Abstract
Improved treatment regimens have reduced fatalities from opportunistic diseases, such as Pneumocystis pneumonia, in AIDS patients. However, serious chronic conditions, including pulmonary hypertension (PH), are increasing in this group. We report here that when CD4 T cells in Pneumocystis-infected mice are temporally depleted and then allowed to return, the extended inflammation results in PH that persists after Pneumocystis is eliminated. Using this model of PH, we have found that i) the onset of PH is correlated with the return of CD4 T cells, but PH persists after CD4 levels diminish; ii) vascular remodeling accompanies PH, but whereas temporary medial hypertrophy is evident with transient PH in immunocompetent mice, persistent PH is associated with perivascular fibrosis; iii) elevated levels of the fibrotic mediator FIZZ1 are found in bronchoalveolar lavage fluid of mice with persistent PH; and iv) although Th2-related mechanisms may be involved in PH etiology, PH still occurs in interleukin-4 receptor-deficient mice under these conditions. Overall, the data presented here demonstrate that the immune response to an infectious disease pathogen, such as Pneumocystis, can, when perturbed and prolonged, lead to later development of a serious chronic condition such as PH.
Recent treatment advances, such as customized antibiotic regimens and highly active anti-retroviral therapy, have reduced fatalities in acquired immune deficiency syndrome (AIDS) patients from opportunistic infectious diseases such as Pneumocystis pneumonia (PCP). At the same time, however, there has been a corresponding increase in other types of pathological conditions in AIDS patients. This includes rapidly developing conditions such as immune reconstitution inflammatory syndrome, in which elements of the reconstituted immune function, typically CD4 T cells, initiate an exuberant and sometimes dangerous response to an immune stimulus, including residual pathogens from previous infections.1,2 More common are chronic complications, often pulmonary in nature, including malignancies (notably Kaposi sarcoma and AIDS-related lymphoma with pulmonary involvement) and pulmonary hypertension (PH), which occurs 1000 times more frequently in HIV-infected individuals than in the general population.3,4 AIDS-related PH shares the same histological signs of pulmonary vascular remodeling as idiopathic PH, including plexogenic and thrombotic arteriopathy.5 AIDS-related PH also carries the same poor prognosis as idiopathic PH: one study found that PH was the final cause of death in 72% of the patients diagnosed with the condition, and 3-year postdiagnosis survival rates ranged from 28 to 47%, depending on the condition of the patients at presentation.6
Although there have been a variety of theories put forward, including cytokine imbalance, alteration of adrenergic tone, and concurrent autoimmune disease,7 the cause of AIDS-related PH is not clear. We wished to examine the hypothesis that previously resolved pulmonary infections create conditions that will predispose to the development of PH. In particular, we wanted to examine the role of prior Pneumocystis infections in the etiology of AIDS-related PH.
In the early years of the AIDS epidemic, PCP was often the heralding event of an AIDS diagnosis, and although various treatments have reduced PCP from its peak levels in 1990, it is still one of the most common opportunistic infections,8 and may represent an early and perhaps pattern-setting chapter in the patient’s pulmonary history. Pathological mechanisms of PCP are typically studied in mice deficient in CD4 T cells (as are untreated AIDS patients, to varying degrees), either via antibody-mediated depletion or the use of genetically modified animals.9,10 In those mice, there is a strong inflammatory response to Pneumocystis that is T1 in nature, characterized by accumulation of macrophages, neutrophils, and CD8 T cells, and host tissue damage is associated with those cells, in particular CD8 T cells.11 However, in view of immune reconstitution inflammatory syndrome, it is becoming evident that experimental explorations of Pneumocystis-related pathology in which CD4 T cells are present are relevant. We have previously shown that when CD4 T cells are present during PCP, such as in B-cell-deficient mice, there is extensive CD4 T-cell-mediated pulmonary damage.12 Even in immunocompetent mice there is a vigorous T2 inflammatory response, characterized by an infiltrate of macrophages, multinucleate giant cells, neutrophils, eosinophils, B cells, and both CD4 and CD8 T cells,13 as well as Th2 cytokine production and hyperplasia of epithelial and mucus-producing cells.13 Although this type of inflammation is typically very effective at clearing Pneumocystis, it also has potential for collateral or long-term changes in the lung. For example, Th2 skewing of responses to subsequent exposure to PC has been observed in humans.14 In other pulmonary conditions, overexuberant Th2 responses are implicated in chronic pathological developments, including asthma15 and fibrotic interstitial lung disease.16 Further, when inflammation is prolonged during PCP that is eventually resolved, as in mice deficient in type I interferon signaling, it results in extensive fibrotic remodeling of the lung.13
These observations have led us to speculate whether a prolonged or delayed CD4 T-cell response to PCP, such as what occurs in AIDS patients in which CD4 function improves in the course of therapy, might cause inflammatory changes in the lung sufficient to initiate vascular remodeling that persists after the infection has been cleared. We report here that when mice are briefly depleted of CD4 T cells and infected with Pneumocystis, and the CD4 cells are then allowed to return, the infection is cleared, but there is indeed lingering PH as determined by significantly elevated right ventricular pressure, and significant increase in right ventricle (RV) mass. In addition, although there is significant medial hypertrophy in pulmonary arterioles early in PCP, the long-term form of vascular remodeling associated with this PH seems to be increased fibrotic perivascular remodeling.
Materials and Methods
Animals
Mice used in this study came from two sources. Many of the BALB/c mice and all of the interleukin (IL)-4 receptor knockout mice (which were on a BALB/c background) were bred in the research animal facility at Montana State University from stock originally obtained from Jackson Laboratories (Bar Harbor, ME). In some cases, BALB/c mice were purchased from the National Cancer Institute (Fredrick, MD). During the course of experimental incubations, mice were kept in isolation rooms, inside ventilated cages receiving HEPA-filtered air, and given autoclaved mouse chow and acidified water.
Depletion of CD4 T Cells and Infection with Pneumocystis: Experimental Model
For depletion of CD4 T lymphocytes, mice were given intraperitoneal injections of 300 μg of the anti-CD4 antibody GK1.5 (American Type Culture Collection, Manassas, VA). The timing of these depletions was such that mice received an injection of GK1.5 2 days before inoculation with Pneumocystis, again at 2 to 3 days after inoculation, and then two to three more times at 3- to 4-day intervals. Inoculation with Pneumocystis was performed with intratracheal inoculation of lightly anesthetized mice with aliquots of lung homogenates from Pneumocystis-infected severe combined immunodeficient mice.17 Evaluations of inflammation, infection, and PH were made at various times after the infection. We found that CD4 T cells slowly began to reappear at 25 to 38 days after infection, Pneumocystis was cleared at 30 to 40 days after infection, PH began to appear at 30 to 42 days after infection, and was persistent at the latest time points measured (50 to 63 days). Once the timing of these events was determined, additional experiments were performed within these time frames.
Right Ventricular Pressure Measurements
Measurements of blood pressure in the RVs of mice were obtained by transthoracic insertion of a fluid-filled cannula.18 Briefly, a hubless 25-gauge needle was inserted into a 20-cm length of Micro-Line tubing [inner diameter (i.d.), 0.02 inches], which was then attached to a Cobe CDXIII pressure transducer (Argon Medical Devices, Athens, TX). The transducer was in turn connected to a Buxco MAX2270 preamplifier and BioSystem XA software (Wilmington, NC). The cannula was filled with heparinized, degassed normal saline, and then before each experiment, the pressure measurement system was calibrated against a mercury sphygmomanometer. At the time of measurement, mice were weighed and injected with 60 μg/g body weight of pentobarbital. As soon as anesthesia was reached (loss of pedal reflex), a small incision was made in the chest fur, the pectoralis muscle was partially cut and retracted, and the needle was inserted while pressure was constantly monitored. When appropriate placement was confirmed, eight 5-second intervals of pressure measurements were recorded. On later analysis, digital recordings of the measurements were carefully examined, and all of the eight recordings in which the pressure waveform was consistent for the 5-second period were averaged, using the parameters of average peak pressure, and average pressure (maximum-minimum).
Bronchoalveolar Lavage (BAL)
After pressure measurements, the anesthetized mice were sacrificed by exsanguination. The trachea of each mouse was nicked with fine scissors, and then a 16-cm length of Micro-Line tubing attached to a 5-ml syringe was inserted. Five 1-ml aliquots of sterile Hanks’ balanced salt solution with 3 mmol/L ethylenediaminetetraacetic acid were then applied to lavage the alveolar contents.19 Aliquots (100 μl) from each lavage were spun onto a slide using a cytospin centrifuge and then stained with Diff-Quick dye (Dade Behring, Newark, DE). Proportions of each cell type were later determined microscopically using a ×100 objective lens. Additional aliquots were taken to count total BAL cells using a hemocytometer. The bronchoalveolar lavage fluid (BALF) supernatant was collected by centrifugation at 900 × g for 10 minutes, and aliquots were saved at −80°C for subsequent assays.
Cardiac Mass Measurements
After lavage, the relative mass of the RV to the left ventricle (LV) plus septum (LV + S) was determined according to standard methodology.20 In brief, the atria were trimmed away from the heart, which was then weighed, after which the RV was cut away from the heart and weighed, as was the remaining LV + S. RV mass is expressed as the percentage LV + S[(RV/LV + S) × 100].
Flow Cytometry and Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
BAL cells were resuspended in a minimal volume (100 to 200 μl) of phosphate-buffered saline with 2% calf serum and an anti-mouse Fc receptor antibody (Trudeau Institute, Saranac Lake, NY) to block nonspecific binding. The cells were then stained with a mixture of fluorophore-conjugated antibodies against the mouse CD antigens CD4, CD8, CD25, and either CD28 or CD19 (PharMingen, San Diego, CA) and then examined on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). Lymphocytes obtained from the spleens of control uninfected mice were used as a basis of comparison and instrument setup. Analysis of cytometry data was performed with CellQuest software (Becton Dickinson), and numbers of relevant cell types (eg, CD4 lymphocytes) was determined by combining flow cytometry data (percentage of a given cell type) with BAL cell counts. Cytokine concentrations in undiluted samples of BALF were measured in some cases using cytometry-based bead ELISA kits (mouse Th1-Th2 and mouse inflammation; Becton Dickinson). A standard ELISA was used to measure the levels of the putative fibrotic mediator FIZZ1 (found in inflammatory zone 1),21,22 using a polyclonal antibody from Alpha Diagnostic International (San Antonio, TX). To quantify the ELISA, known concentrations of a control peptide that represents a 14-amino acid segment of FIZZ1 (Alpha Diagnostic International) was used, and results are thus expressed as nanograms of peptide equivalent.
Enumeration of Pneumocystis
After lavage, the airway was ligated, and two thirds of the lung was removed, placed into 5 ml of sterile Hanks’ balanced salt solution, and homogenized by pushing through a metal mesh screen with a sterile rubber pestle. An aliquot of this material was diluted 1:20 and applied to a glass slide with a cytospin centrifuge. After drying, the slides were stained in Diff-Quick dye (with 20 to 40 minutes in the final nuclear stain). Pneumocystis (cysts and trophozoites) were then enumerated as described10 by counting nuclei until 500 organisms were counted, within a minimum of five ×60 fields. If less than 500 nuclei were observed, a maximum of 50 fields were examined. The average counts were then converted to log Pneumocystis nuclei/lung; with this technique on the microscope used, the limit of detection was (log) 4.43 when 50 fields were counted. Although prior lavage does remove some Pneumocystis organisms from the lung, it is a small fraction of what is present in the lung (<10%), and this was consistent among different groups of mice.
Histology and Morphometry
One-third of the lung (the large left lobe) was fixed for 24 hours in phosphate-buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with either hematoxylin and eosin (H&E) or Masson’s trichrome stain using standard histological techniques. Morphometry of pulmonary vasculature was performed using methods similar to those previously published.23 Sections were examined using differential interference contrast optics on a Axiovert M microscope (Carl Zeiss, Thornwood, NY). Pictures were taken of 20 arterial/arteriolar vessels in the 10 to 100 μm range in each section. These images were then analyzed using the ImageJ version of NIH Image (W.S. Rasband; ImageJ; National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/, accessed November 2006).24 On each vessel profile, three measurements were taken of the vessel outer diameter (o.d.), i.d., and thickness of the medial smooth muscle layer (SM; defined as between the internal and external elastic lamina). The percentage of vessel diameter that is medial smooth muscle was calculated as (average SM × 2/average o.d.) × 100 and expressed as a percentage of o.d.
Pulmonary Soluble Collagen Content
In some experiments, aliquots of the lung homogenate used for Pneumocystis counts were quick frozen for later analysis of soluble collagen content using the Sircol dye method.25 A kit from Biocolor Ltd. (Newtonabby, UK) was used for this procedure: lung homogenates were thawed, centrifuged, and filtered and then subjected to extraction with 0.5 mol/L acetic acid, after which the extracted samples were analyzed for soluble collagen according to the manufacturer’s instructions. The samples were also assayed for total protein using a bicinchoninic acid assay (Pierce, Rockford, IL). Results are expressed as micrograms of collagen per milligrams of total protein.
Statistical Analysis
The software program GraphPad Prism (San Diego, CA) was used for all statistical tests of significance (ie, P ≤ 0.05). When more than two groups were being compared, one-way analysis of variance followed by Tukey’s post hoc pairwise comparisons was performed. When only two groups were compared, we used a two-sided t-test, with Welch’s correction if the groups had unequal variances. In cases in which a deviation from a normal distribution was observed, a nonparametric test (Mann-Whitney test) was also used. In those cases, we found that both the t-test and Mann-Whitney test indicated the same result (ie, both indicated significance or nonsignificance); however, typically one test gave a more conservative (larger, but still <0.05) P value. The P values we report are always the conservative values.
Results
Transient Vascular Remodeling and PH in Immunocompetent Pneumocystis-Infected Mice
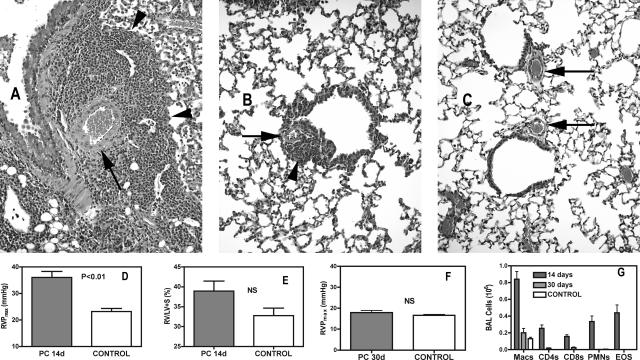
Although immunocompetent mice will clear a Pneumocystis infection in 21 to 28 days, there are often signs of residual perivascular inflammation (Figure 1, A–C). To determine whether this inflammation was sufficient to cause vascular remodeling that results in PH, we infected normal immunocompetent male BALB/c mice with Pneumocystis and measured RVP and RV mass at various intervals after infection. In immunocompetent mice, the peak inflammatory response occurred at ∼14 days after infection; at this time there was significant involvement of both lymphocytes (CD4+ and CD8+) and granulocytes, especially eosinophils (Figure 1G). Interestingly, there was also hypertrophy of both epithelial and vascular structures, with an obvious thickening of arteriolar smooth muscle in areas of active inflammation (Figure 1A). Alteration to the pulmonary vasculature was accompanied by significantly elevated RVP, compared with control noninfected mice; as seen in Figure 1D, control mice at the altitude of Bozeman, MT (1550 meters) exhibited RVP of 23.19 ± 1.15 mmHg (although this can be higher in aged control mice), whereas after 14 days of infection with Pneumocystis, mean RVP was 36.03 ± 2.25 mmHg. There was also a tendency toward increased right ventricular mass (expressed as the ratio of right ventricular mass to LV + septum mass) in these animals; however, this trend was not significant (Figure 1E). When these same assessments were made at 30 days after infection, after clearance of Pneumocystis, it was apparent that in immunocompetent animals, effects of Pneumocystis infection on the function of the pulmonary vasculature were transient. Histological observations showed that although there was still some residual perivascular inflammation, there was no longer the dramatic thickening of the medial layer of the smaller arteries that was seen at 14 days (Figure 1, A–C). Furthermore, RVP in mice after 30 days of Pneumocystis infection was no longer statistically higher than what was seen in control animals (Figure 1F), and there was also no significant long-term effect on right ventricular mass (not shown). This corresponded with significant changes in the proportions of inflammatory cells in the lung; the inflammatory cell influx into the alveolar compartment was sharply reduced, with granulocytes now being almost absent, macrophages being the dominant cell type, and approximately equal but reduced numbers of CD4 and CD8 lymphocytes (Figure 1G).
Figure 1.
PH and perivascular inflammation is transient in immunocompetent mice. A: Pulmonary tissue in an immunocompetent mouse 14 days after inoculation with Pneumocystis showing marked perivascular inflammation (arrowhead) and enlarged medial layers of small pulmonary arteries (arrow). B: Similar view at 30 days after infection with limited residual perivascular inflammation and reduced medial hypertrophy in small arteries. C: Comparable view in an uninfected control. D and E: At 14 days after inoculation with Pneumocystis, mean peak right ventricular pressure (RVP) is significantly elevated, whereas the mass of the RV, expressed as the percentage RV mass is of the combined mass of the left ventricle + septum (LV + S), is slightly, but not significantly higher; n = 9 (infected) or 6 (control). F: At 30 days after infection, RVP is no longer significantly elevated [n = 6 (infected) or 7 (control)]. NS, nonsignificant. G: Inflammatory cells in the BALF are greatly reduced at 30 days compared with 14 days (Macs, macrophages; CD4s and CD8s are lymphocytes only; PMNs, neutrophils; EOS, eosinophils). Values are means ± SEM; P is probability that value is statistically equal to control animals. Original magnifications, ×400.
Extended Inflammatory Responses to Pneumocystis Infection Results in Persistent PH
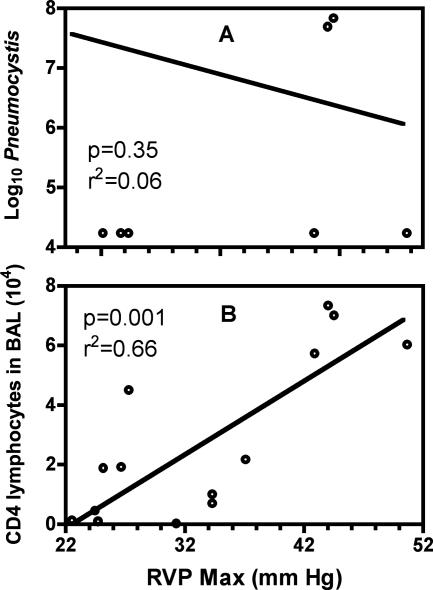
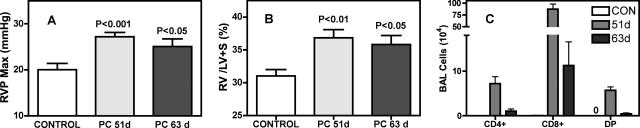
Unlike the situation in immunocompetent patients, when immunocompromised patients contract PCP, the disease is typically prolonged, and there is an extended inflammatory process in the lung. This is also the case in various models of PCP in immunocompromised mice. The particular scenario in which we wanted to examine pulmonary vascular changes during prolonged inflammation in PCP was that of depletion followed by gradual reconstitution of CD4 T cells. To do so, we depleted BALB/c mice of their CD4 T cells early during the period of Pneumocystis infection and then stopped the depletions to allow the CD4 T cells to return. When mice were assessed at 28 to 40 days after infection, RVP was highly variable, ranging from 20 to 45 mmHg, and there was no linear relationship to time after infection. There was also a lack of correlation between RVP and the levels of Pneumocystis organisms, suggesting that elevated pressures are not simply because of the physical presence of large numbers of pathogens exerting pressure on the pulmonary vasculature and increasing resistance to blood flow (Figure 2A). What did appear to be related to higher levels of RVP, during this same period, was the degree to which CD4 T cells were recovering; although the number of CD4 T cells found in the BALF was highly variable, there was a statistically significant correlation between the levels of CD4 T cells and RVP values (Figure 2B). When mice that had been given this short-term depletion of CD4 cells were assessed at later time points (50 days or more after inoculation), the development of PH was clear: although the Pneumocystis had been totally cleared (data not shown), RVP values were consistently elevated, as were relative right ventricular masses (Figure 3, A and B). Furthermore, once PH had been established, the relationship with inflammatory cells was no longer pertinent. Indeed, in the period of 50 to 63 days after infection, the levels of inflammatory cells were dramatically decreasing; total BAL cells at 51 days were 2.65 ± 1.14 × 106 (n = 11), whereas at 63 days this figure had dropped to 0.35 ± 0.38 × 106 (n = 8), which is near control levels. Granulocytes no longer comprised substantial percentages of the BAL cells at this time (<6% total), and there were approximately equal numbers of lymphocytes and macrophages (data not shown). What was clear is that PH was persistent during this period, despite the decline in CD4 T-cell numbers (Figure 3C), suggesting that although CD4 cells are required for the initiation of vascular changes resulting in PH, they are not necessary for PH to continue. Although the drop in total cell number during this period is dramatic, we have observed similar rapid changes in cell numbers throughout a 10 to 15 day period in immunocompetent mice once Pneumocystis has been cleared (not shown). The major notable difference in this case is that CD8 T cells seem to the last inflammatory cell to linger in higher numbers.
Figure 2.
Mean peak RVP at 28 to 38 days after Pneumocystis inoculation with short-term CD4 depletion and Pneumocystis infection were evaluated (20 to 30 days after last anti-CD4 treatment). Recovery of CD4 T cells is highly variable, as was measured RVP. RVP is not correlated to Pneumocystis, but a correlation exists between RVP and CD4 levels. Points represent individual animals (n = 16) pooled from three separate experiments.
Figure 3.
PH persists in BALB/c mice after Pneumocystis infection is cleared, when CD4 T cells have been temporally depleted. Pneumocystis-infected mice were depleted of CD4 cells for the first 10 days of infection; CD4 cells were then allowed to return. Both mean maximum RVP (A) and RV mass (B) remain elevated at 51 and 63 days after infection, even as the numbers of inflammatory cells decrease during this period (C). CD4+ and CD8+ are single positive lymphocyte populations, whereas DP lymphocytes are double positive for both CD4 and CD8. Values are means ± SEM, n = 12 (PC-infected 51 days and control) or n = 9 (infected 63 days); P is probability that value is statistically equal to control, noninfected mice.
Medial Smooth Muscle Hypertrophy of Small Pulmonary Arteries Does Not Seem to Be Related to PH that Occurs in Response to a Prolonged Pneumocystis Infection
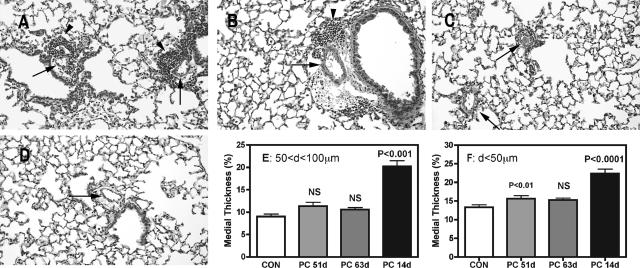
As described above, during the peak inflammatory response to Pneumocystis in immunocompetent mice (at 14 days), there is pronounced medial smooth muscle hypertrophy in many pulmonary arterial vessels. Surprisingly, when the inflammatory response to Pneumocystis was extended by short-term depletion of CD4 T cells, medial vessel hypertrophy at the time of persistent PH was not consistently evident in H&E tissue sections (Figure 4, A–D). These subjective observations were confirmed by a detailed quantitative assessment of medial vascular thickness, which also indicated that although there may have been slight medial hypertrophy at 53 days after infection, there was no longer a significant difference in the medial smooth muscle layer between mice at 63 days after infection and control, noninfected animals (Figure 4, E and F).
Figure 4.
Medial hypertrophy and inflammation in BALB/c mice subsides after Pneumocystis infection has been cleared. In mice with short-term CD4 depletion, perivascular inflammation (arrowhead) and thickness of the medial smooth muscle layer (arrows) are reduced from what was seen in 14-day infected immunocompetent mice (Figure 1A) at 51 (A) and 63 (B) days after infection, although both of these still have slightly greater area than in immunocompetent mice 45 days after infection (C) or control noninfected animals (D). Quantification of the smooth muscle layer confirms these observations (E and F); values represent the percentage of the diameter of the vessels that is medial smooth muscle (see Materials and Methods for details on calculations). E: Vessels larger than 50 μm but smaller than 100 μm in o.d. F: Vessels smaller than 50 μm. Values are means ± SEM, n = 12 (51 days infected and control) or 9 (63 and 14 days infected); P is probability that value is statistically equal to control. NS, nonsignificant.
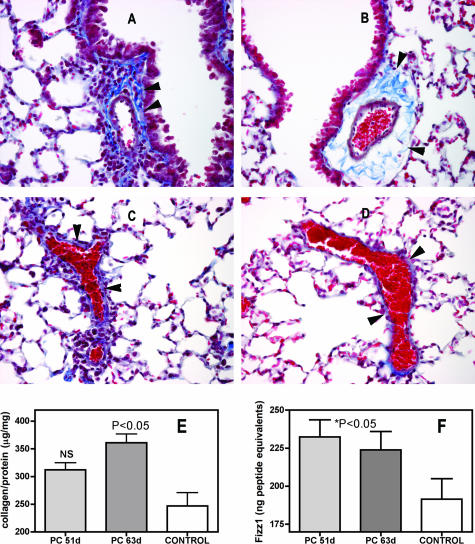
Increased Perivascular Fibrosis Occurs in Pneumocystis-Infected Mice that Have Been Subjected to Short-Term CD4 T-Cell Depletion
Although the H&E sections in Figure 4 show only slight residual perivascular inflammation at the time of persistent PH, similar sections stained with Masson’s trichrome show that fibrotic changes, including increased deposition of collagen, have occurred in many perivascular areas (Figure 5, A–D). Quantitative assessment of pulmonary fibrosis, in the form of total soluble pulmonary collagen, suggests that this fibrosis is progressive because there is moderate but not significant increases in total collagen at 51 days after infection and higher and statistically significant increases in total collagen at 63 days (Figure 5E). We also explored the possible implication of known fibrotic mediators; for example, the cytokine IL-13 has been implicated in the development of fibrosis, and elevated levels of IL-13 are present in the early stages of Pneumocystis infection.13 However, we did not detect significantly elevated levels of IL-13 in the BALF of short-term CD4-depleted and PC-infected mice at 53 and 61 days after infection (not shown). We did, however, find elevated concentrations of the putative fibrotic mediator FIZZ1 (found in inflammatory zone 1), which may act downstream of other inflammatory agents, including IL-13, in the BALF of the Pneumocystis-infected, short-term CD4-depleted mice with PH and perivascular fibrosis (Figure 5F).
Figure 5.
Perivascular fibrosis in BALB/c mice after Pneumocystis infection has been cleared. Masson trichrome stains of sections of pulmonary tissue of Pneumocystis-infected (A and C) and control (B and D) BALB/c mice. Infected mice were CD4 depleted for the first 10 days of infection; samples were taken 63 days after inoculation. Increased staining density of extracellular matrix proteins is evident both in the cross section (A) and tangential section (C) of the infected lung (arrowheads, collagen stains blue with this procedure). Quantification of soluble collagen levels in short-term CD4-depleted mice at 51 and 62 days after inoculation confirms the higher levels of extracellular matrix protein in lung tissue. E: Lung homogenates were assayed for soluble collagen as described in Materials and Methods. Results are expressed as micrograms of soluble collagen per milligram of total protein in the sample, means ± SEM, n = 7 per group. Increases in BALF levels of the fibrotic mediator FIZZ1 coincided with increased fibrosis (F); aliquots of BALF were tested by ELISA for the presence of the putative fibrotic mediator and quantified against standards of FIZZ1 peptide. Values are means ± SEM, n = 8 to 11. Original magnifications, ×400.
IL-4 Signaling Is Not Required for PH to Develop in Pneumocystis-Infected Mice that Have Been Subjected to Short-Term CD4 T-Cell Depletion
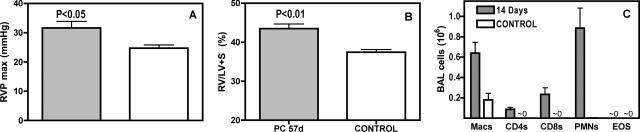
Because pulmonary fibrosis is associated with events such as the production of Th2-type cytokine by Th2 cells (including CD4 cells), and the formation of alternatively activated macrophages that may be instrumental in collagen production,26 we hypothesized that IL-4 signaling would be required for the development of PH in our model. Surprisingly, we found that when mice deficient in the IL-4 receptor, and therefore refractory to Th2 skewing, are subjected to the short-term depletion and Pneumocystis infection regimen, they also develop persistent PH, in the form of elevated RVP and RV/LV + S (Figure 6, A and B). This occurred despite the differences in inflammatory cell recruitment throughout the period of infection, most notably a total lack of eosinophils, compared with observations in wild-type mice (Figure 6C).
Figure 6.
Persistent PH does not depend on IL-4 signaling. IL-4 KO mice were Pneumocystis-infected and depleted of CD4 cells for 10 days and then sampled at 57 days after inoculation. A: RVP of infected and control IL-4 KO mice. B: Relative right ventricular mass of infected and control IL-4 KO mice. These events occur despite different cellular response to Pneumocystis, as indicated by the profile of BAL cells seen 14 days after infection (C); when compared with immunocompetent mice (see Figure 1G), there is no eosinophil response but a higher recruitment of neutrophils. Values are means ± SEM, n = 5; P is probability that value is statistically equal to control.
Discussion
We report here that PH is a sequelae of PCP in mice when natural inflammatory responses to Pneumocystis are prolonged because of temporary depletion followed by reestablishment of CD4 T cells. In addition, although PH can occur in the course of an active Pneumocystis infection, our results show that in this model, Pneumocystis-related PH persists after the pathogen has been cleared and most aspects of PCP-related inflammation have subsided. Furthermore, it seems that in our experimental system, the CD4 cells themselves are instrumental in initiating the processes that result in persistent PH.
These experimental observations are particularly relevant in light of the increased incidence of noninfectious pulmonary complications, including PH, in AIDS patients after partial reconstitution of their own immune function.27,28,29 Several reviews have examined cases of HIV-related PH, and speculated that there are multiple causes and factors involved in the development of this condition,30,31,32 but the role of prior pulmonary infections in these cases has not been clearly resolved. Although many of these reviews show a lack of correlation of PH with active pulmonary infections, only one presented information related to previous infections with opportunistic lung infections and also found a lack of correlation33; however, that study was based on a single patient. These investigations also point out that there is not a correlation between CD4 cell counts at the time of presentation with PH and the incidence of PH.30,31 This does not contradict our study, however, because we show that PH is persistent after CD4 T-cell levels have decreased, and it is only during the onset of PH in Pneumocystis-related PH that a correlation with the reoccurrence of CD4 T cells is relevant.
Furthermore, some of the mechanisms that have been proposed as potential factors in the etiology of HIV-associated PH are also part of the inflammatory processes occurring in the course of Pneumocystis infection. For example, altered secretion of cytokines such as IL-1β, IL-6, and tumor necrosis factor-α are speculated as a causal factor in HIV-associated PH,7 and these cytokines are also involved in host defense against Pneumocystis.34,35,36 It has also been suggested that chronic hypoxia and elevated norepinephrine present during active AIDS infections may result in elevated α-1 adrenergic tone via stimulation of vascular smooth muscle tone.7,37 This may be true during Pneumocystis infections as well because reduced blood oxygen occurs in PCP in both mice11,38 and humans.39,40 Unfortunately, because of a lack of sufficient data, these mechanisms of HIV-associated PH are only speculative at present; however, they are similar to those proposed for other forms of PH in which inflammation is an important factor. For example, inflammatory cytokines (IL-1, IL-6, tumor necrosis factor-α) are elevated in patients with the rare POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes) in which PH invariably occurs.41 Histological examination of pulmonary lesions revealed perivascular accumulation of inflammatory cells, presumed to be a source of inflammatory cytokines, in both primary plexogenic PH in humans,42 and in PH related to systemic sclerosis.43 In addition, the occurrence of PH in Gaucher disease in humans is correlated with markers of inflammation, including C-reactive protein.44 Potential roles for some of these same inflammatory mediators in the development of PH have been definitively shown in the monocrotaline inflammatory model of PH in rodents. These include IL-6,45 IL-1,46 leukotrienes,47 and tumor necrosis factor-α.48 Overall then, our evidence that inflammation associated with prior Pneumocystis infections in mice may create conditions leading to the development of PH suggests that previous history of Pneumocystis infections in HIV patients should be considered as a possible factor in the pathological underpinnings of HIV-related PH.
Vascular remodeling is an essential component of PH, and we present here a somewhat surprising pattern of remodeling concomitant with the development of PH in mice that have had a transitory depletion of their CD4 T cells. We did not observe vascular plexiform lesions in any of our mice. However, although these lesions are frequently observed in lung samples from humans with PH, including HIV-PH, they are only very rarely seen in mouse models of PH.49 In addition, although pronounced medial hypertrophy of vascular smooth muscle was a prominent development in immunocompetent mice, this development was not a persistent feature in our short-term depletion model. Rather, what persisted were fibrotic changes in the perivascular areas of the lung, including increased deposition of collagens near the smaller intrapulmonary arteries. Both of these types of remodeling can contribute to increased pulmonary blood pressure. With medial hypertrophy, increased smooth muscle in the precapillary vessels results in increased vascular tone and reduced vessel lumen diameter, increasing vascular resistance and resulting in elevated pressures.50 The fibrotic changes we observed are probably related to vascular adventitial remodeling, which reduces distensibility and compliance of the vessels, which in turn contributes to increased vessel pressures.51,52
Perivascular fibrotic remodeling may be a response to vascular changes such as medial hypertrophy because there is evidence that increased intravascular pressures will induce connective tissue synthesis53 or it may arise in response to inflammation through related and/or independent mechanisms. Indeed, there is substantial evidence from other animal models that changes occur in adventitial cells (including extracellular matrix protein production) as an early response to perturbations that cause PH, including hypoxia,54 monocrotaline exposure,55 and even with ex vivo exposure to cadmium and transforming growth factor-β.56 Furthermore, the type of perivascular fibrosis we report here is also seen in some human patients with chronic bronchiolar inflammation.57 Therefore, in our report the sustained PH that persists after clearance of both the Pneumocystis infection and most of the inflammatory cells may be attributable to quantitative rather than qualitative differences of the disease process. Specifically, if the inflammation has been prolonged, because of deficiency of CD4 T cells, then as the CD4 cells return, the fibrosis and armoring of the perivascular areas may be extensive, maintaining the elevated pulmonary blood pressures.
We also demonstrate the importance of a subset of the acquired immune system, specifically CD4 T cells, in the onset of Pneumocystis-induced PH. There are of course, many possible functions of CD4 T cells that may contribute to the development of PH. In response to Pneumocystis, there is a greater tendency for CD4 cells to become Th2-type cells, rather than Th1.58 Furthermore, the Th2 cytokines IL-4 and IL-13 are involved in the development of fibrotic changes in the lung.59,60 Our observation of elevated FIZZ1 in the BALF suggested a possible mechanism of CD4 Th2 cells in the onset of perivascular fibrosis via IL-4-mediated promotion of alternatively activated macrophages,61 and FIZZ1,62,63 which in turn promotes pulmonary fibrosis.64 We were somewhat surprised to find that in the absence of IL-4 signaling, there was no significant difference in Pneumocystis-related PH after short-term CD4 T-cell depletion. Clearly, there are many other possible CD4 T-cell-related actions that bear investigation; one possibility is the cytokine IL-13, which is implicated in the development of pulmonary fibrosis.65 Most importantly, some of the profibrotic effects of IL-13 occur independently from IL-4-related pathways.66 Although we did not find IL-13 in the lungs of mice that exhibit PH during the persistent state of the disease, when Pneumocystis organisms have been cleared and inflammation has receded, IL-13 is produced in the lung early in the response to Pneumocystis inoculation.13 Therefore, it is possible that secretion of IL-13 at a particular stage of PCP might initiate processes that result in fibrosis that continues well after IL-13 secretion has diminished. We are currently pursing studies to investigate this possibility.
Overall, these experiments demonstrate how an infectious disease can be instrumental in the etiology of a chronic and potentially deadly noninfectious disease process when the normal immune mechanisms of defense against that pathogen are disrupted. It is notable that in this experimental model, the onset of the chronic condition (PH) is associated with the reconstitution of the immune system component that is required for the elimination of the acute infectious disease (CD4 T cells). These findings may help to highlight the need for potential ancillary therapeutics when normal immune mechanisms are reconstituted in the course of treatment, such as is the case with HIV patients receiving highly active anti-retroviral therapy.
Acknowledgments
We thank Trent Bushmaker, Sara Erickson, Melanie Rutkowski, Quinton King, Nicole Meissner, and Jim Wiley for general help; Gayle Callis for expert histological services; and Tamera Marcotte and the staff of the Animal Resource Center at Montana State University for animal care.
Footnotes
Address reprint requests to Steve D. Swain, Department of Veterinary Molecular Biology, Montana State University, 960 Technology Blvd., Bozeman, MT 59718. E-mail: uvsss@montana.edu.
Supported by the National Institutes of Health (grants RO1HL55002, PO1HL71659, and COBRE 5P20RR020185).
References
- Shelburne SA, III, Hamill RJ. The immune reconstitution inflammatory syndrome. AIDS Rev. 2003;5:67–79. [PubMed] [Google Scholar]
- Stoll M, Schmidt RE. Adverse events of desirable gain in immunocompetence: the immune restoration inflammatory syndromes. Autoimmun Rev. 2004;3:243–249. doi: 10.1016/j.autrev.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Rerkpattanapipat P, Wongpraparut N, Jacobs LE, Kotler MN. Cardiac manifestations of acquired immunodeficiency syndrome. Arch Intern Med. 2000;160:602–608. doi: 10.1001/archinte.160.5.602. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD. Noninfectious pulmonary complications of HIV/AIDS. Curr Opin Pulm Med. 2005;11:208–212. doi: 10.1097/01.mcp.0000159833.28271.00. [DOI] [PubMed] [Google Scholar]
- Kim KK, Factor SM. Membranoproliferative glomerulonephritis and plexogenic pulmonary arteriopathy in a homosexual man with acquired immunodeficiency syndrome. Hum Pathol. 1987;18:1293–1296. doi: 10.1016/s0046-8177(87)80417-3. [DOI] [PubMed] [Google Scholar]
- Nunes H, Humbert M, Sitbon O, Morse JH, Deng Z, Knowles JA, Le Gall C, Parent F, Garcia G, Herve P, Barst RJ, Simonneau G. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2003;167:1433–1439. doi: 10.1164/rccm.200204-330OC. [DOI] [PubMed] [Google Scholar]
- Pellicelli AM, Palmieri F, Cicalini S, Petrosillo N. Pathogenesis of HIV-related pulmonary hypertension. Ann NY Acad Sci. 2001;946:82–94. doi: 10.1111/j.1749-6632.2001.tb03904.x. [DOI] [PubMed] [Google Scholar]
- Kovacs JA, Gill VJ, Meshnick S, Masur H. New insights into transmission, diagnosis, and drug treatment of Pneumocystis carinii pneumonia. JAMA. 2001;286:2450–2460. doi: 10.1001/jama.286.19.2450. [DOI] [PubMed] [Google Scholar]
- Roths JB, Marshall JD, Allen RD, Carlson GA, Sidman CL. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice. Natural history and pathobiology. Am J Pathol. 1990;136:1173–1186. [PMC free article] [PubMed] [Google Scholar]
- Harmsen AG, Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990;172:937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TW, Gigliotti F, Finkelstein JN, McBride JT, An CL, Harmsen AG. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J Clin Invest. 1999;104:1307–1317. doi: 10.1172/JCI6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SD, Meissner NN, Harmsen AG. CD8 T cells modulate CD4 T-cell and eosinophil-mediated pulmonary pathology in Pneumocystis pneumonia in B-cell-deficient mice. Am J Pathol. 2006;168:466–475. doi: 10.2353/ajpath.2006.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner NN, Swain S, Tighe M, Harmsen A, Harmsen A. Role of type I IFNs in pulmonary complications of Pneumocystis murina infection. J Immunol. 2005;174:5462–5471. doi: 10.4049/jimmunol.174.9.5462. [DOI] [PubMed] [Google Scholar]
- Theus SA, Sawhney N, Smulian AG, Walzer PD. Proliferative and cytokine responses of human T lymphocytes isolated from human immunodeficiency virus-infected patients to the major surface glycoprotein of Pneumocystis carinii. J Infect Dis. 1998;177:238–241. doi: 10.1086/517363. [DOI] [PubMed] [Google Scholar]
- Larché M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–463. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the Th1/Th2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SD, Lee SJ, Nussenzweig MC, Harmsen AG. Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo. Infect Immun. 2003;71:6213–6221. doi: 10.1128/IAI.71.11.6213-6221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Wagner R, Slankard ML, Abelmnn WH. Left and right ventricular pressures in mice. J Appl Physiol. 1971;30:424–426. doi: 10.1152/jappl.1971.30.3.424. [DOI] [PubMed] [Google Scholar]
- Harmsen AG. Role of alveolar macrophages in lipopolysaccharide-induced neutrophil accumulation. Infect Immun. 1988;56:1858–1863. doi: 10.1128/iai.56.8.1858-1863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan KA. Selected contribution: pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol. 2001;90:2502–2507. doi: 10.1152/jappl.2001.90.6.2502. [DOI] [PubMed] [Google Scholar]
- Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMα, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065–1067. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]
- Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164:1315–1326. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Shimosawa T, Itakura K, Guanqun X, Ando K, Fujita T. Adrenomedullin can protect against pulmonary vascular remodeling induced by hypoxia. Circulation. 2004;109:2246–2251. doi: 10.1161/01.CIR.0000127950.13380.FD. [DOI] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC, Kwon JJ, Keane MP, Strieter RM. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol. 2004;172:3860–3868. doi: 10.4049/jimmunol.172.6.3860. [DOI] [PubMed] [Google Scholar]
- Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85:173–180. doi: 10.1016/s0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel AS, von Overbeck J, Opravil M, Jenni R, Speich R, Mueller NJ. Long-term survival and interruption of HAART in HIV-related pulmonary hypertension. Eur J Clin Microbiol Infect Dis. 2005;24:153–155. doi: 10.1007/s10096-005-1289-7. [DOI] [PubMed] [Google Scholar]
- Recusani F, Di Matteo A, Gambarin F, D’Armini A, Klersy C, Campana C. Clinical and therapeutical follow-up of HIV-associated pulmonary hypertension: prospective study of 10 patients. AIDS. 2003;17:S88–S95. doi: 10.1097/00002030-200304001-00013. [DOI] [PubMed] [Google Scholar]
- Le Houssine P, Karmochkine M, Ledru F, Batisse D, Piketty C, Kazatchkine MD, Weiss L. [Primary pulmonary hypertension in human immunodeficiency virus infection. Study of 9 cases and review of the literature]. French. Rev Med Intern. 2001;22:1196–1203. doi: 10.1016/s0248-8663(01)00491-x. [DOI] [PubMed] [Google Scholar]
- Pellicelli AM, Barbaro G, Palmieri F, Girardi E, D’Ambrosio C, Rianda A, Barbarini G, Frigiotti D, Borgia MC, Petrosillo N. Primary pulmonary hypertension in HIV patients: a systematic review. Angiology. 2001;52:31–41. doi: 10.1177/000331970105200105. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Edell ES, Dunn WF, Edwards WD. Human immunodeficiency virus infection and pulmonary hypertension: two new cases and a review of 86 reported cases. Mayo Clin Proc. 1998;73:37–45. doi: 10.1016/S0025-6196(11)63616-1. [DOI] [PubMed] [Google Scholar]
- Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-related pulmonary hypertension: analytic review of 131 cases. Chest. 2000;118:1133–1141. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- Aarons EJ, Nye FJ. Primary pulmonary hypertension and HIV infection. AIDS. 1991;5:1276–1277. [PubMed] [Google Scholar]
- Chen W, Havell EA, Moldawer LL, McIntyre KW, Chizzonite RA, Harmsen AG. Interleukin 1: an important mediator of host resistance against Pneumocystis carinii. J Exp Med. 1992;176:713–718. doi: 10.1084/jem.176.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Havell EA, Gigliotti F, Harmsen AG. Interleukin-6 production in a murine model of Pneumocystis carinii pneumonia: relation to resistance and inflammatory response. Infect Immun. 1993;61:97–102. doi: 10.1128/iai.61.1.97-102.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, Lei D, Vazquez C, Odom G, Summer WR, Nelson S, Shellito J. Exacerbation of murine Pneumocystis carinii infection by adenoviral-mediated gene transfer of a TNF inhibitor. Am J Respir Cell Mol Biol. 1997;16:112–118. doi: 10.1165/ajrcmb.16.2.9032117. [DOI] [PubMed] [Google Scholar]
- Pellicelli AM, D’Ambrosio C, Vizza CD, Borgia MC, Tanzi P, Pino P, Zachara E, Soccorsi F. HIV-related pulmonary hypertension. From pathogenesis to clinical aspects. Acta Cardiol. 2004;59:323–330. doi: 10.2143/AC.59.3.2005190. [DOI] [PubMed] [Google Scholar]
- Swain SD, Wright TW, Degel PM, Gigliotti F, Harmsen AG. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect Immun. 2004;72:5722–5732. doi: 10.1128/IAI.72.10.5722-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60–64. doi: 10.1378/chest.93.1.60. [DOI] [PubMed] [Google Scholar]
- Staikowsky F, Lafon B, Guidet B, Denis M, Mayaud C, Offenstadt G. Mechanical ventilation for Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. Is the prognosis really improved? Chest. 1993;104:756–762. doi: 10.1378/chest.104.3.756. [DOI] [PubMed] [Google Scholar]
- Lesprit P, Godeau B, Authier FJ, Soubrier M, Zuber M, Larroche C, Viard JP, Wechsler B, Gherardi R. Pulmonary hypertension in POEMS syndrome: a new feature mediated by cytokines. Am J Respir Crit Care Med. 1998;157:907–911. doi: 10.1164/ajrccm.157.3.9707095. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- Alpert MA, Goldberg SH, Singsen BH, Durham JB, Sharp GC, Ahmad M, Madigan NP, Hurst DP, Sullivan WD. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;68:1182–1193. doi: 10.1161/01.cir.68.6.1182. [DOI] [PubMed] [Google Scholar]
- Elstein D, Nir A, Klutstein M, Rudensky B, Zimran A. C-reactive protein and NT-proBNP as surrogate markers for pulmonary hypertension in Gaucher disease. Blood Cells Mol Dis. 2005;34:201–205. doi: 10.1016/j.bcmd.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Bhargava A, Kumar A, Yuan N, Gewitz MH, Mathew R. Monocrotaline induces interleukin-6 mRNA expression in rat lungs. Heart Dis. 1999;1:126–132. [PubMed] [Google Scholar]
- Voelkel NF, Tuder R. Interleukin-1 receptor antagonist inhibits pulmonary hypertension induced by inflammation. Ann NY Acad Sci. 1994;725:104–109. doi: 10.1111/j.1749-6632.1994.tb39794.x. [DOI] [PubMed] [Google Scholar]
- Tabata T, Ono S, Song C, Noda M, Suzuki S, Tanita T, Fujimura S. Role of leukotriene B4 in monocrotaline-induced pulmonary hypertension. Nihon Kyobu Shikkan Gakkai Zasshi. 1997;35:160–166. [PubMed] [Google Scholar]
- Miyata M, Ueno Y, Sekine H, Ito O, Sakuma F, Koike H, Nishio S, Nishimaki T, Kasukawa R. Protective effect of beraprost sodium, a stable prostacyclin analogue, in development of monocrotaline-induced pulmonary hypertension. J Cardiovasc Pharmacol. 1996;27:20–26. doi: 10.1097/00005344-199601000-00004. [DOI] [PubMed] [Google Scholar]
- Greenway S, van Suylen RJ, Du Marchie Sarvaas G, Kwan E, Ambartsumian N, Lukanidin E, Rabinovitch M. S100A4/Mts1 produces murine pulmonary artery changes resembling plexogenic arteriopathy and is increased in human plexogenic arteriopathy. Am J Pathol. 2004;164:253–262. doi: 10.1016/S0002-9440(10)63115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis. 2002;45:173–202. doi: 10.1053/pcad.2002.130041. [DOI] [PubMed] [Google Scholar]
- Kobs R, Chesler N. The mechanobiology of pulmonary vascular remodeling in the congenital absence of eNOS. Biomech Model Mechanobiol. 2006;5:217–225. doi: 10.1007/s10237-006-0018-1. [DOI] [PubMed] [Google Scholar]
- Tozzi CA, Christiansen DL, Poiani GJ, Riley DJ. Excess collagen in hypertensive pulmonary arteries decreases vascular distensibility. Am J Respir Crit Care Med. 1994;149:1317–1326. doi: 10.1164/ajrccm.149.5.8173773. [DOI] [PubMed] [Google Scholar]
- Tozzi CA, Poiani GJ, Harangozo AM, Boyd CD, Riley DJ. Pressure-induced connective tissue synthesis in pulmonary artery segments is dependent on intact endothelium. J Clin Invest. 1989;84:1005–1012. doi: 10.1172/JCI114221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Dempsey EC, Bouchey D, Reyland ME, Stenmark KR. Chronic hypoxia induces exaggerated growth responses in pulmonary artery adventitial fibroblasts: potential contribution of specific protein kinase c isozymes. Am J Respir Cell Mol Biol. 2000;22:15–25. doi: 10.1165/ajrcmb.22.1.3536. [DOI] [PubMed] [Google Scholar]
- Wilson DW, Segall HJ, Pan LC, Dunston SK. Progressive inflammatory and structural changes in the pulmonary vasculature of monocrotaline-treated rats. Microvasc Res. 1989;38:57–80. doi: 10.1016/0026-2862(89)90017-4. [DOI] [PubMed] [Google Scholar]
- Kasper M, Seidel D, Knels L, Morishima N, Neisser A, Bramke S, Koslowski R. Early signs of lung fibrosis after in vitro treatment of rat lung slices with CdCl2 and TGF-β1. Histochem Cell Biol. 2004;121:131–140. doi: 10.1007/s00418-003-0612-6. [DOI] [PubMed] [Google Scholar]
- Andoh Y, Shimura S, Aikawa T, Sasaki H, Takishima T. Perivascular fibrosis of muscular pulmonary arteries in chronic obstructive pulmonary disease. Chest. 1992;102:1645–1650. doi: 10.1378/chest.102.6.1645. [DOI] [PubMed] [Google Scholar]
- Shellito JE, Tate C, Ruan S, Kolls J. Murine CD4+ T lymphocyte subsets and host defense against Pneumocystis carinii. J Infect Dis. 2000;181:2011–2017. doi: 10.1086/315487. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Nozaki Y, Hatano K, Phan SH. Lung interleukin-4 gene expression in a murine model of bleomycin-induced pulmonary fibrosis. Cytokine. 2001;15:138–147. doi: 10.1006/cyto.2001.0903. [DOI] [PubMed] [Google Scholar]
- Jakubzick C, Choi ES, Joshi BH, Keane MP, Kunkel SL, Puri RK, Hogaboam CM. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J Immunol. 2003;171:2684–2693. doi: 10.4049/jimmunol.171.5.2684. [DOI] [PubMed] [Google Scholar]
- Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, Vollmer E, Muller-Quernheim J, Zissel G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–792. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- Stütz AM, Pickart LA, Trifilieff A, Baumruker T, Prieschl-Strassmayr E, Woisetschlager M. The Th2 cell cytokines IL-4 and IL-13 regulate found in inflammatory zone 1/resistin-like molecule α gene expression by a STAT6 and CCAAT/enhancer-binding protein-dependent mechanism. J Immunol. 2003;170:1789–1796. doi: 10.4049/jimmunol.170.4.1789. [DOI] [PubMed] [Google Scholar]
- Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, McKenzie A, Lukacs NW, Phan SH. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6. J Immunol. 2004;173:3425–3431. doi: 10.4049/jimmunol.173.5.3425. [DOI] [PubMed] [Google Scholar]
- Ma WL, Ye H, Tao XN, Xin JB. Dynamic changes of found in inflammatory zone 1 protein and mRNA expression in the lung with experimental pulmonary fibrosis of the rat. Sheng Li Xue Bao. 2005;57:493–497. [PubMed] [Google Scholar]
- Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, McKenzie A, Wilke CA, Chrisman CJ, Moore BB. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol. 2004;172:4068–4076. doi: 10.4049/jimmunol.172.7.4068. [DOI] [PubMed] [Google Scholar]