Abstract
E-Cadherin (CDH1) expression is reduced in thyroid carcinomas by primarily unknown mechanisms. In several tissues, SNAIL (SNAI1) and SLUG (SNAI2) induce epithelial-mesenchymal transition by altering target gene transcription, including CDH1 repression, but these transcription factors have not been studied in thyroid carcinoma. Recently, our group has provided direct evidence that ectopic SNAI1 expression induces epithelial and mesenchymal mouse tumors. SNAI1, SNAI2, and CDH1 expression were analyzed in thyroid-derived cell lines and samples of human follicular and papillary thyroid carcinoma by reverse transcriptase-polymerase chain reaction, Western blotting, and immunohistochemistry. The effect of SNAI1 expression on CDH1 transcription was analyzed by reverse transcriptase-polymerase chain reaction and Western blotting in ori-3 cells. Thyroid carcinoma development was analyzed in CombitTA-Snail mice, in which SNAI1 levels are up-regulated. SNAI1 and SNAI2 were not expressed in cells derived from normal thyroid tissue, or in normal human thyroid samples, but were highly expressed in cell lines derived from thyroid carcinomas, in human thyroid carcinoma samples, and their metastases. SNAI1 expression in ori-3 cells repressed CDH1 transcription. Combi-TA mice developed papillary thyroid carcinomas, the incidence of which was increased by concomitant radiotherapy. In conclusion, SNAI1 and SNAI2 are ectopically expressed in thyroid carcinomas, and aberrant expression in mice is associated with papillary carcinoma development.
Thyroid cancer consists of several histological subtypes, including follicular and papillary carcinoma. These two histiotypes exhibit both common and individual alterations in gene expression compared with normal thyroid tissue. At the molecular level, alterations in expression of various proteins have been found between normal and tumor tissue including growth factors, eg, transforming growth factor-β1, epidermal growth factor; proteins regulating the cell cycle and apoptosis, eg, retinoblastoma, cyclin D1; cell adhesion molecules, such as E-cadherin (CDH1) and CD44; and tyrosine kinases and their receptors, eg, RET.1
Cadherins are transmembrane proteins mediating homotypic cell-cell adhesion. At a cellular level their expression induces differentiation and growth suppression, which translates at an organ level into the formation of polarized, ordered tissues.2 The prototypical member, CDH1, is highly expressed in the normal thyroid gland and in benign thyroid lesions, including toxic diffuse goiter, multinodular goiter, and follicular adenomas.3 CDH1 expression leads to aggregation of thyrocytes in culture,4 and exposure of thyrocytes to thyroid-stimulating hormone increases CDH1 transcription,5 demonstrating the intimate relationship between CDH1 expression and thyroid differentiation.
CDH1 expression is decreased in classic papillary thyroid carcinoma3,6,7,8,9,10 and its diffuse sclerosing variant9 and also in follicular carcinoma.3,7,8,11,12,13 Rarely, this decrease in expression is attributable to mutation of the CDH1 gene,7,9 loss of heterozygosity,7 or hypermethylation of the CDH1 promoter.9 In most tumors the mechanism of CDH1 down-regulation is, however, unknown.
Several transcriptional repressors have been shown to modulate cadherin expression, including Snail family members SNAIL (SNAI1)14,15 and SLUG (SNAI2).16,17 These transcription factors contain zinc finger motifs and bind to E-boxes in the CDH1 promoter, repressing gene transcription14,15 and producing changes in cell phenotype consistent with epithelial to mesenchymal transition including migration, invasion, resistance to cell death, and induction of angiogenesis.18
SNAI1 and SNAI2 are ectopically expressed in several tumor cell lines.14 Furthermore, SNAI1 has been found to be expressed in a number of human tumors derived from epithelial tissues, including gastric and hepatic carcinoma,19,20,21 with expression in the latter correlating with both tumor invasiveness and prognosis.20,21 Recently, we provided the first direct experimental evidence that SNAI1 was linked to tumorigenesis by showing that mice in which SNAI1 was overexpressed by 20% developed both epithelial and mesenchymal tumors.22 Embryonic fibroblasts derived from these mice were subsequently shown to induce tumor formation in nude mice.22
We sought to investigate the pattern of SNAI1 and SNAI2 expression in the normal human thyroid gland, papillary and follicular thyroid cancers, and transformed lines derived from thyroid carcinomas. We subsequently examined the effects of SNAI1 expression in thyroid cells and analyzed thyroid carcinoma development in mice overexpressing SNAI1.
Materials and Methods
Human Tissue Specimens
Formalin-fixed, paraffin-embedded sections of follicular thyroid carcinoma (n = 31) and papillary thyroid carcinoma (n = 32) were obtained from the pathology archives of the Royal Infirmary of Edinburgh, Edinburgh, UK, and University Hospital Birmingham, Birmingham, UK. Sections of formalin-fixed normal human skin and colorectal and prostate carcinomas were used as positive and negative tissue controls for immunohistochemistry. Ethical approval was obtained for the use of all human material (LREC reference 04/S1101/59). Pathological stage of tumors used was as follows: follicular: minimally invasive, n = 17; invasive, n = 14; papillary: T1, n = 6; T2, n = 11; T3, n = 5; T4, n = 10.
Antibodies, Tissue Culture, and Plasmids
Primary antibodies were as follows. CDH1 antibody (clone 36) was obtained from BD Biosciences, Oxford, UK, and used at a concentration of 1:300 for immunohistochemistry, 1:300 for immunofluorescence, and 1:5000 for Western blotting. SNAI1 and SNAI2 antibodies and specific blocking peptides were obtained from Autogen Bioclear, Wiltshire, UK. SNAI1 (clone E18) was used at a dilution of 1:10 for immunohistochemistry, 1:50 for immunofluorescence, and 1:100 for Western blotting. SNAI2 (clone G18) was used at 1:20 for immunohistochemistry, 1:50 for immunofluorescence, and 1:100 for Western blotting. Monoclonal anti-SNAI1 antibody was a gift from Antonio Garcia Herreros (Unitat de Biologia Cellular i Molecular, Barcelona, Spain) and has been previously described23 and was used at 1:1000 for Western blotting. Mouse monoclonal anti-β-actin (clone AC-15) was obtained from Abcam (Cambridge, UK) and used at 1:10,000 in Western blotting. Secondary antibodies were as follows: goat anti-mouse IgG-horseradish peroxidase-conjugated (Upstate, Dundee, Scotland) and rabbit anti-goat IgG-horseradish peroxidase-conjugated (Autogen Bioclear) were used at 1:5000 for Western blotting. Fluorescein isothiocyanate-linked anti-mouse IgG2a (Sigma, Gillingham, UK) was used at a 1:800 dilution and fluorescein isothiocyanate-linked rabbit anti-goat IgG (Sigma) was used at a 1:200 dilution, both for immunofluorescence.
K1 and ori-3 cells were a gift from Professor D. Wynford-Thomas, Department of Pathology, University of Cardiff, Cardiff, UK. B-CPAP, CAL-62, FTC-133, and 8305C cells were a gift from Professor M. d’Armiento, Department of Experimental Medicine and Pathology, University of Rome “La Sapienza,” Rome, Italy. Lines were derived from the following human tissues: ori-3, SV40-transformed normal thyrocytes; B-CPAP, K1, papillary thyroid carcinoma; FTC-133, follicular thyroid carcinoma; CAL-62, 8305C, anaplastic thyroid carcinoma. Culture media for individual lines was as follows: B-CPAP, K1, ori-3, 8305C, RPMI 1640 medium + 10% fetal calf serum; CAL-62, Dulbecco’s modified Eagle’s medium + 5% fetal calf serum; FTC-133, Dulbecco’s modified Eagle’s medium + Ham’s F12 (1:1) + 10% fetal calf serum + 2 mmol/L glutamine. All culture media, serum, and glutamine were purchased from Life Technologies, Paisley, UK, and lines were maintained in a 5% CO2 humidified atmosphere at 37°C. The plasmid pCAG-hSnail was a gift from Professor S. Tsukita, Department of Cell Biology, University of Kyoto, Kyoto, Japan, and has been described previously.24
Cell Transfection, RNA Extraction, and cDNA Formation
To prepare for transfection, 2 × 105 cells were cultured in six-well plates 12 hours before transfection. Cells were then transfected with the plasmid pCAG-hSnail using Fugene 6 reagent (Roche Diagnostics, Lewes, UK) at a plasmid/DNA ratio of 3:2, according to the manufacturer’s instructions. Cells were separated from the culture dish by scraping 24 hours after transfection, and RNA was harvested using TRIzol reagent (Invitrogen, Paisley, UK) according to the manufacturer’s protocol. cDNA was produced using a reverse transcription kit (Promega, Southampton, UK) after prior treatment of RNA with 1 U of DNase (Promega) per reaction with incubation at 37°C for 30 minutes. Synthesized cDNA was used in subsequent polymerase chain reactions. Cell transfection experiments were performed on three separate occasions, after initial transfection efficiencies of 70 to 80% were documented in ori-3 cells.
Total RNA from mice was isolated in two steps using TRIzol (Life Technologies, Inc., Grand Island, NY) followed by RNeasy mini-kit (Qiagen Inc., Valencia, CA) purification following the manufacturer’s protocols. RNA was cleaned up with the optional on-column DNase treatment. The integrity and quality of RNA were verified by electrophoresis, and RNA concentration was measured. To analyze expression of CombitTA-Snail in mice, reverse transcriptase (RT) was performed according to the manufacturer’s protocol in a 20-μl reaction containing 50 ng of random hexamers, 3 μg of total RNA, and 200 U of Superscript II RNase H− reverse transcriptase (Gibco/BRL, Paisley, UK).
Polymerase Chain Reaction
Oligonucleotides (TAGN, Gateshead, UK) used for amplification from cell lines and polymerase chain reaction (PCR) conditions were as follows, with an initial denaturation step of 94°C for 5 minutes in all cases. CDH1: forward 5′-TGCCCAGAAAATGAAAAAGG-3′, reverse 5′-GTGTATGTGGCAATGCGTTC-3′, with 35 cycles of 94°C × 30 seconds, 58°C × 1 minute, and 72°C × 1 minute. SNAI1: forward 5′-AATCGGAAGCCTAACTACAAG-3′, reverse 5′-AGGAAGAGACTGAAGTAGAG-3′ with 35 cycles of 94°C × 1 minute, 53°C × 1 minute, and 72°C × 1 minute. SNAI2: forward 5′-GCCTCCAAAAAGCCAAACTA-3′, reverse 5′-CACAGTGATGGGGCTGATG-3′ with 35 cycles of 94°C × 1 minute, 53°C × 1 minute, and 72°C × 1 minute. PCR reagent (Promega) concentrations per reaction were as follows: oligonucleotides, 25 pmol; MgCl2, 15 mmol/L; and Taq polymerase, 2 U.
To analyze expression of CombitTA-Snail in mice, reverse transcription was performed according to the manufacturer’s protocol in a 20-μl reaction containing 50 ng of random hexamers, 3 μg of total RNA, and 200 U of Superscript II RNase H− reverse transcriptase (Gibco/BRL). Exogenous SNAI1 gene product expression in mice was analyzed with mSnailF and Combi-polyA-B1 primers: Combi-polyA-B1: 5′-TTGAGTGCATTCTAGTTGTG-3′; mSnailF: 5′-CAGCTGGCCAGGCTCTCGGT-3′. The PCR conditions used to amplify CombitTA-Snail were as follows: 94°C × 1 minute, 56°C × 1 minute, and 72°C × 2 minutes for 40 cycles. Mouse E-cadherin expression was analyzed with the following primers: mEcadherin forward: 5′-GGACGTCCATGTGTGTGA-3′; mEcadherin reverse: 5′-CTTCTACACACTCAGGGA-3′. The PCR conditions used to amplify E-cadherin were as follows: 94°C × 1 minute, 52°C ×1 minute, and 72°C × 2 minutes for 40 cycles. The PCR products were confirmed by hybridization with specific internal probes. Amplification of β-actin RNA served as a control to assess the quality of each RNA sample from both cell lines and mice.
Immunohistochemistry for SNAI1, SNAI2, and CDH1
Immunohistochemistry for CDH1 was performed as follows. Sections were dewaxed, incubated in methanol:hydrogen peroxide (H2O2) (10:1) for 5 minutes, and antigen retrieval was performed in 0.01 mol/L sodium citrate three times for 5 minutes each in a microwave. Sections were incubated in normal goat serum for 30 minutes and then in primary antibody for 60 minutes. After washing in Tris-buffered saline (TBS) sections were incubated in horseradish peroxidase Envision solution (DAKO, Ely, UK) for 60 minutes. Diaminobenzidine tetrahydrochloride (DAKO) was then used as a substrate for the peroxidase reaction at a concentration of 1 mg/ml + 1 μl/ml H2O2, and slides were dipped in hematoxylin, dehydrated, and mounted.
Immunohistochemistry for SNAI1 and SNAI2 was performed as follows. Slides were immersed in W-cap buffer (Bio-Optica, Milan, Italy) and cycled in a Pixel antigen retriever (CellPath, Newtown, UK) for 60 minutes, washed in running water, and placed in methanol/hydrogen peroxide (10:1) for 5 minutes. Sections were then incubated in primary antibody in TBS 7.5 × buffer (Bios Europe Ltd., Skelmersdale, UK) at 4°C overnight, washed with TBS, and reacted with peroxidase-linked rabbit anti-sheep antibody (DAKO) at a 1:100 dilution in TBS for 1 hour. The immunoreactivity was revealed as above using diaminobenzidine tetrahydrochloride. Slides were then dipped in hematoxylin, dehydrated, and mounted. To control for SNAI1 and SNAI2 antibody specificity, primary antibodies were combined with a 10-fold excess of blocking peptide, incubated for 2 hours at room temperature, and then used as previously described for immunohistochemistry.
Intensity and subcellular localization of reactivity was scored as previously described.25 In brief, intensity of staining (graded 0 to 3) and percentage of positively staining follicles per lesion, subcellular localization of immunoreactivity, and correlation between positive immunoreactivity, tissue phenotype/morphology, and localization within tumor (ie, centrally or at invasive front) were recorded. The presence of morphologically normal follicles within each section served as additional internal negative/positive controls depending on antibody used. Correlation of staining with tumor characteristics was assessed using Fisher’s exact test.
Immunofluorescence for SNAI1, SNAI2, and CDH1
Cells were grown on coverslips, and then immunofluorescence was performed on confluent cells as described. Cells were washed with phosphate-buffered saline (PBS), fixed with ice-cold methanol/acetone (1:1 ratio) for 5 minutes, and then washed with PBS. Cells were treated with a 0.1% solution of Triton-X for 20 minutes then blocked with a 5% solution of normal goat serum for 20 minutes. Primary antibodies were constituted in 5% normal goat serum and applied for 1 hour; secondary antibody was used for 30 minutes, with multiple washes with 1% bovine serum albumin after both primary and secondary antibody stages. 4,6-Diamidino-2-phenylindole (DAPI) (Sigma) was used at a concentration of 1:10,000 for 1 minute to stain nuclei. Coverslips were then mounted onto slides with immunofluorescence mounting media (Sigma). A Leica fluorescence microscope (Leica, Wetzlar, Germany) was used to analyze sections.
Protein Lysate Formation and Western Blotting
Protein lysates were made from cell lines by washing in PBS then lysing in radioimmunoprecipitation assay buffer containing protease inhibitors (Sigma). Protein concentrations were determined using a Bio-Rad protein assay kit (Bio-Rad, Hemel Hempstead, UK). For Western blotting, equal concentrations of protein lysates were separated on 8% (CDH1) or 15% (SNAI1 and SNAI2) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, and proteins were transferred onto Hybond polyvinylidene difluoride nitrocellulose membrane (Amersham, High Wycombe, UK). Membranes were blocked in 10% Marvel in TBS for 20 minutes and then incubated with primary antibodies in TBS-Tween at 4°C overnight. Blots were washed extensively with TBST, probed with secondary antibody, washed in TBS-Tween, and reacted with enhanced chemiluminescence reagent (Amersham) according to the manufacturer’s instructions.
Mice and Treatments
Animals were housed under nonsterile conditions in a conventional animal facility. CombitTA-Snail mice have been previously described.22 Similar phenotypic features were seen in all assays for both of the CombitTA-Snail transgenic lines generated. To study the effect of irradiation on thyroid carcinoma development, 30 CombitTA-Snail and 30 control mice 5 to 6 weeks of age were irradiated with a dose of 400 rads and maintained in microisolator cages on sterilized food and acidified sterile water.
For the effect of SNAI1 suppression in thyroid carcinomas after radiation treatment, stock solutions of 4 mg/ml doxycycline (Sigma) were prepared fresh in water and sucrose and administered to mice in the drinking water. Mice were started on doxycycline or placebo (the same volume of diluent) beginning on the day after thyroid carcinoma was confirmed (day 0). Mice tolerated the treatment well, and no interruption of therapy was necessary. Mice were followed clinically three times a week. The death endpoint was determined either by spontaneous death of the animal or by elective sacrificing of the animal because of signs of pain or suffering according to established criteria.
Histological Analysis of Mice
Mice included in this study were subjected to standard necropsy. All major organs were closely examined under the dissecting microscope, and samples of each organ were processed into paraffin, sectioned, and examined histologically. All tissue samples were taken from homogenous and viable portions of the resected sample by the pathologist and were fixed within 2 to 5 minutes of excision. Hematoxylin and eosin (H&E)-stained sections of each tissue were reviewed by a single pathologist (T.F.). For comparative studies, age-matched mice were used (wild-type or CombitTA-Snail mice in the continuous presence of tetracycline). Statistical analysis of rates of tumor development in wild type compared with CombiTA-Snail mice was analyzed using Fisher’s exact test.
Cell Transfers and Tumorigenicity Assay
To test the tumorigenicity of the various CombitTA-Snail thyroid cancers, 4- to 6-week-old athymic (nude) male mice were injected subcutaneously on both flanks with 106 cells suspended in 200 μl of PBS. The animals were examined for tumor formation once a week and were sacrificed for histopathological studies and collection of tissues for DNA analyses when moribund.
Results
SNAI1 and SNAI2 mRNA and Protein Are Expressed in Thyroid Carcinoma Cell Lines
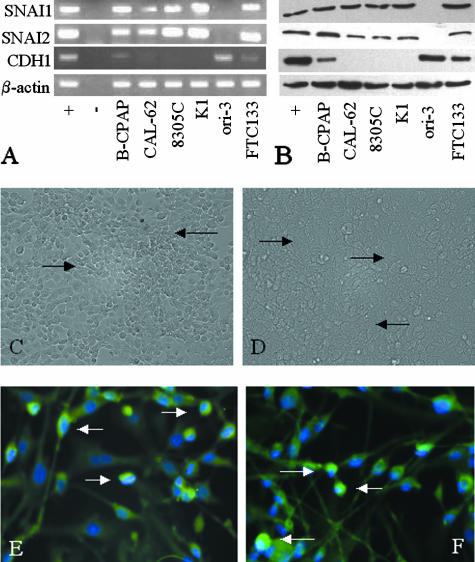
To test the initial hypothesis that Snail family transcription factors would be expressed in thyroid carcinoma, a panel of human thyroid cell lines were screened for SNAI1 and SNAI2 mRNA expression by reverse transcription-polymerase chain reaction (RT-PCR) and protein expression by Western blot. As can be seen from Figure 1, A and B, all lines expressed both SNAI1 and SNAI2 mRNA and protein except ori-3 cells, the only line tested that does not originate from a thyroid carcinoma. These data therefore support the hypothesis that SNAI1 and SNAI2 are aberrantly transcribed in thyroid carcinoma. The expression of CDH1 mRNA and protein was also analyzed in these lines. Ori-3 cells expressed abundant CDH1 mRNA and protein, as may be anticipated, whereas B-CPAP and FTC-133 cells demonstrated weaker CDH1 mRNA and protein expression. No CDH1 mRNA or protein expression was seen in lines CAL-62, 8305C, and K1.
Figure 1.
Expression of SNAI1, SNAI2, and CDH1 in thyroid-derived cell lines. cDNA (A) and protein (B) expression of SNAI1, SNAI2, and CDH1 in thyroid lines were analyzed by RT-PCR with products resolved on 2% agarose gels (for cDNA) and protein electrophoresis on 8% SDS-PAGE gels (for CDH1) and 15% SDS-PAGE gels (for SNAI1 and SNAI2). β-Actin amplification was used to ensure equal sample loading. Positive controls were LNCaP cells (SNAI1, SNAI2) and MCF-7 cells (CDH1). Negative controls were reactions without cDNA. Antibodies E18 and G18, already described, were used in Western blotting for SNAI1 and SNAI2, respectively. SNAI1 and SNAI2 cDNA and protein were expressed in all lines derived from thyroid carcinomas (B-CPAP, CAL-62, FTC-133, K1, and 8305C) but not in ori-3 cells derived from normal thyrocytes. CDH1 cDNA and protein was strongly expressed in ori-3 cells, with weaker expression in B-CPAP and FTC-133 cells. Cell lines expressing SNAI1 and SNAI2 did not form monolayers with close cell contacts, even at confluence, illustrated by the gaps between 8305C cells (C, arrows). Ori-3 cells did, however, form a confluent monolayer without discernable gaps between cells (D, arrows). Both SNAI1 and SNAI2 demonstrated a mixture of nuclear and cytoplasmic localization in all cell lines in which they were expressed, with nuclear staining being much more intense, as shown by colocalization of SNAI1 and SNAI2 (fluorescein isothiocyanate) with DAPI nuclear marker (blue) (E and F, respectively, nuclear localization demonstrated by arrows, FTC-133 cells shown). Original magnifications: ×20 (C and D); ×60 (E and F).
We next analyzed whether the morphology of cultured cells expressing SNAI1 and SNAI2 was different from ori-3 cells, which do not express these proteins. All lines expressing SNAI1 and SNAI2 demonstrated a lack of close cellular contact, even at confluence (Figure 1C), compared with ori-3 cells that grew as a cell monolayer with close apposition of cells at confluence (Figure 1E). For SNAI1 and SNAI2 protein to be functional in thyroid tumor cell lines, they would be expected to show nuclear localization. We therefore used immunofluorescence to study this, with counterstaining of nuclei with DAPI, a nuclear marker. SNAI1 and SNAI2 were predominantly localized to cell nuclei, with weaker cytoplasmic reactivity (Figure 1, E and F, respectively), as shown by co-localization with DAPI. Thus SNAI1 and SNAI2 are localized to the nuclei in the thyroid carcinoma cells analyzed, and their presence correlates with alterations in cell morphology expected for cells with impaired cell-cell adhesion.
SNAI Expression Is Associated with Modulation of CDH1 Expression in Thyrocytes in Vitro
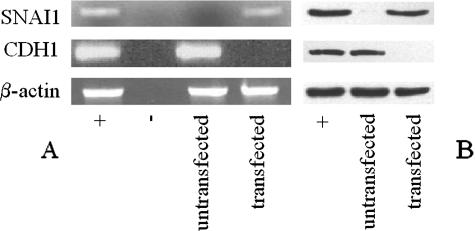
We next assessed whether expression of SNAI1 in ori-3 cells leads to a modulation in CDH1 mRNA and protein levels. The plasmid pCAG-hSnail was used to express transiently human SNAI1 in ori-3 cells, and CDH1 mRNA and protein levels were assessed before transfection and 24 hours after transfection by RT-PCR on extracted RNA or Western blotting of protein lysates. As can be seen, CDH1 mRNA (Figure 2A) and protein (Figure 2B) were present in untransfected ori-3 cells, but expression of SNAI1 repressed both CDH1 mRNA (Figure 2A) and protein (Figure 2B) to an undetectable level at 24 hours. This demonstrates that SNAI1 seems to be active in ori-3 thyrocytes with respect to repression of CDH1 transcription.
Figure 2.
Effect of transfection of SNAI1 on CDH1 expression in thyrocytes. Ori-3 cells were transfected with SNAI1; CDH1 and SNAI1 cDNA expression was analyzed by RT-PCR 24 hours after transfection (A), and protein expression was analyzed by electrophoresis on 8% SDS-PAGE gels (for CDH1) and 15% SDS-PAGE gels (for SNAI1) (B). β-Actin amplification was used to ensure equal sample loading. Positive controls were LNCaP cells (SNAI1) and MCF-7 cells (CDH1). Negative controls were reactions without cDNA. At 24 hours after transfection, SNAI1 cDNA and protein are expressed, associated with transcriptional silencing of CDH1 and corresponding loss of cDNA and protein expression.
SNAI1 and SNAI2 Are Expressed in Human Thyroid Carcinomas
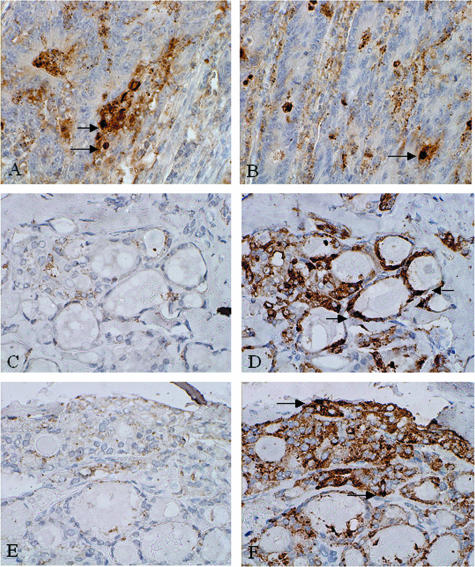
Antibodies to SNAI1 and SNAI2 were initially characterized. Strong nuclear staining in colorectal adenocarcinomas was seen with antibodies to both SNAI1 (Figure 3A) and SNAI2 (Figure 3B), as has been previously shown.26 Omission of secondary antibody led to an absence of immunoreactivity (Figure 3C), compared with when secondary antibody was used (Figure 3D). Addition of a specific SNAI1- or SNAI2-blocking peptide to the primary antibody similarly led to a lack of staining (Figure 3E) compared with reactions without blocking peptide (Figure 3F). Follicular (n = 31) and papillary (n = 32) thyroid carcinomas were then stained for SNAI1, SNAI2, and CDH1. Expression of these proteins was also analyzed in morphologically normal areas of thyroid tissue in each section.
Figure 3.
Characterization of antibodies to SNAI1 and SNAI2. Positive reactivity is demonstrated by brown staining. SNAI1 (A) and SNAI2 (B) demonstrate nuclear reactivity in colorectal adenocarcinoma. Omission of secondary antibody leads to an absence of SNAI staining (C) compared with sections where secondary antibody is used (D). Use of a specific blocking peptide to SNAI1 antibody similarly produces an absence of staining (E) compared with sections stained in the absence of blocking peptide (F) (arrows indicate nuclear staining). Identical results were obtained for SNAI2 antibody with omission of secondary antibody and use of blocking peptide. Original magnifications, ×40.
Immunoreactivity for neither SNAI1 nor SNAI2 was seen in morphologically normal follicles in any specimen analyzed (Figure 4, A and B); thus SNAI1 and SNAI2 are not expressed in normal thyroid tissue. Histologically normal thyroid gland demonstrated CDH1 reactivity along epithelial cell membranes, as has been previously described,3 in all cases (Figure 4C).
Figure 4.
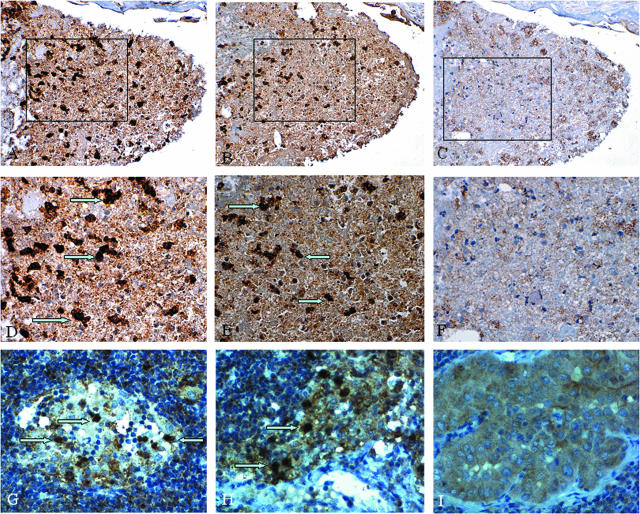
Expression of SNAI1, SNAI2 and CDH1 in normal human thyroid tissue and follicular carcinomas. Positive reactivity is demonstrated by brown staining. SNAI1 and SNAI2 are not expressed in normal thyroid follicles (A and B), whereas CDH1 is expressed along basolateral cell membranes (C). SNAI1 is expressed in the nuclei and cytoplasm of thyrocytes from follicular thyroid carcinomas (D and G, arrows indicate positive nuclei), as is SNAI2 (E and H, arrows indicate positive nuclei). CDH1 expression is markedly reduced in cells from follicular carcinomas (F and I) with nuclear SNAI1 and SNAI2 expression compared with normal thyroid follicles. Original magnifications: ×40 (A–C, G–I); ×20 (D–F).
SNAI1 was expressed in neoplastic areas of 18 of 31 follicular carcinomas. On analysis of expression by tumor stage, SNAI1 was expressed in 9 of 17 minimally invasive and 9 of 14 invasive lesions. There was therefore no correlation of SNAI1 expression with tumor stage (P = 0.39). Reactivity was seen in nuclear and cytoplasmic localizations in positively staining cases (Figure 4, D and G). SNAI2 reactivity was seen in 22 of 31 follicular carcinomas, 12 of 17 minimally invasive, and 10 of 14 invasive lesions; again SNAI2 expression did not correlate with tumor stage (P = 0.63). Similarly to SNAI1, expression was restricted to cell nuclei and cytoplasm (Figure 4, E and H). SNAI1 and SNAI2 are therefore both highly expressed in follicular carcinomas of all pathological stages. A reduction in CDH1 expression was seen in 15 of 31 follicular cancers, 6 of 17 minimally invasive, and 10 of 14 invasive tumors (Figure 4F). Inverse correlation between SNAI1/SNAI2 and CDH1 expression was seen in 13 of 31 cases (9 of 17 minimally invasive and 4 of 14 invasive) in focal tumor areas. CDH1 expression is therefore more frequently lost in invasive compared with minimally invasive lesions (P = 0.09). In three minimally invasive carcinomas, SNAI1 and SNAI2 expression seemed to be restricted to the invasive tumor front, with concomitant reduced CDH1 reactivity.
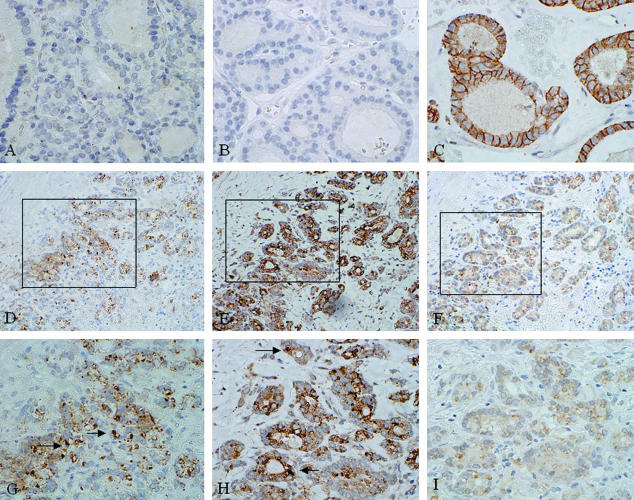
Expression of SNAI1 was seen in 28 of 32 papillary cancers (T1, n = 4; T2, n = 10; T3, n = 4; T4, n = 10). There was no statistical difference in T1 and T2 tumors compared with T3 and T4 cases (P = 0.35). Immunoreactivity was seen in both nuclear and cytoplasmic locations, similar to follicular carcinomas (Figure 5, A and D). SNAI2 expression was seen in all tumors analyzed, but expression was only nuclear in location in three cases (T2, n = 1; T4, n = 2) (Figure 5, B and E) and was almost entirely cytoplasmic in nature in the other cases. CDH1 expression was reduced compared with reactivity in normal tissue in 15 of 32 papillary cancers in our series (T1, n = 2; T2, n = 2; T3, n = 4; T4, n = 7), with loss of expression correlating with advanced tumor stage (P = 0.063) (Figure 5, C and F). SNAI1 nuclear positivity and reduction of CDH1 reactivity occurred in follicles of 12 tumors. In two tumors, there seemed to be increased SNAI1 expression at the invasive tumor front, with concomitant reduction in CDH1 expression.
Figure 5.
Expression of SNAI1, SNAI2, and CDH1 in papillary thyroid carcinomas and their lymph node metastases. Positive reactivity is demonstrated by brown staining. SNAI1 is expressed in the nuclei and cytoplasm of both papillary carcinomas (A and D, arrows indicate positive nuclei) and their lymph node metastases (G, arrows indicate positive nuclei), as is SNAI2 (B, E, and H; arrows indicate positive nuclei). CDH1 reactivity is reduced in cells from this carcinoma (C and F) and its nodal metastasis (I) demonstrating nuclear SNAI1 and SNAI2 expression, compared with normal follicles. Original magnifications: ×20 (A–C); ×40 (D–I).
The data on papillary carcinomas prompted us to analyze SNAI1 and SNAI2 expression in lymph node metastases from these tumors. Of four metastases studied, all expressed both SNAI1 and SNAI2 in nuclear and cytoplasmic locations (Figure 5, G and H). Concomitant with this, diffuse weak cytoplasmic CDH1 staining was noted, but with no membrane reactivity (Figure 5I).
SNAI1 Expression in Transgenic Mice Is Associated with Thyroid Carcinoma Development
The data shown above demonstrate that SNAI1 and SNAI2 are ectopically expressed in both human thyroid carcinoma cell lines and follicular and papillary thyroid cancer specimens. Furthermore, SNAI1 expression in thyrocytes is associated with suppression of CDH1 transcription. On the basis of the above results, we wished to determine whether SNAI1 expression in transgenic mice is associated with thyroid carcinoma development. To test this, we analyzed mice expressing hypomorphic tetracycline-repressible SNAI1 transgenes that increase SNAI1 expression to 20% greater than normal levels using the CombiTA-SNAI1 transgene. These mice have previously been shown to develop both epithelial and mesenchymal tumors.22 Papillary thyroid carcinomas developed in 3 of 61 CombiTA-Snail mice analyzed (5%) compared with 0 of 30 wild-type mice (P = 0.296) (Figure 6A). Tumors developed between 7 and 11 months of age. Cervical radiotherapy is a known risk factor for thyroid cancer,27 and we were therefore interested to analyze whether the addition of radiotherapy to SNAI1 overexpression in mice led to an increased incidence of thyroid cancer development. Seven of 30 (23%) mice thus analyzed developed papillary carcinoma, confirming that SNAI1 overexpression and radiotherapy were synergistic tumorigenic factors (Table 1). Furthermore, tumor development in these mice was greatly accelerated, occurring between 3 and 5 months of age, and in two cases was associated with multiple lymph node metastases. The tumorigenicity of these thyroid cancers was confirmed by injection into nude mice. Last, we wished to assess the effect of reducing SNAI1 expression in CombiTA-Snail mice that had received radiotherapy and developed thyroid cancer. Administering tetracycline abolished SNAI1 expression, as expected (Figure 6B), with concomitant CDH1 re-expression. Tumor growth was not retarded in any case, however, demonstrating that tumor growth could not be reversed by inhibition of SNAI1 expression.
Figure 6.
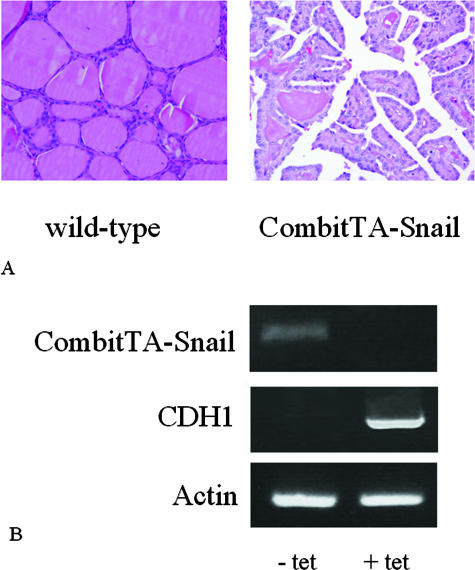
A: Thyroid carcinoma development in CombitTA-Snail mice. Histological analysis of thyroid of wild type and CombitTA-Snail mice is demonstrated. Representative tissue sections from wild-type and CombitTA-Snail mice were stained with H&E. The histological section of a wild-type mouse shows normal follicle formation, whereas the CombitTA-Snail thyroid shows the presence of a papillary thyroid carcinoma. B: Tetracycline suppresses SNAI1 expression in mouse thyroid glands. RT-PCR was used to analyze CombitTA-Snail and CDH1 expression in the presence and absence of tetracycline in mouse drinking water using mouse RNA derived from thyroid tissue, with actin as a loading control. In the presence of tetracycline, there is strong CDH1 expression, but removal of tetracycline induces SNAI1 expression, with concomitant loss of CDH1 expression. Original magnifications, ×20.
Table 1.
Thyroid Carcinoma Development in CombitTA-Snail Mice
| Mice | Mice studied* | Mice with thyroid carcinoma (%)† | Age in months at tumor onset | Tumor type (%) | Sex ratio (female:male) |
|---|---|---|---|---|---|
| CombitTA-Snail | 61 | 3 (5) | 7 to 11 | Papillary | 2:1 |
| CombitTA-Snail + Rx‡ | 30 | 7 (23) | 3 to 5 | Papillary¶ | 5:2 |
| CombitTA-Snail + Rx + Dox∥ | 7 | 7 | Papillary | 5:2 |
Number of mice during or after the period of cancer.
Number of mice sacrificed with cancer and percentage of tumor incidence.
Effect of irradiation on thyroid carcinoma development in young CombitTA-Snail mice. Thirty CombitTA-Snail mice were irradiated at 400 rads to determine thyroid carcinoma development. No thyroid carcinoma development was observed in controls irradiated with equal dosage.
Effect of Snail suppression in papillary thyroid carcinomas developed after radiation exposure. Mice were evaluated for disease progression after administration of tetracycline (4 g/L in the drinking water for 2 weeks). This dose is sufficient to suppress exogenous Snail expression (see Figure 6).
Multiple nodes were detected in two cases.
Discussion
This study provides the first evidence that Snail family transcription factors are involved in thyroid carcinoma development. First, we have shown SNAI1 and SNAI2 are expressed in human thyroid carcinoma samples and in cell lines derived from thyroid cancers, but not in normal thyroid tissue or in a cell line derived from normal thyrocytes. Second, SNAI1 is active in the ori-3 model of normal thyrocytes as regards repression of CDH1 transcription. Third, expression of SNAI1 in mouse thyroid glands is associated with papillary carcinoma development.
Analysis of thyroid cell lines demonstrated that SNAI1 and SNAI2 mRNA and protein were expressed in all lines derived from thyroid carcinoma analyzed. This included lines of papillary (B-CPAP, K1), follicular (FTC-133), and anaplastic (CAL-62, 8305C) carcinoma origin. Interestingly, however, neither SNAI1 nor SNAI2 mRNA or protein was expressed in ori-3 cells, derived from SV-40-transformed normal thyrocytes. Of note, ori-3 cells grow in a manner representing normal thyroid follicles and retain functional evidence of thyroid origin (iodide-trapping and thyroglobulin production),28 whereas none of the other lines studied adopts either a regular morphology in culture or thyroid functional characteristics. CDH1 mRNA and protein were strongly expressed in ori-3 cells, correlating with their retention of thyroid follicular function and morphology, thus demonstrating an inverse expression to SNAI1 and SNAI2 mRNA. The lines B-CPAP and FTC-133 expressed CDH1 mRNA and protein weakly, whereas in the other lines analyzed it was absent. B-CPAP cells do show features of thyroid cells such as thyroglobulin production,29 and FTC-133 cells have previously been shown to express CDH1.30
We next analyzed whether SNAI1 and SNAI2 were located in the nuclei of thyroid carcinoma cells, as may be expected if they are active in their role as transcription factors in this situation. In all lines analyzed, expression was indeed predominantly nuclear in location, even in B-CPAP and FTC-133 cells, which express both SNAI1 and SNAI2 and CDH1. It seems therefore that there must be additional, unknown, factors inhibiting SNAI1- and SNAI2-mediated repression of CDH1 transcription in these two cell lines. As has already been mentioned, a variety of other cell lines demonstrate lack of inverse expression of SNAI1/SNAI2 and CDH1,14,16 likely representing the fact that multiple regulators of CDH1 transcription exist. Consistent with the role of SNAI1 and SNAI2 in producing a mesenchymal cell phenotype, cell lines expressing these transcription factors did not form cell monolayers with close cell-cell contacts. This even included the lines B-CPAP and FTC-133, and therefore it seems that in these lines CDH1 membranous expression does not lead to monolayer formation in the presence of SNAI1 and SNAI2 nuclear expression. A possible explanation for this phenomenon is either posttranslational modification of CDH1 in these cells, for example phosphorylation, or alteration in CDH1 binding partners needed for strong cell-cell adhesion, both of which have been shown to lead to reduced cell-cell adhesion in vitro.31
Next, we wished to assess whether expression of SNAI1 in thyroid cells led to transcriptional repression of known SNAI1 target genes. The ori-3 line was selected, as it is SNAI1- and SNAI2-negative and CDH1-positive. CDH1 transcription is repressed by SNAI1 in cells from a variety of tissue types,14,15 and this was therefore chosen to analyze ability of SNAI1 to repress gene transcription in thyrocytes. In this line, SNAI1 did indeed lead to transcriptional silencing of CDH1 within 24 hours, confirming that these cells represent a suitable thyroid-derived model for analysis of effects of SNAI1 expression. In this context it is interesting to note that a recent report by Rao and colleagues32 demonstrated that treatment of FTC-133 cells with lithium chloride led to up-regulation of SNAI1 expression with concomitant CDH1 down-regulation, agreeing with our observation that SNAI1 can modulate CDH1 expression in thyrocytes. Transfection efficiencies of 70 to 80% were achieved in ori-3 cells, and it seems that, at this level of SNAI1 expression, CDH1 transcription is too low to be detected by RT-PCR or Western blotting of these transfected cells.
On analyzing expression of SNAI1 and SNAI2 within follicular and papillary thyroid carcinoma specimens, it became apparent that both transcription factors were highly expressed in these tumor types, with nuclear and cytoplasmic staining being apparent in most cases; however, SNAI2 demonstrated nuclear expression less commonly in papillary carcinomas. Yang and colleagues33 have shown that subcellular localization of SNAI1 correlates with transcriptional repressive activity, and it is likely that only nuclear transcription factor immunoreactivity represents potentially functionally active protein in the tumors studied. Interestingly, in a small number of tumors, SNAI1 and SNAI2 immunoreactivity was most marked at the invasive front, associated with CDH1 down-regulation. This fits with the proposed roles of SNAI1 and SNAI2 in tumor invasion.34 For both tumor types, there was an inverse correlation between SNAI1/SNAI2 and CDH1 expression in some cases only. Furthermore, SNAI1 and SNAI2 were both expressed in lymph node metastases from papillary carcinomas. This suggests that ectopic expression of these transcription factors occurs early on in the neoplastic process, being present in microfoci of papillary carcinoma and minimally invasive follicular carcinomas, with expression being maintained through to invasive carcinoma and lymphomatous metastases. In agreement with previous observations in mice overexpressing SNAI1 and SNAI2,22,35 these observations indicate SNAI1/SNAI2 can participate in both tumor formation and dissemination during tumor metastasis.
The coexpression of SNAI1 and SNAI2 in thyroid carcinomas raises the question as to whether one or other transcription factor shows dominant activity in tumor cells. From the data presented here, it cannot be shown that either factor is active in thyroid tumors in vivo, although nuclear localization of the transcription factors is suggestive of functionality. Recent evidence demonstrates that SNAI1 and SNAI2 regulate expression of both overlapping and unique genes in an in vitro model36 and perform different roles during breast cancer progression.34
The above experiments provided circumstantial evidence that SNAI1 and SNAI2 may be involved in thyroid carcinogenesis. We wished, however, to obtain more direct proof of any link. SNAI1 demonstrated nuclear localization more commonly than SNAI2 from our immunohistochemical data, and therefore we chose to concentrate on SNAI1 in further experiments. We have previously shown that CombiTA-Snail mice develop a range of epithelial and mesenchymal tumors not seen in wild-type mice, with concordant increased SNAI1 expression in a variety of tissues. This therefore was chosen as a model to assess whether SNAI1 expression led to thyroid carcinomas in vivo. We first confirmed that SNAI1 RNA was present in thyroid glands from Combi-TA mice (Figure 6B) and absent from wild-type mice (not shown). SNAI1 expression was associated with repression of CDH1 transcription in the thyroid (Figure 6B). Thyroid carcinoma development was then assessed in both wild-type and Combi-TA mice. As has been seen, papillary thyroid carcinomas developed in 3 of 61 CombiTA-Snail mice analyzed (5%) compared with 0 of 30 wild-type mice, providing a direct link between SNAI1 expression in the thyroid and papillary carcinoma development. Of interest, irradiation led to an increased rate of tumor development, which also occurred at an earlier age. Furthermore, once thyroid carcinoma had developed, repression of SNAI1 in the thyroid gland to undetectable levels (Figure 6B) did not arrest tumor development, demonstrating that SNAI1 down-regulation does not prevent established tumor growth.
In conclusion, we have demonstrated that SNAI1 and SNAI2 are highly expressed in human thyroid carcinomas and cell lines derived thereof, and SNAI1 expression in mouse thyroid glands is associated with papillary carcinoma development.
Footnotes
Address reprint requests to R.G. Hardy, Tissue Injury and Repair Group, Centre for Regenerative Medicine, University of Edinburgh, Division of Clinical and Surgical Sciences, Room FU501, Chancellors Bldg, 49 Little France Crescent, Edinburgh EH16 4SB, UK. E-mail: r.hardy@ed.ac.uk.
Supported by the Urquhart Trust, UK (to R.G.H.), Fondo Europeo de Desarrollo Regional (to I.S.-G.), Ministerio de Educacion y Ciencia (grants SAF2006-03726, and Proyectos de Estimulo a la Transferencia de Resultados de Investigacion N° 95-0913.OP to I.S.-G.), Junta de Castilla y León (grant CSI03A05), Fondo de Investigacion Sanitaria (grants PI050087 and PI050116), the Fundación de Investigación Mutua Madrilena Automovilista, the Federación de Cajas de Ahorro Castilla y León (I Convocatoria de Ayudas para Proyectos de Investigación Biosanitaria con Células Madre), and Consorcio para el Desarrollo de Tecnologicas Avanzadas para la Medicina (Consorcias Estrategicos Nacionales de Investigacion Tecnica-Ingenio 2010) project.
References
- Segev DL, Umbricht C, Zeiger MA. Molecular pathogenesis of thyroid cancer. Surg Oncol. 2003;12:69–90. doi: 10.1016/s0960-7404(03)00037-9. [DOI] [PubMed] [Google Scholar]
- Mege RM, Matsuzaki F, Gallin WJ, Goldberg JI, Cunningham BA, Edelman GM. Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc Natl Acad Sci USA. 1988;5:7274–7278. doi: 10.1073/pnas.85.19.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant G, Hoang-Vu C, Cetin Y, Dralle H, Scheumann G, Molne J, Hansson G, Jansson S, Ericson LE, Nilsson M. E-cadherin: a differentiation marker in thyroid malignancies. Cancer Res. 1993;53:4987–4993. [PubMed] [Google Scholar]
- Yap AS, Stevenson BR, Keast JR, Manley SW. Cadherin-mediated adhesion and apical membrane assembly define distinct steps during thyroid epithelial polarization and lumen formation. Endocrinology. 1995;136:4672–4680. doi: 10.1210/endo.136.10.7664688. [DOI] [PubMed] [Google Scholar]
- Brabant G, Hoang-Vu C, Behrends J, Cetin Y, Potter E, Dumont JE, Maenhaut C. Regulation of the cell-cell adhesion protein, E-cadherin, in dog and human thyrocytes in vitro. Endocrinology. 1995;136:3113–3119. doi: 10.1210/endo.136.7.7789339. [DOI] [PubMed] [Google Scholar]
- Serini G, Trusolino L, Saggiorato E, Cremona O, De Rossi M, Angeli A, Orlandi F, Marchisio PC. Changes in integrin and E-cadherin expression in neoplastic versus normal thyroid tissue. J Natl Cancer Inst. 1996;88:442–449. doi: 10.1093/jnci/88.7.442. [DOI] [PubMed] [Google Scholar]
- Soares P, Berx G, van Roy F, Sobrinho-Simoes M. E-cadherin gene alterations are rare events in thyroid tumors. Int J Cancer. 1997;70:32–38. doi: 10.1002/(sici)1097-0215(19970106)70:1<32::aid-ijc5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cerrato A, Fulciniti F, Avallone A, Benincasa G, Palombini L, Grieco M. β- and γ-Catenin expression in thyroid carcinomas. J Pathol. 1998;185:267–272. doi: 10.1002/(SICI)1096-9896(199807)185:3<267::AID-PATH113>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Rocha AS, Soares P, Seruca R, Maximo V, Matias-Guiu X, Cameselle-Teijeiro J, Sobrinho-Simoes M. Abnormalities of the E-cadherin/catenin adhesion complex in classical papillary thyroid carcinoma and in its diffuse sclerosing variant. J Pathol. 2001;194:358–366. doi: 10.1002/path.905. [DOI] [PubMed] [Google Scholar]
- Kapran Y, Ozbey N, Molvalilar S, Sencer E, Dizdaroglu F, Ozarmagan S. Immunohistochemical detection of E-cadherin, α- and β-catenins in papillary thyroid carcinoma. J Endocrinol Invest. 2002;25:578–585. doi: 10.1007/BF03345079. [DOI] [PubMed] [Google Scholar]
- von Wasielewski R, Rhein A, Werner M, Scheumann GF, Dralle H, Potter E, Brabant G, Georgii A. Immunohistochemical detection of E-cadherin in differentiated thyroid carcinomas correlates with clinical outcome. Cancer Res. 1997;57:2501–2507. [PubMed] [Google Scholar]
- Naito A, Iwase H, Kuzushima T, Nakamura T, Kobayashi S. Clinical significance of E-cadherin expression in thyroid neoplasms. J Surg Oncol. 2001;76:176–180. doi: 10.1002/jso.1031. [DOI] [PubMed] [Google Scholar]
- Smyth P, Sheils O, Finn S, Martin C, O’Leary J, Sweeney EC. Real-time quantitative analysis of E-cadherin expression in ret/PTC-1-activated thyroid neoplasms. Int J Surg Pathol. 2001;9:265–272. doi: 10.1177/106689690100900402. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia D, Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- Bolós V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller MA, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161:1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimachi K, Tanaka S, Kameyama T, Taquchi K, Aishima S, Shimada M, Sugimachi K, Tsuneyoshi M. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res. 2003;9:2657–2664. [PubMed] [Google Scholar]
- Miyoshi A, Kitajima Y, Kido S, Shimonishi T, Matsuyama S, Kitahara K, Miyazaki K. Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br J Cancer. 2005;92:252–258. doi: 10.1038/sj.bjc.6602266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Mancera PA, Perez-Caro M, Gonzalez-Herrero I, Flores T, Orfao A, de Herreros AG, Gutierrez-Adan A, Pintado B, Sagrera A, Sanchez-Martin M, Sanchez-Garcia I. Cancer development induced by graded expression of Snail in mice. Hum Mol Genet. 2005;14:3449–3461. doi: 10.1093/hmg/ddi373. [DOI] [PubMed] [Google Scholar]
- Francí C, Takkunen M, Dave N, Alameda F, Gomez S, Rodriguez R, Escriva M, Montserrat-Sentis B, Baro T, Garrido M, Bonilla F, Virtanen I, Garcia de Herreros A. Expression of Snail protein in tumor-stroma interface. Oncogene. 2006;25:5134–5144. doi: 10.1038/sj.onc.1209519. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- Hardy RG, Tselepis C, Hoyland J, Wallis Y, Pretlow TP, Talbot I, Sanders DS, Matthews G, Morton D, Jankowski JA. Aberrant P-cadherin expression is an early event in hyperplastic and dysplastic transformation in the colon. Gut. 2002;50:513–519. doi: 10.1136/gut.50.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pálmer HG, Larriba MJ, Garcia JM, Ordonez-Moran P, Pena C, Peiro S, Puig I, Rodriguez R, de la Fuente R, Bernad A, Pollan M, Bonilla F, Gamallo C, de Herreros AG, Munoz A. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917–919. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- Morris JH, Hardin CA. Thyroid cancer in adult following external irradiation. Arch Intern Med. 1964;113:97–100. doi: 10.1001/archinte.1964.00280070099016. [DOI] [PubMed] [Google Scholar]
- Lemoine NR, Mayall ES, Jones T, Sheer D, McDermid S, Kendall-Taylor P, Wynford-Thomas D. Characterisation of human thyroid epithelial cells immortalised in vitro by simian virus 40 DNA transfection. Br J Cancer. 1989;60:897–903. doi: 10.1038/bjc.1989.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabien N, Fusco A, Santoro M, Barbier Y, Dubois PM, Paulin C. Description of a human papillary thyroid carcinoma cell line. Morphologic study and expression of tumoral markers. Cancer. 1994;73:2206–2212. doi: 10.1002/1097-0142(19940415)73:8<2206::aid-cncr2820730828>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Havekes B, Schroder van der Elst JP, van der Pluijm G, Goslings BM, Romijn JA, Smit JW. Beneficial effects of retinoic acid on extracellular matrix degradation and attachment behaviour in follicular thyroid carcinoma cell lines. J Endocrinol. 2000;167:229–238. doi: 10.1677/joe.0.1670229. [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Rao AS, Kremenevskaja N, Resch J, Brabant G. Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/β-catenin signalling. Eur J Endocrinol. 2005;153:929–938. doi: 10.1530/eje.1.02038. [DOI] [PubMed] [Google Scholar]
- Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Res. 2005;65:3179–3184. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- Côme C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, Savagner P. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–5402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- Pérez-Mancera PA, Gonzalez-Herrero I, Perez-Caro M, Gutierrez-Cianca N, Flores T, Gutierrez-Adan A, Pintado B, Sanchez-Martin M, Sanchez-Garcia I. SLUG in cancer development. Oncogene. 2005;24:3073–3082. doi: 10.1038/sj.onc.1208505. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S, Bolos V, Jorda M, Fabra A, Portillo F, Palacios J, Cano A. Genetic profiling of epithelial cells expressing e-cadherin repressors reveals a distinct role for snail, slug, and e47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543–9556. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]