Abstract
The role of estrogens in the etiology of prostate cancer is controversial. To demonstrate the specific effects of estrogens and androgens on the development of the prostatic epithelial hyperplasia, we used luteinizing hormone receptor knockout mice (LuRKO), which are resistant to pituitary regulation mediated by luteinizing hormone, lack postnatal androgen production, and have rudimentary accessory sex glands, the growth of which can be induced with exogenous androgen replacement. This model is thus ideal for the investigation of direct hormonal effects on the prostate. Testosterone, but not 5α-dihydrotestosterone, replacement from 21 days of life for 8 weeks induced pronounced hyperplasia and inflammation in the prostates of LuRKO mice. Interestingly, 5α-dihydrotestosterone combined with 17β-estradiol did not induce hyperplasia or inflammation, and treatments with inhibitors of estrogen action, aromatase inhibitor, and ICI 182780 further exacerbated testosterone-induced hyperplastic growth. However, the activation of estrogen receptor (ER)-β with a specific agonist, DPN [2,3-bis(4-hydroxyphenol)-propionitrile], prevented the development of prostatic hyperplasia and inflammation in testosterone-treated LuRKO mice. Thus, it seems that in the presence of sufficient androgenic stimulation, it is the balance between ER-α- and ER-β-mediated signaling that determines whether estrogens promote hyperplasia or protect the prostate against hyperplastic changes.
Androgens play a central role in the biology of the prostate. Estrogens, however, can also modulate prostatic growth and development. There is strong experimental evidence that, at least in rodents, excessive or untimely exposure to estrogens can induce prostatic neoplasia.1,2,3,4,5,6 Moreover, aromatizable but not nonaromatizable androgens can cause prostate cancer.7,8 On the other hand, impaired estrogen action can also lead to structural and functional abnormalities in prostatic epithelium, as has been demonstrated in estrogen receptor (ER)-β9 or aromatase-deficient mice.10 Furthermore, transgenic mice overexpressing androgen receptors (AR) in the prostate epithelium present with increased epithelial proliferation and develop prostatic intraepithelial neoplasia,11 and ER-β knockout (ERβKO) mice have increased expression of AR in prostate epithelium,9 indicating that enhanced androgen action in prostate epithelium also promotes the development of prostatic hyperplasia and dysplasia. Taken together, these observations imply that both androgens and estrogens are needed to induce proliferative and precancerous lesions and prostate cancer in rodent models.
The effect of estrogens on the prostate may be indirect and mediated by the inhibition of androgen secretion, or direct action mediated via ERs in the prostate. Both ER subtypes, ER-α (ESR1) and ER-β (ESR2), are expressed in the prostate: ER-α is found in stromal cells of the prostatic urethra,12,13,14 whereas ER-β is highly expressed in rodent and human prostatic stroma and epithelium.15,16 One hypothesis of the endocrinological control of the prostate is that androgens cause proliferation and functional activation (secretion) of the prostatic epithelium via AR and that estrogens suppress proliferation and promote differentiation of the prostatic epithelium via ER-β.9,17 In addition, the stromal ER-α in prostate can induce epithelial changes, specifically squamous epithelial metaplasia, in highly estrogenized animals.18
In the present study, we used luteinizing hormone (LH) receptor knockout mice (LuRKO), which are insensitive to pituitary regulation mediated by LH and lack postnatal androgen production.19 The prostates of LuRKO mice are rudimentary, but they can be induced to grow to the normal size with exogenous androgen replacement.20 Thus, these mice offer an excellent model to study the effects of different hormonal treatments on the growth of the prostate. In this study, LuRKO mouse model was used to demonstrate the role of androgens and estrogens in the progression of hyperplastic lesions. Because ER-β has been shown to regulate prostatic growth and differentiation,9,17 its role was studied by administering to LuRKO mice a specific ER-β agonist. The results revealed a protective role for ER-β in the development of hyperplasia and inflammation in the prostate.
Materials and Methods
Animals
LuRKO mice and their wild-type (WT) littermates were used. In LuRKO mice a targeted deletion of exon 11 of the LH receptor gene totally inactivates LH/LHR function.19 All animals were housed in a controlled environment on an illumination schedule of 12 hours light/12 hours dark and fed with a soy-free diet (RM3; SDS, Witham, UK) and water ad libitum. Mice were genotyped using the polymerase chain reaction-based method described previously.19 For each treatment group four to six mice were used. Because some experiments were repeated, the total number of animals per treatment varied from 4 to 12. All procedures used were performed according to the institutional policy of the University of Turku.
Hormone Treatments
Chemicals
Aromatase inhibitor finrozole, MPV-2213ad (Hormos Medical Ltd., Turku, Finland), which has 4-[3-(4-fluorophenyl)-2-hydroxy-1-(1H-1,2,4-triazol-1-yl) propyl]benzonitrile as a base, is a novel, potent, and selective nonsteroidal aromatase inhibitor that blocks conversion of androgens to estrogens. The potency and specificity of Finrozole has been demonstrated in vitro21 in humans21 and in rodents.22
DPN [2,3-bis(4-hydroxyphenol)-propionitrile] (Tocris Cookson Inc., Ellisville, MO) is a highly potent ER-β agonist with a 70-fold selectivity over ER-α.23,24 The antiproliferative effect of DPN has been shown previously in vitro in mouse tissue.25
ICI 182,780 [7α,17β-[9[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol] (Tocris Cookson Ltd., Northpoint, UK) is a high-affinity ER antagonist (IC50 = 0.29 nmol/L), devoid of any partial agonism both in vitro and in vivo.26,27
Testosterone (T), dihydrotestosterone (DHT), testosterone propionate (TP), and 17β-estradiol dipropionate were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany), and 17β-estradiol (E2) pellets were from Innovative Research of America (Sarasota, FL).
Androgen Replacement
One-cm-long silastic capsules (inner diameter, 1.98 mm) filled with either T or DHT were implanted subdermally under anesthesia into homozygous LuRKO male mice or their WT littermates at the age of 21 days. Silastic tubing (Chase Walton Elastomers; SF Medical, Hudson, MA) sealed at both ends with silicone (Silastic; Medical adhesive silicone, type A; Dow Corning France S.A.S., Lyon, France) was used. Anesthesia was performed using isoflurane (2 to 3%) and buprenorphine (Temgesic; Schering-Plough, Brussels, Belgium; 3 to 5 μg/mouse s.c.) for postoperative analgesia. Androgen replacement was continued for 8 or 16 weeks. In the latter case, capsules were renewed after 8 weeks.
ICI 182,780, Finrozole, and DPN Treatments
Three-week-old WT and LuRKO mice were implanted with T capsules, as described above. ICI 182,780, finrozole, or DPN treatments were started simultaneously with androgen replacement. Mice were injected subcutaneously with ICI 182,780, 0.5 mg/mouse twice per week for 8 weeks. ICI 182,780 was diluted to 10% ethanol/oil-solution. Finrozole and DPN were administered subdermally with silastic tubing filled with either 4 mg of finrozole or 4 mg of DPN. After 4 weeks, finrozole and DPN capsules were removed, and treatments were continued for 4 weeks with daily subcutaneous injection, the dose being in both cases 3 mg/kg body weight/day.
17β-Estradiol Treatment
Three-week-old LuRKO and WT male mice were implanted subdermally simultaneously with DHT silastic capsules as described above and E2 pellets, the daily dose of E2 being 25 μg/animal.
Short Exposure of LuRKO Mice to TP, DHT, or 17β-Estradiol Dipropionate
Three-week-old LuRKO and WT male mice were injected subcutaneously once a day for 3 days with TP (100 μg/g body weight), DHT (10 μg/g body weight), or β-estradiol dipropionate (0.25 μg/g body weight) and then sacrificed. All hormones were diluted to 10% ethanol/oil solution.
Serum T, DHT, and Prolactin Hormone Assays
For serum T determination, 25-μl aliquots were extracted twice with 2 ml of diethyl ether and evaporated under nitrogen to dryness. The residues were reconstituted in phosphate-buffered saline (PBS) and measured using standard radioimmunoassay as described previously.28 DHT was measured using an enzyme-linked immunosorbent assay kit from Alpha Diagnostic International (San Antonio, TX). Serum prolactin was measured by radioimmunoassay, as described previously.29
Preparation of Tissue Samples and Histological Analysis
Animals were euthanized by CO2 suffocation. The prostates were dissected, fixed in 10% neutral formalin for 24 hours at room temperature, dehydrated in ethanol, cleared in xylene, and embedded in paraffin. Prostate blocks were serially cut at 100-μm intervals, and 4-μm sections were mounted on coated slides. The slides were incubated overnight at +37°C and stored at +4°C.
Hematoxylin and eosin (H&E)-stained serial sections were examined under ×10 magnification, and all of the acini from the largest section per lobe were analyzed using Image J software (National Institutes of Health, Bethesda, MD). To determine the relative epithelial area per each acinus, both the epithelial area and total acinus area were determined. The percentages of tufting and bridging acini and acini with intraepithelial inflammation were calculated. Sections containing all prostatic lobes were used for immunohistochemistry. All assessments were performed using a BX-51 microscope (Olympus Corp., Tokyo, Japan). The images were captured by a DP70 digital microscope camera (Olympus Corp.).
Antibodies
Antibodies against ER-α (monoclonal, clone 1D5), progesterone receptor (PR) (rabbit anti-human PR, A0098), and Ki-67 (rat anti-mouse Ki-67, M 7249) were purchased from DAKO A/S (Glostrup, Denmark). Antibodies against AR (N-20), p63 (mouse anti-p63, clone 4A4), and ER-β (MCA1974S) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), BD Biosciences Pharmingen (San Diego, CA), and Serotec Ltd. Scandinavia (Hamar, Norway), respectively.
Immunoperoxidase Staining for ER-α, PR, AR, p63, ER-β, and Ki-67
Slides were dewaxed and rehydrated. After washing in water, sections underwent antigen retrieval in a microwave oven in 10 mmol/L sodium citrate buffer, pH 6.0, for 15 minutes. For ER-β staining 10 mmol/L Tris-buffer (0.05% Tween 20, pH 10) was used as antigen retrieval buffer. The sections were allowed to cool and rinsed with PBS buffer. Then the endogenous peroxidase was blocked by incubating the sections in 1% H2O2 for 20 minutes. After PBS rinses, sections were incubated with primary antibodies overnight at +4°C. ER-β antibody was diluted 1:100, ER-α and Ki-67 antibodies 1:200, PR antibody 1:400, and AR and p63 antibodies 1:1000 in PBS (3% bovine serum albumin, 0.05% Tween 20). The next day, sections were washed with PBS and incubated at room temperature for 30 minutes with secondary antibodies (horseradish peroxidase-conjugated DAKO Envision+ systems) or for Ki-67 staining for 60 minutes with rabbit anti-rat secondary antibody (Rockland Inc., Gilbertsville, PA). Slides were rinsed with PBS. Color was developed with diaminobenzidine substrate (DAKO Envision system). Sections were then slightly counterstained with Mayer’s hematoxylin, dehydrated, and mounted.
Determination of p63-Positive Cells
The images of p63-stained ventral (VP) and dorsal (DP) prostatic lobes of T- and T + DPN-treated LuRKO mice were captured and then analyzed using Image J software. Two thousand epithelial cells per lobe per mouse were evaluated, and percentages of p63-positive cells were calculated. The counting was systemically started from one side, and in most of the cases, all of the acini in one section were analyzed.
Statistical Analysis
Relative epithelial areas, percentages of p63-positive cells, and androgen levels among treatment groups were analyzed. Significant differences were determined by t-test (percentages of p63-positive cells) or by one-way analysis of variance analysis followed by the posthoc Tukey multiple comparison test (relative epithelial areas and androgen levels). The analyses were conducted using SAS 9.1 software (SAS Institute Inc., Cary, NC). Data are expressed as mean ± SEM.
Results
T but Not DHT Induces Prostatic Hyperplasia with Focal Mild Dysplasia and Inflammation
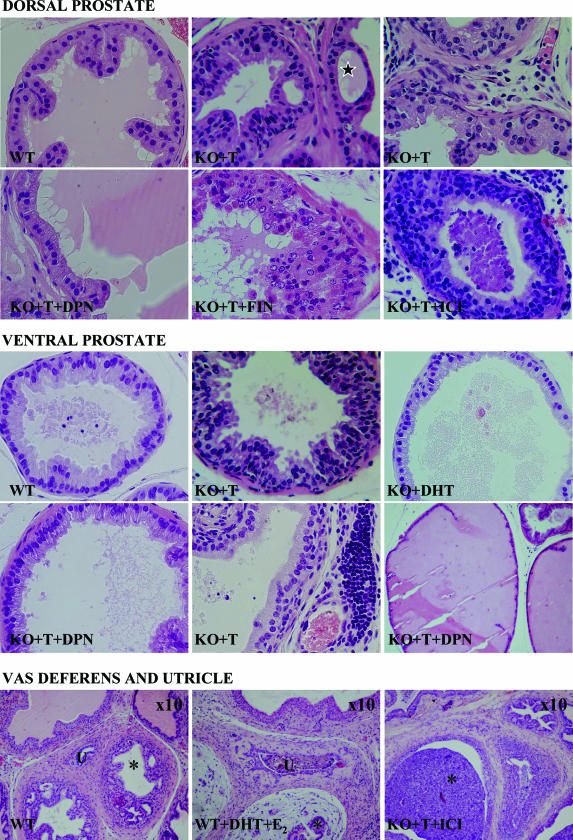
As shown previously,19 the prostates, seminal vesicles, and coagulating glands of the adult intact LuRKO mice were poorly developed, whereas in the T- or DHT-treated LuRKO mice, the sizes of prostate and other accessory sex glands were similar to those of intact WT mice. In addition, pronounced forms of hyperplasia with mild dysplasia (criteria according to Shappell et al30), and inflammation were found in T-treated LuRKO mice (Table 1). These hyperplastic lesions were characterized by either proliferative tufting pattern (Figure 1) or architectural changes, such as intraluminal gland formation, ie, bridging and cribriformic patterns (Figure 1, black star). Longer T treatment (16 weeks) did not exacerbate hyperplastic lesions in LuRKO mice. In contrast to T, DHT induced normal growth or only moderate proliferative changes in the epithelium, seen also in WT mice (Figure 1). Hyperplasia indicated with the relative epithelial area was significantly increased (P < 0.05). Likewise, the percentage of acini with epithelial tufting or bridging was threefold higher in ventral and ninefold higher in dorsal prostates of T-treated LuRKO mice compared with the acini in DHT-treated LuRKO mice (Table 1).
Table 1.
Epithelial Hyperplasia and Inflammation in the Ventral and Dorsal Prostates of Differently Treated WT and LuRKO Mice
| n | Epithelial hyperplasia
|
Inflammation
|
|||
|---|---|---|---|---|---|
| Relative epithelial area (%) | Tufts/bridging (%) | Stromal + to +++ | Intraepithelial (%) | ||
| Ventral prostate | |||||
| WT + T | 6 (257) | 50.40 ± 1.93*(1) | 3.04 | ||
| WT + DHT | 4 (288) | 51.10 ± 3.07*(2) | 2.50 | ||
| LuRKO + T | 8 (235) | 68.75 ± 5.19*(1,2,3,4,5) | 60.85 | ++ to +++ in 4 | 14.89 |
| LuRKO + T + ICI | 6 (177) | 62.90 ± 5.57*(6,7) | 48.19‡ | + to +++ in 5 | 8.80 |
| LuRKO + T + Finrozole | 5 (191) | 61.07 ± 2.42*(8,9) | 22.40 | ++ in 1 | 0.50 |
| LuRKO + T + DPN | 8 (288) | 45.29 ± 3.92*(4,6,8) | 19.44 | + in 1 | |
| LuRKO + DHT | 7 (234) | 42.25 ± 3.18*(3,7,9) | 21.10 | + in 1 | |
| LuRKO + DHT + E2 | 5 (216) | 53.36 ± 4.38*(5) | 6.70† | ||
| Dorsal prostate | |||||
| WT + T | 8 (280) | 53.10 ± 2.38*(1,2,3,4) | 1.00 | ||
| WT + DHT | 4 (179) | 52.24 ± 3.37*(5,6,7) | 0.00 | ||
| LuRKO + T | 8 (209) | 71.62 ± 1.85*(1,5,8,9) | 62.68 | + to +++ in 4 | 16.74 |
| LuRKO + T + ICI | 5 (116) | 70.17 ± 5.18*(2,6,10) | 76.70 | + to +++ in 5 | 9.50 ‡ |
| LuRKO + T + Finrozole | 5 (93) | 75.00 ± 2.49*(3,7) | 61.54 | ||
| LuRKO + T + DPN | 8 (232) | 52.17 ± 0.91*(8,10,11) | 9.05 | + in 1 | 0.50 |
| LuRKO + DHT | 6 (139) | 57.80 ± 2.10*(9) | 7.01 | + in 1 | 6.40 |
| LuRKO + DHT + E2 | 4 (209) | 66.32 ± 3.13*(4,11) | 15.00 | ++ in 1 | 4.50 |
n = number of animals in a treatment group, with the number of acini analyzed per treatment group shown in parentheses. Relative epithelial area (%) describes the epithelial area related to the total acinus area. Acini having tufts and/or branching and bridges are counted and related to the total number of acini per treatment group. The intensity of stromal diffuse inflammation is described as + (weak), ++ (moderate), +++ (strong), and number of affected mice per treatment group is stated. Percentages of acini with intraepithelial inflammation per treatment group are demonstrated. T, testosterone; DHT, dihydrotestosterone; ICI, ICI 182,780; E2, 17β-estradiol.
Significant changes in relative epithelial area (P < 0.05).
In the dorsal prostate of DHT + E2-treated LuRKO mice, 40.5% of the acini are atrophic.
Inflammation in T + ICI-treated mice causes epithelial destruction, thus decreasing the number of tufting/bridging formation. Data expressed as means (±SEM).
Figure 1.
Effects of different treatments on the morphology of the prostate. Morphology of H&E-stained dorsal and ventral prostates and prostatic urethra and vas deferens of WT and with T-, DHT-, T + DPN-, T + finrozole (FIN)-, and T + ICI 178,820 (ICI)-treated LuRKO (KO) mice. Black star indicates bridging formations in prostatic epithelia. Vas deferens is marked with black asterisk and utricle with U. Original magnifications, ×40, if not otherwise stated.
In addition to hyperplastic lesions, T but not DHT treatment induced inflammation in LuRKO but not in WT mice. In T-treated LuRKO mice, prominent inflammation, indicated by the accumulation of lymphocytes (Figure 1, second panel), was observed in the epithelium and surrounding stroma of the vas deferens and coagulating glands and in some distinct foci in the epithelium and focally or diffusely in the stroma of the VP and DP (Table 1).
Estrogens and Prostatic Hyperplasia and Inflammation
The role of estrogens in the prostate was further studied by administering the LuRKO mice with i) DHT combined with E2; ii) T combined with inhibitor of P450 aromatase, finrozole; iii) T combined with ER antagonist, ICI 182,780; or iv) T combined with a potent ER-β-specific agonist, DPN. DHT combined with E2 did not induce hyperplasia in LuRKO mice, but variable estrogenic effects such as atrophy (small lumina lined with single-layer cuboidal epithelium), prominent expression of PR, stromal edema around the vas deferens, and large, squamous metaplastic prostatic utricle (Figure 1, third panel) were detected. ICI 182,780 or finrozole combined with T had no effect or even exacerbated the hyperplasia demonstrated with relative epithelial area and percentage of tufting or cribriformic acini (Figure 1, first panel; Table 1). The T-induced development of hyperplastic lesions and inflammation were, however, prevented by administration of DPN. The relative epithelial area was significantly smaller after combined T and DPN treatment than after T treatment alone in both lobes: 42.3 and 68.8% in VP and 57.8 and 71.6% in DP, respectively (Table 1). In addition, the tufting and bridging appearance decreased (Table 1), and prostatic epithelial cells, especially in VP (Figure 1, second panel) but also in DP (Figure 1, first panel), were tall and had secretory appearance. However, the epithelium was more crowded than in intact WT mice (Figure 1, first and second panels). There were also a number of highly dilatated, secretion filled acini (Figure 1, second panel). Inflammation was exacerbated in LuRKO mice treated with T combined with ICI 182,780 (Figure 1, first and third panels), whereas finrozole and DPN treatments ameliorated inflammation (Table 1). Most of the hyperplastic sites were free from inflammation.
Serum T, DHT, and Prolactin Concentrations
The results of T and DHT measurements are shown in Table 2. In untreated LuRKO mice, the androgen levels were very low or undetectable. The T capsules were expected to increase serum T levels above the normal range of WT mice. Accordingly, the mean T levels in the treated LuRKO and WT mice were 6.40 ± 1.73 ng/ml and 9.10 ± 2.19 ng/ml, respectively, which were ∼7- and 10-fold higher than in WT mice (0.92 ± 0.29 ng/ml). The corresponding DHT levels were 0.33 ± 0.27, 0.91 ± 0.26, and 0.99 ± 0.16 ng/ml for WT, T-treated WT, and LuRKO mice, respectively. The serum DHT concentrations after DHT treatments were 0.19 ± 0.03 ng/ml for WT and 0.74 ± 0.37 ng/ml for LuRKO mice. The mean prolactin level was lower in T-treated LuRKO mice (5.7 ± 0.3 ng/ml) than in WT mice (10.3 ± 1.7 ng/ml).
Table 2.
Androgen Levels in WT and LuRKO Mice
| Serum T (ng/ml)
|
Serum DHT (ng/ml)
|
|||
|---|---|---|---|---|
| n | Mean (SEM) | n | Mean (SEM) | |
| WT | 3 | 0.92 (0.29) | 6 | 0.33 (0.27) |
| WT, T-treated | 5 | 9.10 (2.19) | 6 | 0.91 (0.26) |
| WT, DHT-treated | ND | 6 | 0.19 (0.03)* | |
| LuRKO | 4 | 0.12 (0.02) | 4 | ND |
| LuRKO, T-treated | 5 | 6.40 (1.73) | 7 | 0.99 (0.16)* |
| LuRKO, DHT-treated | ND | 5 | 0.74 (0.37) | |
Amount (ng/ml) of serum testosterone (T) or dihydrotestosterone (DHT) in untreated, T- or DHT-treated WT and LuRKO mice analyzed with either radioimmunoassay (T) or enzyme-linked immunosorbent assay (DHT). n, number of samples analyzed per group. ND, not determined.
Groups differ significantly (P < 0.05) from each other.
Immunoperoxidase Stainings for p63, ER-α, PR, AR, ER-β, and Ki-67 in 11-Week-Old Mice after 8 Weeks of Treatment
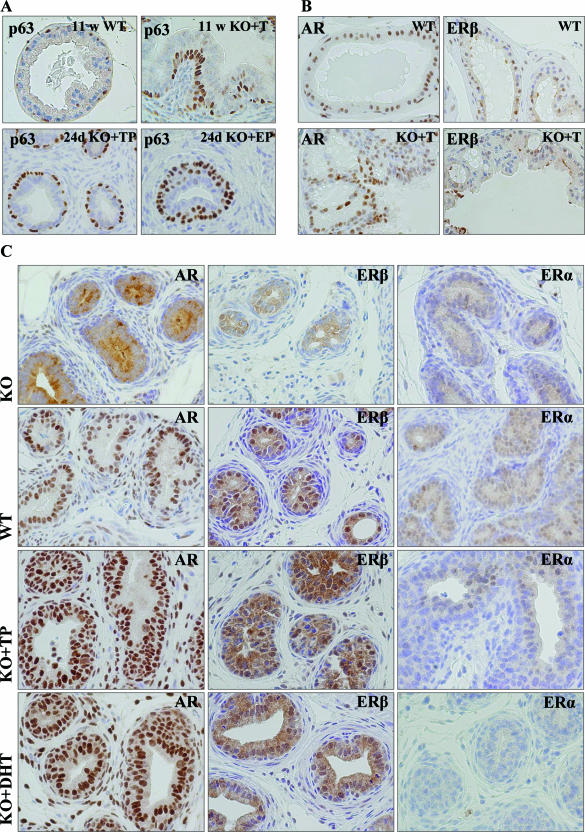
The p63-positive basal epithelial cells are normally detected with a scattered appearance beneath the luminal epithelial cell layer (Figure 2A, top row). In T-treated LuRKO mice, the prostatic epithelium presented with increased number of p63-positive cells in many cellular layers (Figure 2A). Treatment with T combined with DPN prevented this increase, and the number of p63-positive cells was significantly lower in both lobes in T + DPN-treated mice as compared with the mice treated with T alone (Table 3). Immunoreactive ER-β and AR were both present in the hyperplastic lesions of T-treated LuRKO mice, although the expressions were more heterogeneous than in WT mice (Figure 2B) or in LuRKO mice after DHT or combined T and DPN treatments. No differences were observed in the expression of ER-α or PR, both being low/undetectable in prostatic epithelium (data not shown). The number of proliferating Ki-67-positive cells in the sections was very low, and no differences between treatment groups could be observed.
Figure 2.
p63, AR, ER-β, and ER-α expressions in the dorsal prostates of WT and differently treated LuRKO (KO) mice. A: p63 expression in the prostates of 11-week-old WT and T-treated LuRKO mice and of 24-day-old TP- and β-estradiol dipropionate (EP)-treated LuRKO mice. B: Expressions of AR and ER-β in the dorsal prostates of 11-week-old WT and T-treated LuRKO mice. C: Expressions of AR, ER-β, and ER-α in the 24-day-old LuRKO, WT, and TP- and DHT-treated LuRKO mice. Brown-stained nuclei are considered positive. Original magnifications, ×40.
Table 3.
The Percentages of p63-Positive Cells
| Treatment group | n | Ventral prostate (mean ± SEM) | Dorsal prostate (mean ± SEM) |
|---|---|---|---|
| KO + T | 8 | 21.94 ± 0.79* | 21.30 ± 0.94* |
| KO + T + DPN | 8 | 10.94 ± 1.52 | 14.15 ± 1.30 |
p63-positive cells per 2000 epithelial cells per lobe of T-treated versus T- and DPN-treated LuRKO mice were calculated. There were significantly (*P < 0.05) more p63-positive cells in the ventral and dorsal prostatic lobes of T-treated LuRKO mice than in the prostates of T + DPN-treated LuRKO mice. n = number of animals analyzed per treatment group.
Immunoperoxidase Stainings for p63, ER-α, PR, AR, and ER-β in 24-Day-Old Mice after Short Androgen and Estrogen Exposures
In both peripubertal WT and LuRKO mice, TP and DHT induced the growth of prostatic ducts and acini, whereas EP induced primarily the growth of stromal compartments. TP and DHT, but not EP, induced strong AR and ER-β expressions in the prostatic epithelium and stroma (Figure 2C). TP but not DHT treatment caused a weak induction of ER-α (Figure 2C) and PR (data not shown) in the DP epithelium of the LuRKO mice. After T and DHT treatments, distinct basal and luminal cell layers were detected in the prostatic ducts, whereas EP induced an increase and multilayering of the p63-positive epithelial basal cells (Figure 2A, bottom row).
Discussion
The main objective of this study was to elucidate the role(s) of estrogens and ERs in a novel murine model (LuRKO) of T-induced prostatic hyperplasia. The prerequisite of androgens and ARs for the development of proliferative lesions and prostate cancer has been shown convincingly in previous studies.4,31 Only twofold or threefold elevation in circulating T has been shown to be sufficient to induce prostate carcinoma in several rat strains.7,8,32,33 However, also in line with earlier findings, it is likely that androgenic stimulation alone is not the major determinant for the induction of epithelial hyperplasia in LuRKO model because no such changes were induced by a nonaromatizable androgen DHT. There were no major differences between the androgen levels in the different treatment groups (T, DHT) taking into account that the androgenic potency of DHT is ∼10-fold higher than that of T.34 This supports the idea that E2, produced by aromatization of T, promotes the development of prostatic hyperplasia.
The specific roles of the two ER subtypes in normal and pathological growth processes in the prostate are not yet fully known. Interestingly, treatment with the specific ER-β agonist DPN prevented the formation of T-induced epithelial changes in LuRKO model. This strongly suggests that selective transactivation of ER-β protects against prostatic hyperplasia, as has been proposed on the basis of the ER-βKO mouse prostate phenotype,9 as well as studies on the effects of the endogenous ER-β ligand 5α-androstane-3β,17βdiol (3βAdiol).35 Inhibition of hyperplasia by DPN is also in accordance with the antiproliferative effects of ER-β, which have been demonstrated previously in mouse VP9,35 and in mouse mammary cell lines.25,36
On the other hand because E2 is a nonselective ER agonist that promotes the development of hyperplasia, it is likely that ER-α transactivation contributes to this pathological process. We thus hypothesize that ER-β transactivation by 3βAdiol, a metabolite of DHT, and by E2 promotes the maintenance of the normal epithelial architecture. In contrast, transactivation of ER-α by E2 promotes abnormal growth. Further, androgens and AR are needed in both situations. In other words, it seems that it is the balance between ER-α- and ER-β-mediated signaling that determines which epithelial phenotype is favored.
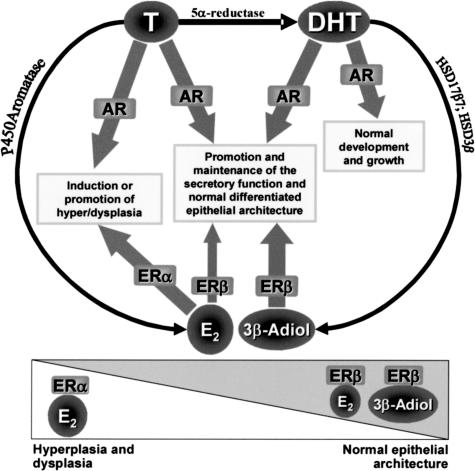
Schematic presentation of the suggested roles of different hormones and receptors in the normal and hyperplastic growth is shown in Figure 3. After treatment with T (and increased level of E2) ER-α-mediated signaling is enhanced, promoting epithelial overgrowth, whereas after DHT alone, or with T combined with DPN, the ER-β-mediated signaling dominates, and normal epithelial architecture is maintained. In line with this hypothesis, treatment with nonselective inhibitors of estrogen action (the pure ER antagonist ICI 182,780 and the aromatase inhibitor finrozole), which attenuate both ER-α- and ER-β-mediated actions, exacerbated T-induced changes in LuRKO model. Furthermore, treatment with DHT + E2 did not induce epithelial hyperplasia, probably because ER-β-signaling was favored. In addition, in another prostate tumor model, namely in Noble rats, DHT seems to have a protective role because DHT + E2 induced markedly less tumors compared with T + E2 treatment.6
Figure 3.
Schematic figure of the androgen and ERs, enzymes, and mechanisms involved in the normal or hyperplastic/dysplastic epithelial growth. In the presence of sufficient androgens/AR, ER-β transactivation (by 3βAdiol and E2) promotes the maintenance of the normal differentiated epithelial architecture, whereas transactivation of ER-α (by E2) promotes the development of hyperplasia/dysplasia. The balance between ER-α- and ER-β-mediated signaling is critical for the induction of pathological processes. E2, 17β-estradiol; 3βAdiol, 5α-androstane-3β,17βdiol; HSD17β7, 17β-hydroxysteroid dehydrogenase type 748; HSD3β, 3β-hydroxysteroid dehydrogenase.35
ER-β-mediated signaling also promoted the differentiation and survival of prostatic epithelial cells as demonstrated with increased number of luminal epithelial cells after DPN treatment in this study. In line with this hypothesis, an opposite effect has been found in ER-βKO mice, in which increased detachment of prostatic epithelial cells has been reported.17 Previous studies with ER-βKO mice have also shown an increase in number of p63-positive basal cells and a reduction in the fraction of cytokeratin-positive fully differentiated cells in the VP.17 Accordingly, the T-induced increase of p63-positive basal cells in the prostates of LuRKO mice was normalized after DPN treatment. However, to determine the early effects of androgens and estrogens on LuRKO mouse prostate, we treated 3-week-old mice with TP, DHT, or 17β-estradiol dipropionate (EP) for 3 days. As expected, in both WT and LuRKO mice, TP and DHT induced the growth of strongly AR-positive prostatic ducts and acini, whereas EP induced primarily the growth of ER-α-positive stromal tissue. Interestingly, only after TP treatment was induction of ER-α and PR found in the DP epithelium of LuRKO mice, further demonstrating the difference in the epithelial response to a treatment with aromatizable versus nonaromatizable androgen. Furthermore, EP induced an increase and multilayering of the p63-positive basal epithelial cells. Increased proliferation of basal cells has been demonstrated also in the DP of adult mice after DES treatment,18 and this effect has been shown to be mediated by ER-α.37
The induction of ER-α in the epithelium by T, and/or lack of sufficient ER-β-stimulation, and the subsequent increase in the number of undifferentiated cells susceptible for the formation of hyperplasia may be the key event initiating the pathological process in the prostate. In addition to the postnatal delay in androgen- and estrogen-induced differentiation, the exceptionally rapid development of hyperplastic lesions in the prostates of T-treated LuRKO mice is likely attributable to the strong proliferative stimulus of high levels of androgens. Likewise, in neonatally castrated rats, the lack of proper androgen/estrogen-induced differentiation combined with peripubertal androgen replacement has been reported to induce prostatic epithelial abnormalities at relatively rapid phase.38 In neonatally estrogenized mice and rats, in which the early postnatal androgen secretion peak is attenuated and which are permanently hypoandrogenemic, prostatic hyperplasia and dysplasia is commonly observed, but not until after 9 months after treatment.1,39 It is noteworthy that dysplasia or even carcinoma can be induced in normal adult rodent prostate with a prolonged T treatment.4,31 The mechanism is probably the same as in LuRKO model, but in the adult prostate the number of undifferentiated cells is apparently much lower than in pubertal LuRKO mouse, and thus the pathological process also slower.
Persistent hyperprolactinemia is associated with prostatic hyperplasia, as well as prostatitis, as demonstrated in transgenic mice overexpressing prolactin.22,40 Excessive estrogen exposure increases prolactin production in male mice,22 which, in turn may play a role in the development of estrogen-induced prostatic neoplasia.41 However, in our study, the structural changes observed in LuRKO mouse prostate are not likely to be induced by prolactin because prolactin levels in T-treated LuRKO mice were not higher than in WT mice.
In addition to pronounced hyperplasia, T but not DHT treatment induced inflammation in LuRKO but not in WT mice. Persistent inflammation is known to be linked with increased risk of cancer in several organs, including the prostate. However, in this study, no obvious link between inflammation and hyperplasia was observed because most of the sites with hyperplastic lesions were free from inflammation. Sexual dimorphism in immunological disease susceptibility is a well-known phenomenon.42 The male immune system seems to develop along with increasing androgen production during puberty because prepubertal castration results in acceleration of a generalized autoimmune disorder in NZB/W male mice.43 Even in the neonatally estrogenized mice, infiltration of immune cells in the prostate commences with puberty.39 Thus, in both neonatally estrogenized WT and LuRKO mice, the maturity of the male immune system may be disturbed because the former has decreased expression of AR,2,44 and the latter lacks androgens after disappearance of fetal Leydig cells at approximately day 10 of life. Although the role of estrogens and ERs in autoimmune diseases is controversial, both ER-deficient mice models, ER-αKO and ER-βKO, have symptoms of autoimmune diseases.45 The role of ER-β as an immunoprotective agent is emphasized in the study in which a wide range of different ER-β-selective agonists were shown to have a beneficial effect in two rat models of inflammation.46 The mechanisms of immunoprotective action of DPN or the other ER-β agonists are not known. In our study, the local effects are possible because ER-β is normally expressed in the periurethral and prostatic sites where inflammation is found. DPN may also exert its effect systemically by modulating the immune system because ER-β has been identified to be the predominant ER in human lymph nodes and spleen, which play a central role in B- and T-cell immune reactions.47 We also found that aromatase inhibitor ameliorated inflammation. We hypothesize, that while aromatase activity is inhibited, more of the T in the prostate is converted to DHT, which is further metabolized to 3βAdiol, an ER-β-ligand. These results, together with exacerbation of inflammation observed after treatments with anti-estrogen ICI 182,780, blocking both ER subtypes, further emphasize the immunoprotective role of ER-β.
Conclusions
In conclusion, the LuRKO mice serve as a model for hormone-induced prostatic hyperplasia, with an exceptionally short induction time needed for the development of hyperplastic lesions. LuRKO prostate is highly sensitive for hormonal stimulus, and T-treated LuRKO mice provide a novel model to study the early events of hormone-induced malignant changes. Our present data, as well as those of others suggests that ERs have a dual role in the prostate. In the presence of a sufficient androgenic stimulation, it is the balance between ER-α- and ER-β-mediated signaling that is critical: ER-α promotes hyperplasia and ER-β the maintenance of the normal epithelial architecture. Induction of ER-α by T, probably via conversion to E2, affecting the cytodifferentiation may thus be one of the events essential for the pathological process in the prostate.
Acknowledgments
We thank Ms. Erja Mäntysalo for managing LuRKO mice; Ms. Teija Hurmerinta, Ms. Liisi Kortela, and Ms. Hannele Rekola for skillful technical assistance; and Hormos Medical Ltd. for providing finrozole for this study.
Footnotes
Address reprint requests to Sari Mäkelä, University of Turku, Functional Foods Forum, FL-20014 University of Turku, Finland. E-mail: sarmak@utu.fi.
Supported by the European Commission (CASCADE Network of Excellence, FOOD-CT-2004 506319 to S.S. and S.M.), the Drug Discovery Graduate School (to S.S.), and the Academy of Finland (grants 211480 and 207028 to T.P., I.H., and M.P.).
References
- Pylkkänen L, Santti R, Newbold R, McLachlan J. Regional differences in the prostate of the neonatally estrogenized mouse. Prostate. 1991;18:117–129. doi: 10.1002/pros.2990180204. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with αERKO and βERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- Risbridger GP, Bianco JJ, Ellem SJ, McPherson SJ. Oestrogens and prostate cancer. Endocr Relat Cancer. 2003;10:187–191. doi: 10.1677/erc.0.0100187. [DOI] [PubMed] [Google Scholar]
- Noble RL. Prostate carcinoma of the Nb rat in relation to hormones. Int Rev Exp Pathol. 1982;23:113–159. [PubMed] [Google Scholar]
- Drago JR. The induction of NB rat prostatic carcinomas. Anticancer Res. 1984;4:255–256. [PubMed] [Google Scholar]
- Leav I, Merk FB, Kwan PW, Ho SM. Androgen-supported estrogen-enhanced epithelial proliferation in the prostates of intact Noble rats. Prostate. 1989;15:23–40. doi: 10.1002/pros.2990150104. [DOI] [PubMed] [Google Scholar]
- Bosland MC, Prinsen MK, Rivenson A, Silverman J, Fiala E, Williams GM, Kroes R, Weisburger JH. Induction of proliferative lesions of ventral prostate, seminal vesicle, and other accessory sex glands in rats by N-methyl-N-nitrosourea: effect of castration, pretreatment with cyproterone acetate and testosterone propionate and rat strain. Prostate. 1992;20:339–353. doi: 10.1002/pros.2990200408. [DOI] [PubMed] [Google Scholar]
- Hoover DM, Best KL, McKenney BK, Tamura RN, Neubauer BL. Experimental induction of neoplasia in the accessory sex organs of male Lobund-Wistar rats. Cancer Res. 1990;50:142–146. [PubMed] [Google Scholar]
- Weihua Z, Mäkelä S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor β in the regulation of growth of the ventral prostate. Proc Natl Acad Sci USA. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson SJ, Wang H, Jones ME, Pedersen J, Iismaa TP, Wreford N, Simpson ER, Risbridger GP. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142:2458–2467. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- Stanbrough M, Leav I, Kwan PW, Bubley GJ, Balk SP. Prostatic intraepithelial neoplasia in mice expressing an androgen receptor transgene in prostate epithelium. Proc Natl Acad Sci USA. 2001;98:10823–10828. doi: 10.1073/pnas.191235898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtimäki J, Mäkelä S, Viljamaa J, Yagi A, Paranko J, Santti R. Neonatal estrogenization of the male mouse results in urethral dysfunction. J Urol. 1996;156:2098–2103. [PubMed] [Google Scholar]
- Schulze H, Barrack ER. Immunocytochemical localization of estrogen receptors in the normal male and female canine urinary tract and prostate. Endocrinology. 1987;121:1773–1783. doi: 10.1210/endo-121-5-1773. [DOI] [PubMed] [Google Scholar]
- Bødker A, Balslev E, Iversen HG, Meyhoff HH, Andersson KE. The expression of receptors for estrogen and epithelial growth factor in the male rabbit prostate and prostatic urethra following castration. Scand J Urol Nephrol. 1997;31:15–18. doi: 10.3109/00365599709070295. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JÅ. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA. Human estrogen receptor B-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- Imamov O, Morani A, Shim GJ, Omoto Y, Thulin-Andersson C, Warner M, Gustafsson JA. Estrogen receptor β regulates epithelial cellular differentiation in the mouse ventral prostate. Proc Natl Acad Sci USA. 2004;101:9375–9380. doi: 10.1073/pnas.0403041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbridger GP, Wang H, Frydenberg M, Cunha GR. The metaplastic effects of estrogen on mouse prostate epithelium: proliferation of cells with basal cell phenotype. Endocrinology. 2001;142:2443–2450. doi: 10.1210/endo.142.6.8171. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- Pakarainen T, Zhang F-P, Mäkelä S, Poutanen M, Huhtaniemi I. Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology. 2005;146:596–606. doi: 10.1210/en.2004-0913. [DOI] [PubMed] [Google Scholar]
- Ahokoski O, Irjala K, Huupponen R, Halonen K, Salminen E, Scheinin H. Hormonal effects of MPV-2213ad, a new selective aromatase inhibitor, in healthy male subjects. A phase I study. Br J Clin Pharmacol. 1998;45:141–146. doi: 10.1046/j.1365-2125.1998.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Nokkala E, Yan W, Streng T, Saarinen N, Wärri A, Huhtaniemi I, Santti R, Mäkelä S, Poutanen M. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142:2435–2442. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-β potency-selective ligands: structure-activity relationships studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor α- and β-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- Helguero LA, Faulds MH, Gustafsson JA, Haldosen LA. Estrogen receptors alfa (ERα) and beta (ERβ) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene. 2005;24:6605–6616. doi: 10.1038/sj.onc.1208807. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- Howell A, Osborne CK, Morris C, Wakeling AE. ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer. 2000;89:817–825. doi: 10.1002/1097-0142(20000815)89:4<817::aid-cncr14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Nikula H, Rannikko S. Treatment of prostatic cancer with a gonadotropin-releasing hormone agonist analog: acute and long term effects on endocrine functions of testis tissue. J Clin Endocrinol Metab. 1985;61:698–704. doi: 10.1210/jcem-61-4-698. [DOI] [PubMed] [Google Scholar]
- Bergendahl M, Perheentupa A, Huhtaniemi I. Effect of short-term starvation on reproductive hormone gene expression, secretion and receptor levels in male rats. J Endocrinol. 1989;121:409–417. doi: 10.1677/joe.0.1210409. [DOI] [PubMed] [Google Scholar]
- Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- Isaacs JT. Role of androgens in prostatic cancer. Vitam Horm. 1994;49:433–502. doi: 10.1016/s0083-6729(08)61152-8. [DOI] [PubMed] [Google Scholar]
- Pollard M, Luckert PH, Schmidt MA. Induction of prostate adenocarcinomas in Lobund Wistar rats by testosterone. Prostate. 1982;3:563–568. doi: 10.1002/pros.2990030605. [DOI] [PubMed] [Google Scholar]
- Pour PM, Stepan K. Induction of prostatic carcinomas and lower urinary tract neoplasms by combined treatment of intact and castrated rats with testosterone propionate and N-nitrosobis(2-oxopropyl)amine. Cancer Res. 1987;47:5699–5706. [PubMed] [Google Scholar]
- Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126:1165–1172. doi: 10.1210/endo-126-2-1165. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Lathe R, Warner M, Gustafsson JÅ. An endocrine pathway in the prostate, ERβ AR, 5α-androstane-3β,17β-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci USA. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor α in breast cancer cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- Risbridger G, Wang H, Young P, Kurita T, Wang YZ, Lubahn D, Gustafsson JA, Cunha G. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev Biol. 2001;229:432–442. doi: 10.1006/dbio.2000.9994. [DOI] [PubMed] [Google Scholar]
- Corbier P, Martikainen P, Pestis J, Härkönen P. Experimental research on the morphofunctional differentiation of the rat ventral prostate: roles of the gonads at birth. Arch Physiol Biochem. 1995;103:699–714. doi: 10.3109/13813459508998139. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- Wennbo H, Kindblom J, Isaksson OG, Tornell J. Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology. 1997;138:4410–4415. doi: 10.1210/endo.138.10.5461. [DOI] [PubMed] [Google Scholar]
- Lane KE, Leav I, Ziar J, Bridges RS, Rand WM, Ho SM. Suppression of testosterone and estradiol-17β-induced dysplasia in the dorsolateral prostate of Noble rats by bromocriptine. Carcinogenesis. 1997;18:1505–1510. doi: 10.1093/carcin/18.8.1505. [DOI] [PubMed] [Google Scholar]
- Brick JE, Wilson DA, Walker SE. Hormonal modulation of response to thymus-independent and thymus-dependent antigens in autoimmune NZB/NZW mice. J Immunol. 1985;134:3693–3698. [PubMed] [Google Scholar]
- Roubinian J, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB7NZW F1 mice. J Exp Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajfer J, Coffey DS. Sex steroid imprinting of the immature prostate. Long-term effects. Invest Urol. 1978;16:186–190. [PubMed] [Google Scholar]
- Shim GJ, Wang L, Andersson S, Nagy N, Kis LL, Zhang Q, Makela S, Warner M, Gustafsson JA. Disruption of the estrogen receptor beta gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci USA. 2003;100:6694–6699. doi: 10.1073/pnas.0731830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H, Albert L, Leathurby Y, Malamas M, Mewshaw R, Miller C, Kharode Y, Marzolf J, Hkomm B, Winneker R, Frail D, Henderson R, Zhu Y, Keith J. Evaluation of an estrogen receptor β agonist in animal models of human disease. Endocrinology. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- Shim GJ, Gherman D, Kim HJ, Omoto Y, Iwase H, Bouton D, Kis LL, Andersson CT, Warner M, Gustafsson JA. Differential expression of oestrogen receptors in human secondary lymphoid tissues. J Pathol. 2006;208:408–414. doi: 10.1002/path.1883. [DOI] [PubMed] [Google Scholar]
- Törn S, Nokelainen P, Kurkela R, Pulkka A, Menjivar M, Ghosh S, Coca-Prados M, Peltoketo H, Isomaa V, Vihko P. Production, purification, and functional analysis of recombinant human and mouse 17β-hydroxysteroid dehydrogenase type 7. Biochem Biophys Res Commun. 2003;305:37–45. doi: 10.1016/s0006-291x(03)00694-6. [DOI] [PubMed] [Google Scholar]