in water/methanol/acetic acid solution (6:3:1 ratio), and then stained for 1 hour with 0.15% Coomassie Blue R-250 in water/methanol/acetic acid (4.5:4.5:1 ratio). Gels were transferred to water/methanol/acetic acid solution (6:3:1 ratio) to allow gradual destaining and MMP bands were identified by reference to an MMP control sample derived from cell culture medium from phorbol 12-myristate 13-acetate-stimulated human skin fibroblasts (catalog no. M 2928; Sigma). MMP activity was determined by densitometry analysis of each band using computer-assisted image analysis software (Multi-Gauge version 3.0 software). Calcium dependence of gelatinase activity was demonstrated by its inhibition by addition of 5 mmol/L ethylenediamine tetraacetic acid to duplicate gels.
Renal TGF-β1 Measurement
TGF-β1 content of kidney protein extracts was measured using a mouse TGF-β1 ELISA kit (DuoSet; R&D Systems Inc., Minneapolis, MN). Kidney extracts were diluted 1 to 2 in lysis buffer, activated with acetic acid/urea, and assayed according to the manufacturer’s instructions. Plates were read using a microplate reader (Bio-Rad) at 450 nm, and TGF-β1 concentrations were calculated from the standard curve by the plate reader software. Concentrations in individual renal extracts were standardized according to their total protein content.
Statistical Analysis
Results are expressed as the mean ± SEM (SEM). Data were demonstrated to be normally distributed, and statistical comparisons between groups were performed using an unpaired two-tailed Student’s t-test (GraphPad Prism; GraphPad Software Inc., San Diego, CA) for two group comparisons. Proteinuria data were analyzed by analysis of variance, followed by Tukey’s multiple comparison test. P < 0.05 was considered to indicate a statistically significant difference between groups.
Results
PAR-2-Deficient Mice Are Protected from Development of CGN
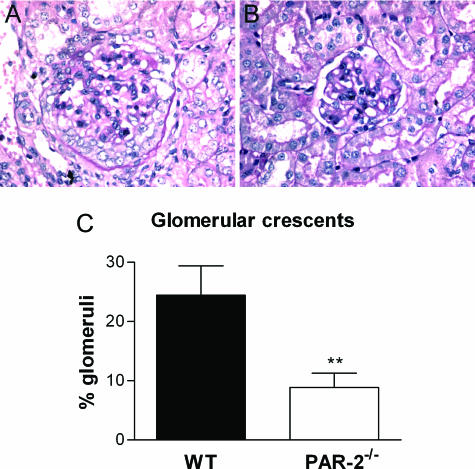
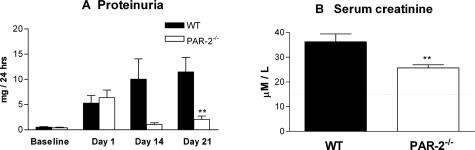
PAR-2−/− mice showed significant protection from development of CGN by both histological and functional assessments. Histological assessment, 21 days after initiation of GN, showed that PAR-2−/− mice had less severe glomerular injury (Figure 1, A and B) with significantly reduced glomerular crescent formation (PAR-2−/−, 8.9 ± 2.4% of glomeruli affected; WT, 24.5 ± 4.9% of glomeruli affected; P < 0.01) (Figure 1C), although there were no qualitative differences in basement membrane integrity or mesangial matrix compared with WT mice. PAR-2−/− mice were also protected from functional injury as determined by significant reductions in proteinuria (PAR-2−/−, 2.1 ± 0.7 mg/24 hours; WT, 11.5 ± 2.9 mg/24 hours; P < 0.01) (Figure 2A) and reduced serum creatinine (PAR-2−/−, 25.7 ± 1.3 μmol/L; WT, 36.3 ± 3.2 μmol/L; P < 0.01) (Figure 2B) at day 21 of disease.
Figure 1.
Photomicrographs demonstrating typical glomerular histology of CGN in WT (A) and PAR-2-deficient (B) mice on day 21 of the disease (periodic acid-Schiff-stained sections; magnification, ×200). PAR-2-deficient mice developed significantly fewer crescentic glomeruli than WT mice at day 21 (C, **P < 0.01).
Figure 2.
Functional assessment of renal injury. PAR-2-deficient mice had significantly less proteinuria on day 21 of disease (A, **P < 0.01). Serum creatinine on day 21 was reduced in PAR-2-deficient mice compared with WT mice (**P < 0.01). The dotted line represents the mean serum creatinine in normal mice (B).
Production of Nephritogenic Antibodies and Glomerular Effector Cell Recruitment Are Unaffected in PAR-2-Deficient Mice
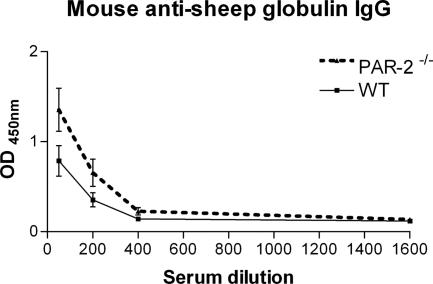
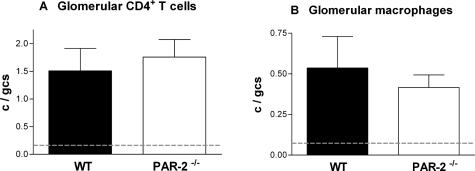
The humoral immune response to the nephritogenic antigen (sheep globulin) indicated by circulating levels of autologous mouse anti-sheep globulin IgG were not significantly different between PAR-2-deficient and WT mice (Figure 3). In addition, glomerular recruitment of the cellular effectors of crescentic glomerular injury (CD4+ T cells: PAR-2−/−, 1.8 ± 0.3 cells per glomerular cross section; WT, 1.5 ± 0.4 cells per glomerular cross section; and macrophages: PAR-2−/−, 0.4 ± 0.1 cells per glomerular cross section; WT, 0.5 ± 0.2 cells per glomerular cross section) was similar in both groups indicating that the cellular immune response was unaffected by the absence of PAR-2 (Figure 4).
Figure 3.
Serum levels of autologous mouse anti-sheep globulin IgG, measured by ELISA, were not significantly different between PAR-2-deficient and WT mice on day 21 of diseases over a range of dilutions from 1:50 to 1:1600.
Figure 4.
Glomerular accumulation of CD4+ T cells demonstrated by staining with mAb GK1.5 (A) and CD68+ macrophages demonstrated by staining with mAb FA/11 (B) was not different in PAR-2-deficient and WT mice on day 21 of disease. The dotted lines represent the mean glomerular T cell and macrophage numbers in normal mice.
Glomerular NO Levels Are Unaffected in PAR-2-Deficient Mice
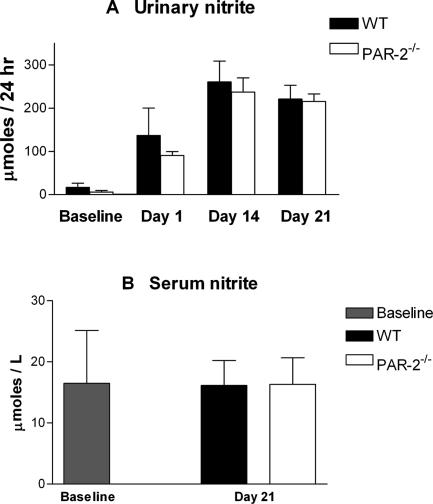
Urinary nitrite in GN reflects production of nitric oxide by glomerular endothelium and recruited macrophages. The vasodilatory effects of PAR-2 in the glomerular afferent arterioles are (in part) mediated by inducing NO production and expression.41 Activated macrophages are also a potent source of urinary nitric oxide. Urinary nitrite was measured at days 14 and 21 to assess the effects of PAR-2 deficiency on glomerular NO production by endothelial cells and macrophages. No difference was found between urinary nitrite levels in PAR-2-deficient and WT mice at any time point (Figure 5A), suggesting that the net NO production by macrophages and endothelial cells in nephritic glomeruli is unaffected by PAR-2 deficiency. Serum nitrite levels were not increased above normal in mice with GN (Figure 5B), reflecting the absence of significant systemic NO generation and inflammatory response in this model.
Figure 5.
Urinary nitrite levels were similar in PAR-2-deficient and WT mice on days 14 and 21 of disease, indicating that PAR-2 deficiency did not affect renal NO production during development of CGN (A). Serum nitrite levels were not increased above baseline in either WT or PAR-2-deficient mice with CGN (B).
Glomerular Cell Proliferation Is Unaffected in PAR-2-Deficient Mice
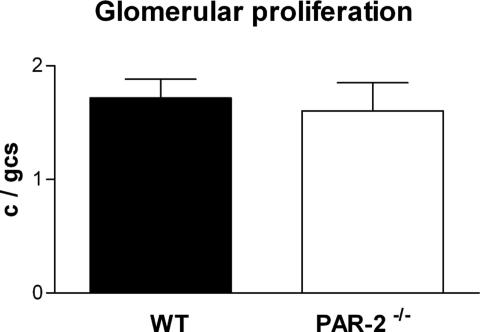
Because PAR-2 agonist peptides have been reported to stimulate mesangial cell proliferation in vitro,20 the effect of PAR-2 deficiency on the numbers of proliferating cells in glomeruli was assessed by staining for Ki-67. PAR-2-deficient and WT mice showed similar numbers of proliferating cells in nephritic glomeruli (Ki-67-positive cells: PAR-2−/−, 1.7 ± 0.16 cells per glomerular cross section; WT, 1.6 ± 0.25 cells per glomerular cross section), indicating no significant effect of PAR-2 on mesangial cell proliferation in vivo (Figure 6).
Figure 6.
The numbers of proliferating cells in glomeruli (demonstrated by staining using the Ki-67 antibody) was similar in PAR-2-deficient and WT mice with CGN on day 21.
PAR-2-Deficient Mice Have Reduced Glomerular Fibrin Deposition and Renal PAI-1 Expression
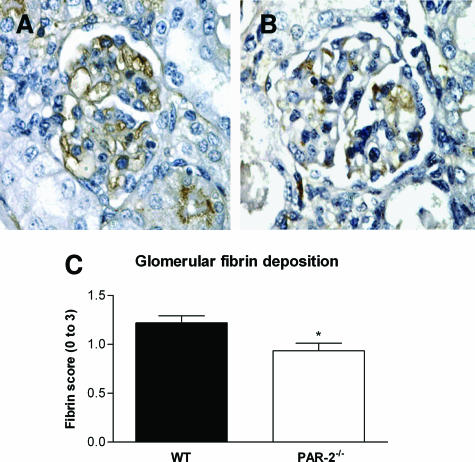
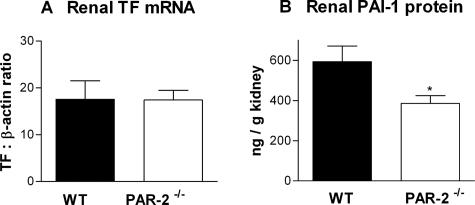
As studies in fibrinogen-deficient mice have demonstrated that fibrin is an important mediator of injury in murine CGN,34 glomerular fibrin deposition was assessed by immunohistological staining. Glomerular fibrin deposition was significantly reduced in PAR-2-deficient mice 21 days after initiation of GN (fibrin score: PAR-2−/−, 0.9 ± 0.1; WT, 1.2 ± 0.1; P < 0.05) (Figure 7, A and B). Reduced fibrin deposition could account for the reduction of histological and functional injury in PAR-2-deficient mice. To explore the reasons for reduced glomerular fibrin deposition, renal TF mRNA and PAI-1 protein were measured by real time PCR and ELISA, respectively. TF is the major initiator of coagulation in vivo and plays a pivotal role in initiation of fibrin deposition in crescentic GN.42 However, no difference in renal TF mRNA expression was observed between PAR-2-deficient and WT mice with CGN (PAR-2−/−, 17.4 ± 2.1 pg of TF/pg of β-actin mRNA; WT, 17.6 ± 4.0 pg of TF/pg of β-actin mRNA; P > 0.98) (Figure 8A). PAI-1 inhibits fibrinolysis and augments net fibrin deposition by inhibiting plasminogen activators, urokinase, and tissue-type plasminogen activator. Deficiency of PAI-1 has previously been shown to be protective in murine CGN, and overexpression was shown to augment injury.39 In the current studies, PAR-2-deficient mice showed significantly reduced renal PAI-1 expression compared with WT mice (PAR-2−/−, 386 ± 38 pg of PAI-1/mg of kidney; WT, 593 ± 78 pg of PAI-1/mg of kidney; P < 0.05) (Figure 8B). Reduced renal PAI-1 expression in PAR-2-deficient mice could contribute to augmented fibrin deposition and CGN in this model.
Figure 7.
Immunoperoxidase staining with a rabbit anti-mouse fibrinogen antibody (magnification, ×200) demonstrated that glomerular fibrin deposition was substantially reduced on day 21 of CGN in PAR-2-deficient mice (B) compared with WT mice (A). Semiquantitative scoring demonstrated a significant reduction in glomerular fibrin in PAR-2-deficient mice (C, *P < 0.05).
Figure 8.
Renal TF mRNA, quantified by real-time PCR, demonstrated no difference in renal TF gene expression between PAR-2-deficient and WT mice on day 21 of CGN (A). Renal PAI-1 levels measured by ELISA were significantly reduced in the PAR-2-deficient mice compared with WT mice at the same time point of CGN (B, *P < 0.05).
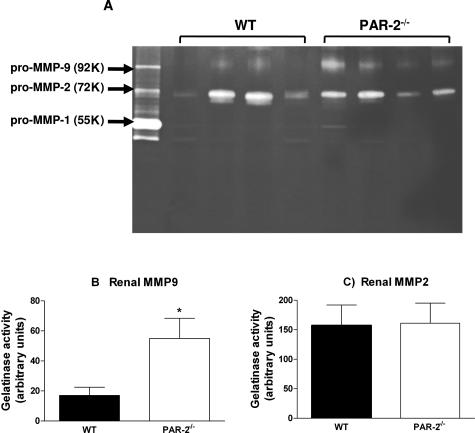
PAR-2 Deficiency Enhances Renal MMP-9 Activity in CGN but Does Not Affect TGF-β1 Expression
Because PAI-1 deficiency has been shown to increase MMP-9 activity in airway inflammation,43 the effect of PAR-2 deficiency and reduced PAI-1 on renal MMP activity in CGN was assessed (Figure 9A). MMP-9 activity (molecular weight 92,000) was clearly detectable and was significantly greater in PAR-2-deficient mice compared with WT mice (MMP-9 activity/20 μg of kidney extract: PAR-2−/−, 55.0 ± 13.4; WT, 17 ± 5.5, P < 0.02) (Figure 9B). Strong lytic activity at 72 kd corresponding to MMP-2 was detected at similar levels in both WT and PAR-2-deficient mice developing CGN (MMP-2 activity/20 μg of kidney extract: PAR-2−/−, 161 ± 34; WT, 157 ± 35) (Figure 9C). Weak bands corresponding to MMP-1 activity were detected at 55,000 and 52,000, but these were not different between WT or PAR-2-deficient mice. TGF-β1 protein in renal extracts were similar in PAR-2-deficient and WT mice at day 21 of CGN (PAR-2−/−, 172 ± 31 pg of TGF-β1/mg of kidney; WT, 185 ± 19 pg of TGF-β1/mg of kidney).
Figure 9.
A gelatin zymogram demonstrating MMP-9, MMP-2, and MMP-1 activity in supernatant from phorbol 12-myristate 13-acetate-stimulated fibroblasts (left lane) and renal tissue extracts from four WT and four PAR-2-deficient mice with CGN at day 21 (A). The molecular weights of standards and samples were determined by comparison with protein molecular weight markers (GE Healthcare, Chalfont, Buckinghamshire, UK). MMP-9 activity quantified by densitometry was significantly greater in PAR-2-deficient mice compared with WT mice with CGN (B, *P < 0.05, n = 8 per group). MMP-2 activity was not significantly different in WT and PAR-2-deficient mice with CGN (C).
Discussion
This study demonstrates that PAR-2-deficient mice are protected from histological and functional renal injury in CGN. Crescentic renal injury in this murine model of GN has been demonstrated to be mediated principally by Th1-dependent cellular immune mechanisms44 and can develop in the absence of adaptive humoral immunity.45 In PAR-2-deficient mice, a reduction in crescentic injury was not associated with attenuation of circulating levels of autologous nephritogenic antibody or any reduction on glomerular accumulation of CD4+ T cells, suggesting that protection was not mediated through attenuation of the nephritogenic adaptive humoral or cellular immune response. Furthermore, glomerular macrophage accumulation was similar in PAR-2-deficient mice and WT mice with GN, suggesting that mechanisms of recruitment of the cellular effectors of glomerular injury are not affected by PAR-2 deficiency. This finding differs from the observations of the effects of PAR-1 deficiency in a similar model of CGN, where protection from crescentic renal injury was associated with reduced recruitment of CD4+ T cells and macrophages into glomeruli.46
In an attempt to define the mechanisms of PAR-2-mediated injury in this model of CGN, we explored aspects of renal pathobiology reported to be influenced by PAR-2, including mesangial cell proliferation20 and nitric oxide-mediated vasodilatation of afferent arterioles.47 We quantified proliferating glomerular cell numbers in PAR-2-deficient and WT mice with CGN and found no differences, suggesting that effects on mesangial cell proliferation are unlikely to explain the injurious role of PAR-2 in this model of CGN. Similarly, urinary nitrite levels measured on days 14 and 21 during the development of CGN were identical in PAR-2-deficient and WT mice. In this model, NO is likely to be derived locally from the endothelium (controlling vascular tone) and from activated macrophages in glomeruli. Serum levels of NO were not elevated, consistent with the absence of a systemic inflammatory response in this disease. The lack of effect of PAR-2 deficiency on urinary nitrite suggests that net NO production by the endothelium and activated macrophages is not significantly influenced by PAR-2.
Glomerular fibrin deposition is a prominent feature of human CGN and is associated with severe renal injury.48,49,50,51 In experimental CGN in rabbits, defibrination is protective,52 and in this murine anti-GBM antibody-induced model, genetic deletion of fibrinogen also affords significant protection from crescent formation and functional injury.34 In the current studies, glomerular fibrin deposition was significantly reduced in PAR-2-deficient mice with CGN, and this could, at least in part, account for the observed reduction in crescent formation and protection of renal function. To investigate the mechanisms of reduced fibrin deposition, we examined molecules with major effects on the procoagulant and fibrinolytic environment in the kidney. TF is the major initiator of coagulation in vivo. TF is abundantly expressed by infiltrating macrophages and intrinsic glomerular cells in CGN and inhibition of TF significantly prevents glomerular fibrin deposition and renal injury.42 However, renal TF mRNA expression in PAR-2-deficient mice was similar to WT mice with CGN, suggesting that the procoagulant environment in glomeruli is equivalent and that PAR-2 is not a key modulator of TF expression by infiltrating macrophages or intrinsic glomerular cells in this model.
PAI-1 is a major regulator of fibrinolysis via its capacity to inhibit the key fibrinolytic molecules (tissue-type plasminogen activator and urokinase). In murine CGN, deficiency of PAI-1 has been shown to be protective and overexpression to exacerbate renal injury.39 Demonstration of the capacity of PAR-2 agonists to induce dose-dependent increases in PAI-1 expression by cultured proximal tubular cells in vitro (without altering urokinase expression)32 raises the prospect that changes in renal PAI-1 expression may contribute to reduced glomerular fibrin deposition in the absence of PAR-2 stimulation. Our results demonstrate significantly reduced renal PAI-1 protein in PAR-2-deficient mice with CGN. Augmented fibrinolysis due to reduced PAI-1 expression together with an unchanged glomerular procoagulant environment could account for reduced glomerular fibrin accumulation in PAR-2-deficient mice.
MMP-2 and MMP-9 are gelatinases that degrade basement membrane collagens types IV and V. Glomerular expression of MMP-9 is increased in rats developing CGN53 and in human mesangial proliferative GN.54 Impaired expression of MMP-9 has been suggested to contribute to mesangial expansion in rat models of GN,55 and CGN is augmented in MMP-9-deficient mice, due to impaired fibrinolysis.56 Our results showed significantly augmented renal MMP-9 activity in PAR-2-deficient mice with CGN but no change in MMP-2 activity. Histological assessment of glomerular basement membrane integrity and mesangial matrix did not reveal any qualitative differences between WT and PAR-2-deficient mice with GN. These results are consistent with a protective role for MMP-9 through its potential to directly augment fibrinolysis.56
It is likely that increased renal MMP-9 activity in PAR-2-deficient mice with CGN is due to their decreased PAI-1 expression, as PAI-1 deficiency has been shown to increase lung MMP-9 activity in a murine model of asthma.43 In addition to its ability to augment PAI-1 expression in renal tubular and mesangial cells in vitro, PAR-2 activation can also augment TGF-β.32 However, in the current study, renal TGF-β levels were unaffected in PAR-2-deficient mice with CGN.
Local activation of coagulation by TF plays an important proinflammatory role in CGN by generating activated coagulation factors VIIa, Xa, and thrombin, which result in glomerular fibrin deposition. In addition, these activated coagulation factors have the capacity to augment renal injury by activating protease-activated receptors PAR-1 and PAR-2. Proinflammatory effects of PAR-1 in CGN have been previously demonstrated, and the current studies provide the first in vivo demonstration of a proinflammatory role for PAR-2 in renal inflammation. Protection from CGN in the absence of PAR-2 in this murine model was associated with reduced crescent formation, reduced functional renal injury, and less accumulation of fibrin in glomeruli. The reduction in glomerular fibrin deposition may be attributable to augmented fibrinolysis resulting from reduced renal expression of PAI-1 and augmented MMP-9 activity. Activation of PAR-2 by locally activated coagulation factors resulting in augmentation of PAI-1 and subsequent inhibition of MMP-9 may be an additional important pathway for the injurious effects of TF in CGN.
Acknowledgments
Breeding stock to establish our PAR-2-deficient mouse colony was generously provided by Prof. Shaun Coughlin. The assistance of Dr. Anthony Longano with assessment of renal histology is gratefully acknowledged.
Footnotes
Address reprint requests to Prof. Peter Tipping, Monash University Department of Medicine, Level 5, Block E, Monash Medical Centre, 246 Clayton Rd., Clayton, 3168, VIC, Australia. E-mail: peter.tipping@med.monash.edu.au.
Supported by a program grant from the National Health and Medical Research Council of Australia.
References
- Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X- dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, Henry PJ, Carr MJ, Hamilton JR, Moffatt JD. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- D’Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade-Gordon P. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- Bohm SK, Kong W, Bromme D, Smeekens SP, Anderson DC, Connolly A, Kahn M, Nelken NA, Coughlin SR, Payan DG, Bunnett NW. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem J. 1996;314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt JD, Cocks TM. Endothelium-dependent and -independent responses to protease-activated receptor-2 (PAR-2) activation in mouse isolated renal arteries. Br J Pharmacol. 1998;125:591–594. doi: 10.1038/sj.bjp.0702157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari B, Guerin S, Far DF, Breitmayer JP, Belhacene N, Peyron JF, Rossi B, Auberger P. Thrombin and trypsin-induced Ca2+ mobilization in human T cell lines through interaction with different protease-activated receptors. FASEB J. 1996;10:309–316. doi: 10.1096/fasebj.10.2.8641564. [DOI] [PubMed] [Google Scholar]
- Howells GL, Macey MG, Chinni C, Hou L, Fox MT, Harriott P, Stone SR. Proteinase-activated receptor-2: expression by human neutrophils. J Cell Sci. 1997;110:881–887. doi: 10.1242/jcs.110.7.881. [DOI] [PubMed] [Google Scholar]
- Déry O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Ramakrishnan V, Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells: comparison with the thrombin receptor. J Biol Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kubo S, Nakaya Y, Ishiki T, Kuroda R, Sekiguchi F, Kawao N, Nishikawa H. Distinct roles for protease-activated receptors 1 and 2 in vasomotor modulation in rat superior mesenteric artery. Cardiovasc Res. 2004;61:683–692. doi: 10.1016/j.cardiores.2003.11.030. [DOI] [PubMed] [Google Scholar]
- McGuire JJ, Hollenberg MD, Andrade-Gordon P, Triggle CR. Multiple mechanisms of vascular smooth muscle relaxation by the activation of proteinase-activated receptor 2 in mouse mesenteric arterioles. Br J Pharmacol. 2002;135:155–169. doi: 10.1038/sj.bjp.0704469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean PG, Aston D, Sarkar D, Ahluwalia A. Protease-activated receptor-2 activation causes EDHF-like coronary vasodilation: selective preservation in ischemia/reperfusion injury: involvement of lipoxygenase products: VR1 receptors, and C-fibers. Circ Res. 2002;90:465–472. doi: 10.1161/hh0402.105372. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hirano K, Nishimura J, Takahashi S, Kanaide H. Mechanism of trypsin-induced endothelium-dependent vasorelaxation in the porcine coronary artery. Br J Pharmacol. 2001;134:815–826. doi: 10.1038/sj.bjp.0704318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hollenberg MD, Loutzenhiser R. Redundant signaling mechanisms contribute to the vasodilatory response of the afferent arteriole to proteinase-activated receptor-2. Am J Physiol. 2005;288:F65–F75. doi: 10.1152/ajprenal.00194.2004. [DOI] [PubMed] [Google Scholar]
- Koo BH, Chung KH, Hwang KC, Kim DS. Factor Xa induces mitogenesis of coronary artery smooth muscle cell via activation of PAR-2. FEBS Lett. 2002;523:85–89. doi: 10.1016/s0014-5793(02)02948-4. [DOI] [PubMed] [Google Scholar]
- Chambers LS, Black JL, Poronnik P, Johnson PR. Functional effects of protease-activated receptor-2 stimulation on human airway smooth muscle. Am J Physiol. 2001;281:L1369–L1378. doi: 10.1152/ajplung.2001.281.6.L1369. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Arai H, Liu N, Nogaki F, Nomura K, Kasuno K, Oida E, Kita T, Ono T. Role of coagulation factor Xa and protease-activated receptor 2 in human mesangial cell proliferation. Kidney Int. 2005;67:2123–2133. doi: 10.1111/j.1523-1755.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- Cenac N, Garcia-Villar R, Ferrier L, Larauche M, Vergnolle N, Bunnett NW, Coelho AM, Fioramonti J, Bueno L. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J Immunol. 2003;170:4296–4300. doi: 10.4049/jimmunol.170.8.4296. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Mencarelli A, Palazzetti B, Distrutti E, Vergnolle N, Hollenberg MD, Wallace JL, Morelli A, Cirino G. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci USA. 2001;98:13936–13941. doi: 10.1073/pnas.241377298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, Smith AJ, Hunter GD, McLean JS, McGarry F, Ramage R, Jiang L, Kanke T, Kawagoe J. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Tao X, Gopal A, Ligon B, Andrade-Gordon P, Steer ML, Perides G. Protection against acute pancreatitis by activation of protease-activated receptor-2. Am J Physiol. 2005;288:G388–G395. doi: 10.1152/ajpgi.00341.2004. [DOI] [PubMed] [Google Scholar]
- Matsuda N, Nishihira J, Takahashi Y, Kemmotsu O, Hattori Y. Role of macrophage migration inhibitory factor in acute lung injury in mice with acute pancreatitis complicated by endotoxemia. Am J Respir Cell Mol Biol. 2006;35:198–205. doi: 10.1165/rcmb.2005-0272OC. [DOI] [PubMed] [Google Scholar]
- Pawlinski R, Pedersen B, Schabbauer G, Tencati M, Holscher T, Boisvert W, Andrade-Gordon P, Frank RD, Mackman N. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103:1342–1347. doi: 10.1182/blood-2003-09-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazerani HR, Plevin R, Kawagoe J, Kanke T, Furman BL. Lack of effect of proteinase-activated receptor-2 (PAR-2) deletion on the pathophysiological changes produced by lipopolysaccharide in the mouse: comparison with dexamethasone. J Pharm Pharmacol. 2004;56:1015–1020. doi: 10.1211/0022357043923. [DOI] [PubMed] [Google Scholar]
- Camerer E, Cornelissen I, Kataoka H, Duong DN, Zheng YW, Coughlin SR. Roles of protease-activated receptors in a mouse model of endotoxemia. Blood. 2006;107:3912–3921. doi: 10.1182/blood-2005-08-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicala C, Pinto A, Bucci M, Sorrentino R, Walker B, Harriot P, Cruchley A, Kapas S, Howells GL, Cirino G. Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circulation. 1999;99:2590–2597. doi: 10.1161/01.cir.99.19.2590. [DOI] [PubMed] [Google Scholar]
- Grandaliano G, Pontrelli P, Cerullo G, Monno R, Ranieri E, Ursi M, Loverre A, Gesualdo L, Schena FP. Protease-activated receptor-2 expression in IgA nephropathy: a potential role in the pathogenesis of interstitial fibrosis. J Am Soc Nephrol. 2003;14:2072–2083. doi: 10.1097/01.asn.0000080315.37254.a1. [DOI] [PubMed] [Google Scholar]
- Holdsworth SR, Kitching AR, Tipping PG. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55:1198–1216. doi: 10.1046/j.1523-1755.1999.00369.x. [DOI] [PubMed] [Google Scholar]
- Drew AF, Tucker HL, Liu H, Witte DP, Degen JL, Tipping PG. Crescentic glomerulonephritis is diminished in fibrinogen-deficient mice. Am J Physiol. 2001;281:F1157–F1163. doi: 10.1152/ajprenal.2001.281.6.F1157. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Kahn ML, Coughlin SR, Sambrano GR, Schauble E, Bernstein D, Foy D, Hafezi-Moghadam A, Ley K. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- Tipping PG, Huang XR, Berndt MC, Holdsworth SR. P-selectin directs T lymphocyte-mediated injury in delayed-type hypersensitivity responses: studies in glomerulonephritis and cutaneous delayed-type hypersensitivity. Eur J Immunol. 1996;26:454–460. doi: 10.1002/eji.1830260228. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Huang XR, Holdsworth SR, Tipping PG. Evidence for delayed-type hypersensitivity mechanisms in glomerular crescent formation. Kidney Int. 1994;46:69–78. doi: 10.1038/ki.1994.245. [DOI] [PubMed] [Google Scholar]
- Kitching AR, Kong YZ, Huang XR, Davenport P, Edgtton KL, Carmeliet P, Holdsworth SR, Tipping PG. Plasminogen activator inhibitor-1 is a significant determinant of renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:1487–1495. doi: 10.1097/01.asn.0000065550.13931.00. [DOI] [PubMed] [Google Scholar]
- Hawkes SP, Li H, Taniguchi GT. Zymography and reverse zymography for detecting MMPs, and TIMPs. Methods Mol Biol. 2001;151:399–410. doi: 10.1385/1-59259-046-2:399. [DOI] [PubMed] [Google Scholar]
- Trottier G, Hollenberg M, Wang X, Gui Y, Loutzenhiser K, Loutzenhiser R. PAR-2 elicits afferent arteriolar vasodilation by NO-dependent and NO-independent actions. Am J Physiol. 2002;282:F891–F897. doi: 10.1152/ajprenal.00233.2001. [DOI] [PubMed] [Google Scholar]
- Erlich JH, Holdsworth SR, Tipping PG. Tissue factor initiates glomerular fibrin deposition and promotes major histocompatibility complex class II expression in crescentic glomerulonephritis. Am J Pathol. 1997;150:873–880. [PMC free article] [PubMed] [Google Scholar]
- Oh CK, Ariue B, Alban RF, Shaw B, Cho SH. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun. 2002;294:1155–1160. doi: 10.1016/S0006-291X(02)00577-6. [DOI] [PubMed] [Google Scholar]
- Tipping PG, Huang XR, Qi M, Van GY, Tang WW. Crescentic glomerulonephritis in CD4- and CD8-deficient mice: requirement for CD4 but not CD8 cells. Am J Pathol. 1998;152:1541–1548. [PMC free article] [PubMed] [Google Scholar]
- Li S, Holdsworth SR, Tipping PG. Antibody independent crescentic glomerulonephritis in mu chain deficient mice. Kidney Int. 1997;51:672–678. doi: 10.1038/ki.1997.97. [DOI] [PubMed] [Google Scholar]
- Cunningham MA, Rondeau E, Chen X, Coughlin SR, Holdsworth SR, Tipping PG. Protease-activated receptor 1 mediates thrombin-dependent, cell-mediated renal inflammation in crescentic glomerulonephritis. J Exp Med. 2000;191:455–462. doi: 10.1084/jem.191.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y, Loutzenhiser R, Hollenberg MD. Bidirectional regulation of renal hemodynamics by activation of PAR1 and PAR2 in isolated perfused rat kidney. Am J Physiol. 2003;285:F95–F104. doi: 10.1152/ajprenal.00396.2002. [DOI] [PubMed] [Google Scholar]
- Kincaid-Smith P. Coagulation and renal disease. Kidney Int. 1972;2:183–190. doi: 10.1038/ki.1972.93. [DOI] [PubMed] [Google Scholar]
- McCluskey RT, Vassalli P, Gallo G, Baldwin DS. An immunofluorescent study of pathogenic mechanisms in glomerular diseases. N Engl J Med. 1966;274:695–701. doi: 10.1056/NEJM196603312741301. [DOI] [PubMed] [Google Scholar]
- Paronetto F, Koffler D. Immunofluorescent localization of immunoglobulins, complement, and fibrinogen in human diseases. I. Systemic lupus erythematosus. J Clin Invest. 1965;44:1657–1664. doi: 10.1172/JCI105272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Kanatsu K, Doi T, Sekita K, Nagai H, Muso E, Yoshida H, Tamura T, Kawai C. Immunoelectron microscopic localization of fibrin-related antigen in human glomerular diseases. Nephron. 1989;52:238–243. doi: 10.1159/000185649. [DOI] [PubMed] [Google Scholar]
- Thomson NM, Moran J, Simpson IJ, Peters DK. Defibrination with ancrod in nephrotoxic nephritis in rabbits. Kidney Int. 1976;10:343–347. doi: 10.1038/ki.1976.120. [DOI] [PubMed] [Google Scholar]
- Kuroda T, Yoshida Y, Kamiie J, Kovalenko P, Nameta M, Fujinaka H, Yaoita E, Endo T, Ishizuka S, Nakabayashi K, Yamada A, Nagasawa T, Yamamoto T. Expression of MMP-9 in mesangial cells and its changes in anti-GBM glomerulonephritis in WKY rats. Clin Exp Nephrol. 2004;8:206–215. doi: 10.1007/s10157-004-0289-8. [DOI] [PubMed] [Google Scholar]
- Urushihara M, Kagami S, Kuhara T, Tamaki T, Kuroda Y. Glomerular distribution and gelatinolytic activity of matrix metalloproteinases in human glomerulonephritis. Nephrol Dial Transplant. 2002;17:1189–1196. doi: 10.1093/ndt/17.7.1189. [DOI] [PubMed] [Google Scholar]
- Tomita M, Koike H, Han GD, Shimizu F, Kawachi H. Decreased collagen-degrading activity could be a marker of prolonged mesangial matrix expansion. Clin Exp Nephrol. 2004;8:17–26. doi: 10.1007/s10157-003-0258-7. [DOI] [PubMed] [Google Scholar]
- Lelongt B, Bengatta S, Delauche M, Lund LR, Werb Z, Ronco PM. Matrix metalloproteinase 9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. J Exp Med. 2001;193:793–802. doi: 10.1084/jem.193.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]