Abstract
The ability of neonatal and adult cardiomyocytes to activate the nuclear factor (NF)-κB pathway in response to lipopolysaccharide and interleukin-1β challenge has been investigated and compared with that of peritoneal macrophages. The activation of the IκB kinase and the phosphorylation and degradation of IκBα and IκBβ was much lower in adult cardiomyocytes than in the neonatal counterparts and macrophages. This restricted activation of the NF-κB pathway resulted in a significant reduction in the time of nuclear activation of NF-κB, as deduced by electrophoretic mobility shift assays and in the transcription of target genes, such as IκBα, cyclooxygenase-2 (COX-2) and nitric-oxide synthase-2 (NOS-2). Studies on chromatin immunoprecipitation showed binding of NF-κB proteins to the regulatory κB sites identified in the promoters of the IκBα, COX-2, and NOS-2 genes in macrophages and, to a lower extent, in neonatal cardiomyocytes. The binding to these κB sites in adult cardiomyocytes was observed only in the IκBα promoter and was minimal or absent in the COX-2 and NOS-2 promoters, respectively, suggesting a restricted activation of NF-κB-regulated genes in these cells. These data indicate that the function of the NF-κB pathway in adult cardiomyocytes is limited in time, which results in the expression of a reduced number of genes and provides a functional explanation for the absence of NOS-2 inducibility in these cells under proinflammatory conditions.
The ability of the heart to respond to proinflammatory cytokines and pathogen-associated molecular patterns has been reported by several authors.1,2,3 However, in isolated cardiac cells from neonatal or adult animals there are discrepancies regarding the conditions required to observe the expression of genes involved in the inflammatory response such as nitric-oxide synthase-2 (NOS-2), cyclooxygenase-2 (COX-2), and matrix metalloproteinases.4,5,6
The nuclear factor (NF)-κB family of transcription factors plays an essential role in regulating the induction of genes involved in inflammation, and it has been shown that they are important mediators of the inflammatory response in the heart. The presence of p65, p50, IκBα, and IκBβ in the myocardium has been described in postnatal and adult heart,7,8 but less is known regarding the role of c-Rel that mediates late events in the transcriptional activity of the NF-κB/c-Rel complex, preferentially in immune cells.9 The p65 protein is a key transcriptionally active component of NF-κB, but the activation of this factor mainly depends on the phosphorylation of inhibitory molecules including IκBα. However, optimal induction of NF-κB target genes also requires phosphorylation of NF-κB proteins such as p65.10
We have shown that recently isolated neonatal and adult cardiomyocytes are unable to express most of the NF-κB-regulated enzymes related to inflammation, except after a prolonged period of culture in the case of neonatal cells.11 This effect is attributable, at least in part, to a reduced activation of the IκB kinase/NF-κB pathway observed in cardiomyocytes11 but exerts differential responses depending on the genes considered; in particular, the expression of NOS-2 is significantly impaired. To gain insight on the mechanisms that regulate the expression of NF-κB-dependent genes in the cardiomyocyte, we used the in situ method of chromatin immunoprecipitation (ChIP) for the study of the transcription of IκBα, COX-2, and NOS-2, as three genes related to NF-κB activation. Our data show that after lipopolysaccharide (LPS) and interleukin (IL)-1β challenge, the activation of the IκB kinase (IKK) exhibits marked differences among cells, with adult cardiomyocytes being the less activated. Neonatal and adult cardiomyocytes expressed IκB proteins and COX-2. However, adult cardiomyocytes failed to express NOS-2 indicating an inefficient capacity of the NF-κB complex to accomplish the transcription of this gene. This behavior was compared with that of macrophages, which exhibited a robust activation of the NF-κB pathway, resulting in the expression of higher levels of COX-2 and NOS-2.
Materials and Methods
Chemicals
LPS from Salmonella typhimurium and all other reagents not specified were from Sigma Chemical Co. (St. Louis, MO). Cytokines were from Roche (Mannheim, Germany). Collagenase type II was from Worthington (Lakewood, NJ). Antibodies were obtained from Santa Cruz Laboratories (Santa Cruz, CA) or Calbiochem (San Jose, CA). Tissue culture dishes were from Falcon (Lincoln Park, NJ), and culture media were from BioWhittaker (Walkersville, MD).
Isolation and Culture of Neonatal Cardiomyocytes
Neonatal cardiomyocytes (NeoC) were isolated from 1-day-old BALB/c mice as follows. The hearts were pooled, minced, disaggregated mechanically, and digested for 15 minutes at 37°C with 0.8% collagenase (type II, Worthington Biochemical Corp.) in phosphate-buffered saline (PBS), according to a previous protocol of successive digestions.11 The cell pellets were resuspended in Dulbecco’s modified Eagle’s medium/F-12/M-199 medium [4:1 (v/v)], supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin, 100 U/ml; streptomycin, 100 μg/ml). The isolated cells were incubated for 1 hour in an uncoated flask, allowing the nonmyocytic population to differentially attach to the flask. Then, the cell suspension was sedimented by centrifugation at low speed (80 × g), and cells were distributed in plates precoated with a 2% solution of gelatin. After overnight incubation, the dishes were washed with PBS, and the medium was replaced. Usually, cells were plated at 4 × 104 cells/cm2 and used after 3 days in culture.11
Isolation of Adult Cardiomyocytes
Ventricular myocytes (AdtC) were isolated as previously described.12,13 In brief, 8-week-old male mice were anesthetized (92:7 mg/kg ketamine/xylazine) and sacrificed by cervical dislocation. Hearts were rapidly removed and placed into Tyrode buffer (140 mmol/L NaCl, 5.4 mmol/L KCl, 1 mmol/L Na2HPO4, 5 mmol/L HEPES, 10 mmol/L glucose, and 1 mmol/L MgCl2, pH 7.4), containing 1 mmol/L CaCl2 at 4°C and then cannulated via the aorta for retrograde perfusion of the coronary arteries. Hearts were perfused with Tyrode buffer containing 1 mmol/L CaCl2 at 2 ml/minute for 5 minutes at 37°C and then Tyrode buffer without CaCl2 at 2 ml/minute for 5 minutes. Final perfusion continued at 2 ml/minute for 8 to 10 minutes with Tyrode buffer containing 40 μmol/L CaCl2, 20 μg/ml collagenase (Yakult Pharmaceuticals Co., Seoul, Korea), and 4 μg/ml protease (Sigma). Ventricles were then minced in Tyrode buffer containing 1 mmol/L CaCl2, 500 μg/ml collagenase, 100 μg/ml protease, and 2.5% bovine serum albumin (Sigma). Ventricular tissue segments were put into a shaking water bath for 10 to 20 minutes at 37°C to complete dispersion of myocytes. Myocytes were cultured at a density of 1 to 2 × 104 cells/cm2 on laminin-coated tissue cultureware. The cardiomyocytes were cultured in Dulbecco’s modified Eagle’s medium with 10% FBS, 10 μg of transferrin, 10 μg/ml insulin, minimal essential medium with vitamin mix and nonessential amino acids, and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin), and used within the next 24 hours. For both NeoC and AdtC cultures, the viability of the cells was determined by propidium iodide exclusion (>90%), and the purity by immunocytochemistry for α-actin and cardiac actinin (>85% positive).
Cardiomyocyte Cell Line
HL-1 cells are cardiomyocytes derived from a mouse atrial tumor and were kindly provided by Dr. W.C. Claycomb, Louisiana State University Health Science Center, New Orleans, LA, and cultured in Claycomb medium (JRH Biosciences, Lenexa, KS) containing 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mmol/L norepinephrine, and 2 mmol/L l-glutamine. Cells were cultivated at 37°C under 5% CO2 on fibronectin-coated flasks. HL-1 is a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte.14
Preparation of Macrophages
Elicited peritoneal macrophages were prepared from male mice 4 days after intraperitoneal administration of 1 ml of 10% thioglycollate broth.15 Cells were seeded at 2 × 106 in 6-cm plates or 5 × 105 in 24-multiwell plates and cultured with RPMI 1640 medium supplemented with 10% heat-inactivated FBS and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C in an humidified atmosphere with 5% CO2. After incubation for 4 hours, nonadherent cells were removed, and remnant cells were cultured for the indicated times.16
Transfection of Macrophages and Cardiomyocytes
Cells were transfected per triplicate using JetPeI transfection reagent (PolyPlus Transfection, San Marcos, CA) with a JetPeI/DNA ratio of 3:1 and in the presence of 2% FBS. Twenty-four hours after transfection, the culture medium was changed, and cells were activated with the indicated stimuli. The vectors used were p-Luc, (κB)3-Luc, p-p65, c-Rel, and the respective dominant-negative forms p-DN-p65 and p-DN-c-Rel, respectively. These vectors were provided generously by Dr. M. Fresno, Universidad Autónoma de Madrid, Madrid, Spain, and have been described previously.17,18 Reporter activities were assayed using the dual luciferase assay system per the manufacturer’s instructions (Promega, Madison, WI). For normalization of transfection efficiency, cells were co-transfected with the reporter plasmid pTK Renilla (Promega), and luminescence data were expressed as the relative light ratio between both enzymes. A vector encoding the GFP gene was used for the evaluation of the transfection efficiency.
Measurement of Metabolites
The amounts of nitrite and nitrate that accumulate in the culture medium were determined after reduction of nitrates to nitrites followed by the determination of NO2− levels with Griess reagent.11 The levels of PGE2 were determined using a Biotrak kit from Amersham, Arlington Heights, IL, following the instructions of the supplier.
Determination of TLR2/4 Expression Levels by Flow Cytometry
Cells were resuspended in PBS 1× and incubated 15 minutes with a fluorescein isothiocyanate/phycoerythrin-conjugated antibody (Ab) against the indicated TLR (eBioscience, San Diego, CA). The fluorescence intensity was measured using a Quantum MESF kit (Bangs Laboratories, Inc., Fishers, IN).
Preparation of Cytosolic and Nuclear Extracts
Cultured cells were washed with PBS, scraped off the dishes in ice-cold PBS, and centrifuged. Cell pellets were homogenized in 200 μl of buffer A (10 mmol/L HEPES, pH 7.9, 1 mmol/L ethylenediamine tetraacetic acid (EDTA), 1 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 10 mmol/L KCl, 1 mmol/L dithiothreitol, 0.5 mmol/L phenylmethyl sulfonyl fluoride, 40 μg/ml leupeptin, 2 μg/ml tosyl-lysyl-chloromethane, 5 mmol/L NaF, 1 mmol/L NaVO4, and 10 mmol/L Na2MoO4), and Nonidet P-40 was added to reach 0.5% (v/v). After 15 minutes at 4°C, the tubes were gently vortexed for 15 seconds, and nuclei were collected by centrifugation at 8000 × g for 15 minutes. The supernatants were stored at −80°C (cytosolic extracts), and the pellets were resuspended in 50 μl of buffer A supplemented with 20% (v/v) glycerol and 0.4 mol/L KCl, and mixed for 30 minutes at 4°C. Nuclear proteins were obtained by centrifugation at 13,000 × g for 15 minutes, and aliquots of the supernatant (nuclear extracts) were stored at −80°C. For Western blot analysis, cytosolic and nuclear proteins were boiled in Laemmli sample buffer, and equal amounts of protein (30 to 15 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Measurement of IKK Activity
Macrophages and NeoC and AdtC cells (5 to 6 × 106) were stimulated, and at the indicated times the cells were homogenized in 1 ml of buffer A and centrifuged for 10 minutes in a microcentrifuge. The IKK from equal amounts of protein from these supernatants was immunoprecipitated with 1 μg of anti-IKK2 Ab. After washing with 4 ml of buffer A, the pellet was resuspended in kinase buffer (20 mmol/L HEPES, pH 7.4, 0.1 mmol/L EDTA, 100 mmol/L NaCl, 1 mmol/L dithiothreitol, 0.5 mmol/L phenylmethyl sulfonyl fluoride, 2 μg/ml aprotinin, 10 μg/ml leupeptin, 2 μg/ml TLCK, 5 mmol/L NaF, 1 mmol/L NaVO4, 10 mmol/L Na2MoO4, and 10 nmol/L okadaic acid). Kinase activity was assayed in 100 μl of kinase buffer containing 50 ng of precipitated protein. Myelin basic protein was used as substrate in the presence of 50 μmol/L [γ-32P]ATP (0.5 μCi). Reactions were stopped with 1 ml of ice-cold buffer A supplemented with 5 mmol/L EDTA, and after retention onto a P81 Whatman filter and counted.
Electrophoretic Mobility Shift Assays
The oligonucleotide sequence corresponding to the NF-κB site of the rat COX-2 promoter (5′-GGCAAGGGGATTCCCTTAGTT-3′)11 was annealed with the complementary sequence by incubation for 5 minutes at 85°C in 10 mmol/L Tris-HCl, pH 8.0, 50 mmol/L NaCl, 10 mmol/L MgCl2, 1 mmol/L dithiothreitol. Aliquots (100 ng) were end-labeled with Klenow enzyme fragment in the presence of 50 μCi of [α-32P]dCTP and the other unlabeled dNTPs in a final volume of 50 μl. DNA probe (5 × 104 dpm) was used for each binding assay: 5 μg of nuclear protein were incubated for 15 minutes at 4°C with the probe and with 1 μg of poly (dI-dC), 5% glycerol, 1 mmol/L EDTA, 10 mmol/L KCl, 5 mmol/L MgCl2, 1 mmol/L dithiothreitol, and 10 mmol/L Tris-HCl (pH 7.8) in a final volume of 20 μl. The DNA-protein complexes were separated on native 6% polyacrylamide gels in 0.5% Tris-borate-EDTA buffer. Supershift assays were performed by incubating the nuclear extracts (3 μg of protein) with the indicated antibodies against NF-κB proteins (p50, p65, and c-Rel) before the addition of the probe.
Western Blot Analysis
The protein levels of IκBα, IκBβ, p65, c-Rel, and α/β-actin were determined in cytosolic or nuclear extracts as indicated. Equal amounts of protein (15 to 30 μg) were size-fractionated in a 10% acrylamide gel, transferred to a Hybond P membrane (Amersham), and after blocking with 5% nonfat dry milk, were incubated with the corresponding antibodies and visualized by enhanced chemiluminescence. Different exposure times were performed with each blot to ensure the linearity of the band intensities. Band intensities were measured on a densitometric scanner (Amersham) and expressed in arbitrary units.
Quantitative Real-Time-Polymerase Chain Reaction (Q-RT-PCR)
Total RNA was extracted from frozen cells by using TRIzol reagent (Life Technologies, Inc., Grand Island, NY). Total RNA (1 μg per sample) was reverse-transcribed with oligo(dT) as primer using Expand Reverse Transcriptase (Roche, Basel, Switzerland) according to the manufacturer’s protocol. Q-RT-PCR was performed using SYBR Green PCR kit (PE Applied Biosystems, Foster City, CA) in an Applied Biosystems 7700 sequence detector. Primer sequences were: NOS-2 (mouse) forward, 5′-CAGCTGGGCTGTACAAACCTT-3′, reverse 5′-CATTGGAAGTGAAGCGTTTCG-3′; COX-2 (mouse) forward, 5′-GCTGTACAAGCAGTGGC AAAG-3′, reverse 5′-GCGTTTGCGGTACTCATTGAGA-3′; IκBα (mouse) forward, 5′-GCTCCGAGACTTTCGAGGAA-3′, reverse 5′-TTGTAGTTGGTGGCCTGCA G-3′. All samples were analyzed in the same run for 18S expression for normalization: forward, 5′-AACACGGGAAACCTCACCC-3′ and reverse, 5′-CCACCAACTAAGAACGGCCA-3′. PCR parameters were 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The quantitative expression values were extrapolated from standard curves for 18S mRNA. The replicates were then averaged, and fold induction was determined, considering the value at zero time as 1.
ChIP Assay
ChIP assay was performed according to Saccani and colleagues.19,20 In brief, after stimulation, cells were fixed by adding directly to the medium formaldehyde solution to reach a final concentration of 1%. After incubation for 15 minutes, the cells were washed extensively with PBS, scraped off the dishes, and centrifuged. Cells were resuspended in ice-cold buffer I (10 mmol/L HEPES, pH 7.9, 10 mmol/L EDTA, 0.5 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, and 0.25% Triton X-100), centrifuged, and the pellet resuspended in buffer II (10 mmol/L HEPES, pH 7.9, 1 mmol/L EDTA, 0.5 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, and 200 mmol/L NaCl). After centrifugation, nuclei were lysed with ice-cold lysis buffer (50 mmol/L Tris-HCl, pH 8.0, 10 mmol/L EDTA, 1% SDS, and protease inhibitors). Chromatin was sheared by sonication (5 × 18 seconds each at 10% power), centrifuged, and diluted five times with the IP dilution buffer (20 mmol/L Tris-HCl, pH 8.0,; 2 mmol/L EDTA, 1% Triton X-100, 150 mmol/L NaCl, and protease inhibitors). The extracts were precleared for 30 minutes with a 50% suspension of salmon sperm-saturated protein A/G agarose. Immunoprecipitations with anti-p65 or anti-c-Rel Abs (Santa Cruz Biotechnologies) or with no Ab, were performed overnight at 4°C. Immune complexes were collected with protein A/G agarose and washed sequentially for 5 minutes each in buffer W150 (20 mmol/L Tris-HCl, pH 8.0, 2 mmol/L EDTA, 1% Triton X-100, 0.1% SDS, and 150 mmol/L NaCl), buffer W500 (20 mmol/L Tris-HCl, pH 8.0, 2 mmol/L EDTA, 1% Triton X-100, 0.1% SDS, and 500 mmol/L NaCl), and buffer WIII (10 mmol/L Tris-HCl, pH 8.0, 1 mmol/L EDTA, 0.25 mol/L LiCl, 1% Nonidet P-40, and 1% deoxycholate). Precipitates were then washed twice with TE (10 mmol/L Tris-HCl, pH 8.0, and 1 mmol/L EDTA), extracted with TES (25 mmol/L Tris-HCl, pH 7.5, 10 mmol/L EDTA, and 0.5% SDS) and heated at 65°C to elute the chromatin. After proteinase K digestion, chromatin DNA was extracted with phenol-chloroform followed by ethanol precipitation. PCR reactions were performed with promoter-specific primers and normalized with the input controls. Primer sequences are shown in Table 1. After amplification, the PCR products were electrophoresed in a 2% agarose gel and visualized by ethidium bromide staining.
Table 1.
Primers for ChIP Assays
| Gene | Primers |
|---|---|
| COX-2 | Forward 5′-TTAACCGGTAGCTGTGTGCGT-3′ |
| Reverse 5′-CCCGGGATCTAAGGTCCTAACT-3′ | |
| NOS-2 | Forward 5′-GACCATGCGAAGATGAGTGGA-3′ |
| Reverse 5′-TGAAAGTGAAATATTGACAGTGTT AGGG-3′ | |
| IκBα (1) | Forward 5′-GACTTTCCAGCCACTCAGGG-3′ |
| Reverse 5′-CCTAAACCCAGGGCCGG-3′ | |
| IκBα (2) | Forward 5′-TACTGAGTGCAGGCTGCAGG-3′ |
| Reverse 5′-GGTCATGCACAGGGAACTTTTT-3′ | |
| IκBα (3) | Forward 5′-ACCCCAGGGAAAGAAGGGTT-3′ |
| Reverse 5′-CTGGAAAGTCCTCCCGACC-3′ |
Statistical Analysis
Unless otherwise stated, data are expressed as mean ± SD. To compare means between two independent experiments the Mann-Whitney rank sum test was used. Statistical analysis of mRNA expression data was performed by using the two-tailed homoscedastic t-test. The results were considered significant at P < 0.05. Data were analyzed by SPSS for Windows statistical package version 9.0.1 (SPSS Inc., Chicago, IL).
Results
Differential Degradation of IκB Proteins after Proinflammatory Challenge
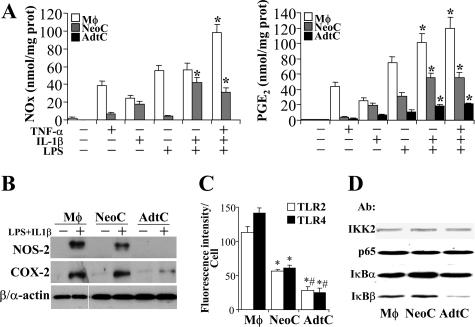
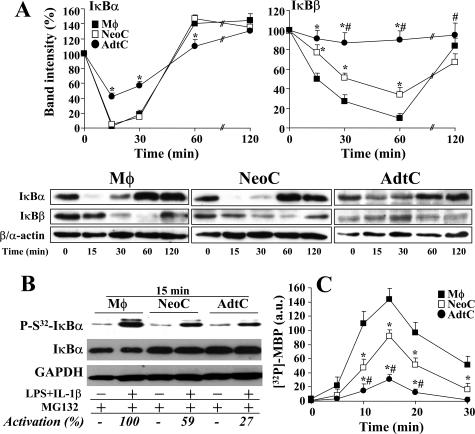
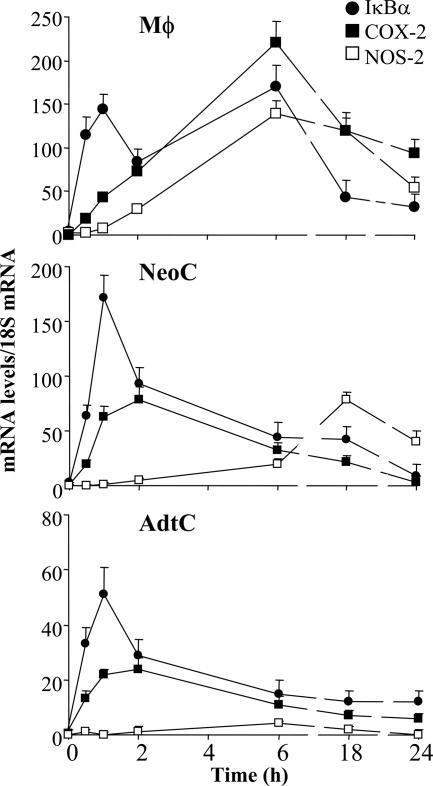
The response of primary cultures of neonatal (NeoC) and adult (AdtC) cardiomyocytes to tumor necrosis factor (TNF)-α, IL-1β, and LPS in terms of NOS-2 and COX-2 expression (two well-characterized NF-κB-dependent genes) and nitrite and PGE2 synthesis was analyzed and compared with that of peritoneal macrophages treated under identical conditions. Figure 1A shows the response of these cells to combinations of these proinflammatory stimuli, and maximal accumulation of NOx and/or PGE2 in the culture medium was obtained when LPS and IL-1β were combined. TNF-α promoted a significant apoptosis in cardiomyocytes after 24 hours of culture and for this reason it was omitted in the proinflammatory mixture. As Figure 1, A and B, shows, AdtC failed to express NOS-2, and only moderate levels of COX-2 were detected, whereas NeoC and macrophages exhibited a robust expression of both NOS-2 and COX-2. Agreement was observed between the levels of NOx and PGE2 and the corresponding protein levels determined by Western blot. In addition to these measurements, the relative levels of TLR4 and TLR2 in the cell membrane were determined in intact cultured cells. Macrophages exhibited approximately twofold and fourfold higher levels than those measured in NeoC and AdtC, respectively (Figure 1C); however, the levels of IKK2, p65, and IκBα were quite similar in the three cell types, and only a minor content of IκBβ was observed in AdtC (Figure 1D). In view of these data, we compared the ability of these cells to activate the NF-κB pathway after proinflammatory challenge. As Figure 2A shows, on normalization to the basal levels, macrophages and NeoC exhibited a rapid degradation of IκBα and IκBβ, as a result of the activation of IKK and phosphorylation of IκBα in S32 in response to LPS and IL-1β (Figure 2B). However, AdtC exhibited a moderate phosphorylation of IκBα in S32 and a decrease of both IκB proteins, minimal in the case of IκBβ, followed by a rapid resynthesis of IκBα. When the activation of IKK was determined in these cells, the activation in AdtC represented only 21% and 32% of the activity in macrophages and NeoC, respectively, after 15 minutes of stimulation (Figure 2C). These differences in IKK activity and IκB degradation and recovery resulted in different responses in the mRNA levels of the genes studied: IκBα, NOS-2, and COX-2, with a significantly attenuated response in the case of the NOS-2 gene in AdtC (Figure 3; to note the difference in scales).
Figure 1.
Differential expression of NOS-2, COX-2, and TLR2/4 in cardiomyocytes. A: Primary cultures of macrophages (Mφ), neonatal cardiomyocytes (NeoC), and adult cardiomyocytes (AdtC) were maintained in culture and stimulated for 24 hours with 10 ng/ml TNF-α, 20 ng/ml IL-1β, 200 ng/ml LPS, or combinations of these, and the accumulation of NOx and PGE2 in the culture medium was determined. B: The levels of NOS-2 and COX-2 were determined by Western blot in cells treated with LPS and IL-1β. C: The membrane levels of TLR2 and TLR4 were determined quantitatively by flow cytometry using calibrated beds. D: The levels of IKK2, p65, and IκB proteins from equal amounts of cytosolic proteins were determined by Western blot. β- and α-actin were used for the normalization of the blots corresponding to macrophages and cardiomyocytes, respectively. Results show a representative blot (B and D) and the mean ± SD of the metabolites or fluorescence distribution of four independent experiments; A: *P < 0.01 versus the condition of only one proinflammatory stimulus; C: *P < 0.01 versus the Mφ condition; #P < 0.05 for AdtC versus NeoC.
Figure 2.
Time course of IκB degradation and IKK activity in macrophages and cardiomyocytes challenged with LPS/IL-1β. Primary cultures of macrophages (Mφ), neonatal cardiomyocytes (NeoC), and adult cardiomyocytes (AdtC) were treated as described in Figure 1 and the time course of the levels of IκBα, IκBβ, and β- and α-actin were determined by Western blot. A: The means of the levels of IκB proteins after normalization for the β- and α-actin content in macrophages and cardiomyocytes, respectively, were plotted as percentage versus 0 minutes (n = 4 experiments) (a representative blot is shown). B: To evaluate the phosphorylation in S32 of IκBα, cells were stimulated with LPS/IL-1β for 15 minutes in the presence of 20 μmol/L MG132 to prevent proteasomal degradation, and the levels of phospho-S32-IκBα were determined by Western blot, using GAPDH for the normalization of the blots, and expressed as the percentage of activation measured in macrophages. C: IKK activity was determined after immunoprecipitation of cell extracts and in vitro assay of the kinase activity using myelin basic protein as substrate. Results show the mean ± SD of at least three experiments. *P < 0.01 and #P < 0.01 versus the corresponding condition in macrophages and NeoC, respectively.
Figure 3.
Time course of IκBα, COX-2, and NOS-2 mRNA levels in activated cardiomyocytes. Cells were treated as described in Figure 1, and total RNA was prepared at the indicated times. The mRNA levels of the indicated genes were determined by Q-RT-PCR after normalization with the 18S mRNA content and considering as 1 the value at zero time. Results show the mean of three experiments assayed per duplicate.
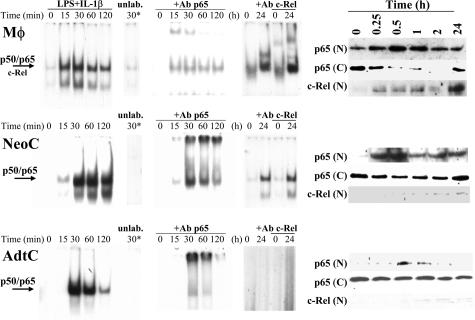
To gain insight on the nuclear translocation of NF-κB in these cells after LPS/IL-1β challenge, serial electrophoretic mobility shift assays were performed. As Figure 4 shows, the binding of the κB complexes was most restricted in time in AdtC than in macrophages or NeoC. Moreover, supershift studies showed that the complexes at early times after activation involved p50 and p65 proteins, whereas at 24 hours the presence of c-Rel was detected in nuclear extracts from activated macrophages and to a lesser extent in NeoC. In agreement with these data, the presence of p65 in the nucleus was higher in macrophages and NeoC than in AdtC, with a minimal contribution of c-Rel in the case of NeoC.
Figure 4.
Nuclear translocation of NF-κB in activated cardiomyocytes. Primary cultures of macrophages (Mφ), neonatal cardiomyocytes (NeoC), and adult cardiomyocytes (AdtC) were stimulated with LPS/IL-1β for the indicated periods of time, and nuclear extracts were prepared. The binding activity of the NF-κB complex was determined by electrophoretic mobility shift assays. A 100-fold excess of unlabeled DNA probe was added to samples obtained after 30 minutes of stimulation and used to ensure specificity of the binding (lane 30*). Supershift assays were performed after incubation of the nuclear cell extracts with anti-p65 or anti-c-Rel Abs, and the corresponding p50/p65 complex was identified. The cytosolic (C) and nuclear (N) levels of p65 and c-Rel were determined by Western blot, and blots were normalized with β- and α-actin (C) and Sp1 (N) to ensure equal lane charge (not shown). Results show a representative experiment of three.
Analysis of NF-κB Complexes in the Promoter Region of Selected NF-κB-Dependent Genes
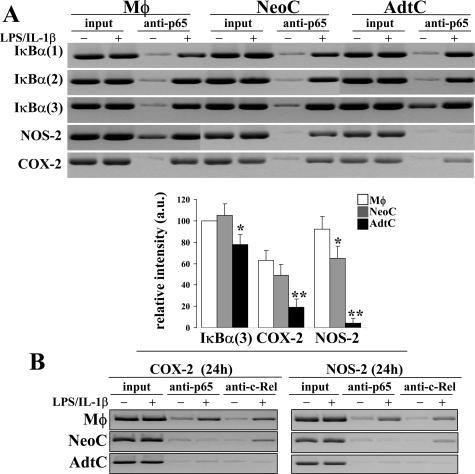
Further to determine the binding of NF-κB proteins to previously recognized regulatory sequences in the promoters of IκBα, NOS-2, and COX-2 ChIP assays were performed in samples obtained after 2 hours and 24 hours of activation with LPS/IL-1β. As Figure 5 shows, binding of p65 complexes was observed in the three motifs investigated of the IκBα promoter, regardless of the nature of the cells. When the binding to the distal κB site from the murine NOS-2 promoter was investigated, a significant and reproducible presence of p65 was observed in macrophages and NeoC; however, AdtC completely failed to show such binding, and only a modest recognition was present in the case of the κB site from the COX-2 promoter. ChIP analysis was also performed on the NOS-2 and COX-2 promoters after 24 hours of stimulation, including anti-c-Rel Abs in the immunoprecipitation reaction. As Figure 5 (bottom) shows, c-Rel was absent in AdtC, and only a minor binding in the case of the COX-2 promoter was observed in NeoC. Opposite to this behavior, macrophages exhibited a clear presence of c-Rel at 24 hours in both promoters.
Figure 5.
ChIP and evaluation of κB-motifs occupancy. Primary cultures of macrophages (Mφ), neonatal cardiomyocytes (NeoC), and adult cardiomyocytes (AdtC) were maintained in culture and activated with LPS/IL-1β. ChIP analysis was performed in samples obtained after 2 hours (A) and 24 hours (B) of activation using anti-p65 and anti-c-Rel Abs for the precipitation, respectively. The following κB sites were investigated in the murine promoters (versus start of transcription): IκBα(1) = −219, IκBα(2) = −252, IκBα(3) = −313, NOS-2 = −962, and COX-2 = −393. Results show a representative blot of three and the mean ± SD of three experiments expressed as percentage of the intensity of the IκBα(3) site from macrophages. *P < 0.05 and **P < 0.01 versus the corresponding levels in macrophages.
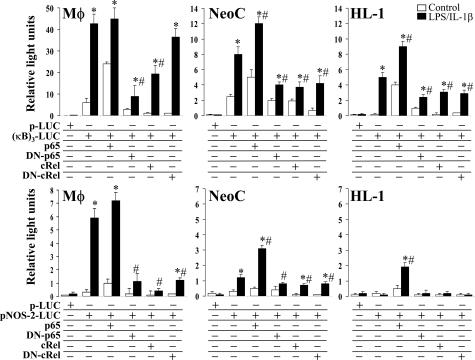
To investigate the possibility of a restoration of the NF-κB response via the expression of p65 and c-Rel proteins, macrophages, NeoC, AdtC, and the adult cardiomyocytic cell line HL-1 were transfected with a κB reporter vector or with the 1.1-kb NOS-2 promoter linked to a luciferase gene and various proteins of the p65 and c-Rel family. After transfection and stimulation with LPS/IL-1β, the reporter activity was measured. The efficiency for the transfection of AdtC was quite low (1 to 4%), and therefore, the experiments were performed with the HL-1 cell line (see Discussion). As Figure 6 shows, the activity of the κB-reporter increased in the three types of cells after LPS/IL-1β challenge, although with significant quantitative differences, with HL-1 being the less responsive cells. Cotransfection with p65 restored the activation of the promoter, whereas c-Rel decreased the reporter activity. The expression of the dominant-negative forms of p65 and c-Rel abolished significantly the inducibility of the reporter luciferase gene. When the experiment was performed in cells transfected with the NOS-2 promoter vector, only macrophages exhibited a robust response, whereas NeoC cells induced a moderate expression, and failure was observed in the case of HL-1 cells. Interestingly, overexpression of p65, but not c-Rel, enhanced notably the activity of the NOS-2 promoter in NeoC and HL-1 cells, which suggests that the activation of the NF-κB pathway accomplished in these cells after LPS/IL-1β challenge is insufficient to direct the typical high expression of this gene observed in macrophages and other cells.
Figure 6.
Effect of p65 and c-Rel transfection on the NOS-2 promoter activity. Primary cultures of macrophages (Mφ), neonatal (NeoC), and the adult cardiomyocytic cell line HL-1 were cotransfected with the indicated vectors, and the luciferase activity was determined after 24 hours of culture. Results are expressed as the luminescence ratio between the light corresponding to the reporter activity versus the light of Renilla luciferase used as normalization vector. Data show the mean ± SD of four experiments. *P < 0.01 versus the control condition; #P < 0.01 versus the condition in the absence of p65 and c-Rel proteins.
Discussion
In this work we have studied quantitative aspects of NF-κB activation in cardiomyocytes using as reference peritoneal macrophages that exhibit a robust response of this pathway after proinflammatory stimulation.21,22 Our data show a differential occupancy and transcriptional activity of various NF-κB-regulated genes in cardiomyocytes, a situation that is dependent on the age of the animals: whereas IκBα was rapidly degraded and re-expressed in the three types of cells studied, COX-2 and NOS-2 expression was attenuated in NeoC with respect to macrophages. Moreover, in AdtC only COX-2 was expressed. Of note, although IκBα was processed in all these cells, IκBβ remained virtually stable in AdtC, and only in macrophages was it fully degraded and resynthesized, which implies a differential regulation of NF-κB activity among these cells. In agreement with these data, previous work showed that AdtC exhibit a moderate response in the expression of genes depending on NF-κB activity, despite containing similar levels of most of the proteins of the pathway, such as IKK, IκBα, and p65.11,23 To explain this loss of expression of κB-dependent genes in AdtC, we investigated the contribution of early signaling, leading to NF-κB activation and the existence of potential transcriptional silencing mechanisms once the complex is located in the nucleus (ie, cooperative actions of histone deacetylases, HDACs).
Regarding the proinflammatory stimulation, we investigated the effect of LPS and IL-1β that provided the most potent response in terms of IκBα degradation, almost identical to that observed when TNF-α was included as stimulus. However, TNF-α was excluded in this study because this cytokine promoted apoptotic death of AdtC and to a lesser extent in NeoC. Co-treatment with IFN-γ did not influence NF-κB activation or the expression of NOS-2 in AdtC (not shown). The profile of genes altered in the heart after systemic administration of LPS is known, and a reversible cardiac dysfunction starting 24 hours after endotoxin administration has been reported.24 These data show a spatial and temporal heterogeneity in the changes of the expression of genes that are important to understand the complexity of the sepsis in the whole organ, including the infiltration of inflammatory cells,25,26 but most of these effects are lost when the organ is digested to prepare cardiomyocytic cultures, suggesting a major contribution of infiltrated nonmyocytic cells to the inflammatory response in the heart.23
The role of TLR2 and TLR4 in the expression of inflammatory mediators in the cardiomyocyte is well documented.26,27,28 Our data show that TLR2 and TLR4 are present in the cell membrane of NeoC and AdtC, although at concentrations that are less than twofold and fourfold those present in the macrophages, respectively. It can be suggested accordingly that the initial downstream signaling is attenuated because the activation of IKK and accumulation of phosphorylated IκBα in AdtC treated for 20 minutes with LPS/IL-1β represents only 20 and 27%, respectively, of the levels reached in macrophages. Moreover, these data suggest that the rapid resetting of IκBα protein after stimulation of AdtC is responsible, at least in part, for the decreased activation of the NF-κB pathway as deduced from the results obtained in cells transfected with a p65 expression vector. In addition to this, it has been reported that the levels of TLR2 and TLR4 can be up-regulated and expressed in the cell membrane on proinflammatory challenge, and in this way a second round of stimulation on cells expressing higher levels of TLRs can result in a more effective response in the case of the AdtC.28,29 Indeed, the mRNA levels of TLR4 increased three times after 24 hours of LPS administration (results not shown),28 and transfection with constitutively active forms of TLR4 induced NF-κB activity but not the transcription of some promoters, such as NOS-2.28 The transcription of NOS-2 in rodent cells is strictly dependent on NF-κB activity, and the distal κB site, which has been used in our ChIP study, confers inducibility in response to interferon (IFN)-γ.30,31 According to our data, the NF-κB proteins remained unbound to this κB site required for the expression of NOS-2, even in the presence of IFN-γ during the stimulation (not shown), at the time that equivalent sites, in particular those found in the IκBα promoter, were occupied and transcriptionally active in view of the rapid up-regulation and accumulation of IκBα mRNA and proteins after LPS/IL-1β challenge. The question remains if prolonged exposure to proinflammatory stimuli, a condition that enhances the expression of TLRs, would be sufficient to improve NF-κB responsiveness leading to a behavior similar to that observed in NeoC. In this regard, we described in a previous work that prolonged culture of cardiomyocytes enhanced notably the expression of genes related to inflammation, specifically the expression of NOS-2 in NeoC in response to LPS/IL-1β challenge.11
We also investigated the contribution of p65 and c-Rel proteins in the particular case of NOS-2 transcription. c-Rel has been detected in macrophages and other immune cells where it mediates late and sustained responses in the activation of specific promoters.32,33 As expected, macrophages expressed c-Rel, and only minor levels were detected at 24 hours in NeoC, but not in AdtC. However, transfection with c-Rel of macrophages, NeoC, and the HL-1 cell line, which behaves very closely to AdtC, resulted in a clear inhibitory effect in the promoter activity of NOS-2 in the three cell systems assayed, precluding a potential inducing effect of this transcription factor. This was not the case of p65 that, as expected, restored a notable activation of the NOS-2 promoter in the three cell types analyzed.
The attenuated response of the NF-κB pathway in cardiomyocytes seems to have important physiopathological consequences. On the one hand, our data suggest that the activation of the NF-κB pathway only induces a moderate expression of some of the genes involved in the release of potent effector molecules, such as NO and prostanoids that have been described to contribute to heart dysfunction.34 On the other hand, data from various groups have stressed the relevance of this activation of the NF-κB pathway to mediate cell protection and function under pathological circumstances; for example, in animals infected with Trypanosoma cruzi, there is a selective impairment of the apoptotic response to TNF-α in cardiomyocytes and neurons via the activation of the NF-κB pathway, and this response seems to be specific because T cells undergo apoptosis after infection.35,36 In agreement with this view, hypertrophic heart can be considered as a condition in which NF-κB is activated and cells are protected against apoptotic death.37 This situation can be pharmacologically prevented with salicylate analogues, such as triflusal, that are potent inhibitors of the NF-κB pathway and, therefore, efficient in preventing cardiac hypertrophy.38 The mechanisms by which the activation of NF-κB protects cardiomyocytes from apoptosis seems to include, in addition to the expression of common anti-apoptotic targets,22,39,40,41 the specific inhibition in the heart of the hypoxia-inducible death factor BNIP3.42 Moreover, initial experiments to evaluate the contribution of HDAC activity on the p65-dependent transactivation revealed that the association between HDAC1 and p65 is minimal in AdtC, and inhibitors of HDAC, such as trichostatin A, failed to enhance the expression of NOS-2 and COX-2 in AdtC or to increase the binding of NF-κB to the sites studied in the ChIP experiments (not shown).
Finally, it should be mentioned that in the myocytic cell line C2C12, derived from skeletal muscle, LPS is able to induce a significant expression of NOS-2 and other genes involved in the inflammatory response; however, in the cardiomyocytic cell line HL-1 as well as in AdtC we failed to observe such an effect, reinforcing the view that these cells have an attenuated response in the NF-κB pathway.43 In summary, our data suggest that adult cardiomyocytes display a moderate capacity to activate the NF-κB pathway, which might be important for the pathophysiology of the organ. Indeed, various studies suggest that the major input of proinflammatory stimuli and effector molecules, such as TNF-α, NO, and prostaglandins that are present in the heart under infectious or inflammatory conditions are released by infiltrating macrophages and neutrophils,23,26 supporting the view that NF-κB activation in adult cardiac cells is involved in other cell functions, distinct from the acute inflammatory response. Ancillary to this conclusion, pharmacological achievements to regulate heart dysfunction under sepsis should take into account the relative contribution of the distinct types of cells present in the heart, as well as those infiltrating from the circulatory system, to the whole inflammatory response in the organ.
Footnotes
Address reprint requests to Lisardo Boscá, IIB-Alberto Sols, Arturo Duperier 4, 28029 Madrid, Spain. E-mail: lbosca@iib.uam.es.
Supported by the Ministerio de Educación y Ciencia (grant SAF2005-03022), Recava, Biociencia, the Fundación Mutua Madrileña, and Centro Nacional de Investigaciones Cardiovasculares-Bancaja (to J.C.).
J.C. and N.G. contributed equally.
References
- Koyanagi M, Egashira K, Kitamoto S, Ni W, Shimokawa H, Takeya M, Yoshimura T, Takeshita A. Role of monocyte chemoattractant protein-1 in cardiovascular remodeling induced by chronic blockade of nitric oxide synthesis. Circulation. 2000;102:2243–2248. doi: 10.1161/01.cir.102.18.2243. [DOI] [PubMed] [Google Scholar]
- Knuefermann P, Nemoto S, Baumgarten G, Misra A, Sivasubramanian N, Carabello BA, Vallejo JG. Cardiac inflammation and innate immunity in septic shock: is there a role for toll-like receptors? Chest. 2002;121:1329–1336. doi: 10.1378/chest.121.4.1329. [DOI] [PubMed] [Google Scholar]
- Frishman WH, Ismail AA. Role of infection in atherosclerosis and coronary artery disease: a new therapeutic target? Cardiol Rev. 2002;10:199–210. doi: 10.1097/00045415-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Bradford Sanders D, Hunter K, Wu Y, Jablonowski C, Bahl JJ, Larson DF. Modulation of the inflammatory response in the cardiomyocyte and macrophage. J Extra Corpor Technol. 2001;33:167–174. [PubMed] [Google Scholar]
- Kinugawa K, Shimizu T, Yao A, Kohmoto O, Serizawa T, Takahashi T. Transcriptional regulation of inducible nitric oxide synthase in cultured neonatal rat cardiac myocytes. Circ Res. 1997;81:911–921. doi: 10.1161/01.res.81.6.911. [DOI] [PubMed] [Google Scholar]
- Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, Feldman AM. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor α can be modulated by anti-tumor necrosis factor α therapy. Proc Natl Acad Sci USA. 2000;97:12746–12751. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz S, Fraccarollo D, Wagner H, Behr TM, Jung P, Angermann CE, Ertl G, Bauersachs J. Sustained activation of nuclear factor κB and activator protein 1 in chronic heart failure. Cardiovasc Res. 2003;57:749–756. doi: 10.1016/s0008-6363(02)00723-x. [DOI] [PubMed] [Google Scholar]
- Norman DA, Yacoub MH, Barton PJ. Nuclear factor NF-κB in myocardium: developmental expression of subunits and activation by interleukin-1 β in cardiac myocytes in vitro. Cardiovasc Res. 1998;39:434–441. doi: 10.1016/s0008-6363(98)00118-7. [DOI] [PubMed] [Google Scholar]
- Jankowska EA, von Haehling S, Czarny A, Zaczynska E, Kus A, Anker SD, Banasiak W, Ponikowski P. Activation of the NF-κB system in peripheral blood leukocytes from patients with chronic heart failure. Eur J Heart Fail. 2005;7:984–990. doi: 10.1016/j.ejheart.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Goren N, Cuenca J, Martin-Sanz P, Bosca L. Attenuation of NF-κB signalling in rat cardiomyocytes at birth restricts the induction of inflammatory genes. Cardiovasc Res. 2004;64:289–297. doi: 10.1016/j.cardiores.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Poon BY, Ward CA, Giles WR, Kubes P. Emigrated neutrophils regulate ventricular contractility via α4 integrin. Circ Res. 1999;84:1245–1251. doi: 10.1161/01.res.84.11.1245. [DOI] [PubMed] [Google Scholar]
- Fernández-Velasco M, Goren N, Benito G, Blanco-Rivero J, Bosca L, Delgado C. Regional distribution of hyperpolarization-activated current (If) and hyperpolarization-activated cyclic nucleotide-gated channel mRNA expression in ventricular cells from control and hypertrophied rat hearts. J Physiol. 2003;553:395–405. doi: 10.1113/jphysiol.2003.041954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi F, Diaz-Guerra MJ, Casado M, Hortelano S, Leoni S, Bosca L. Bacterial lipopeptides induce nitric oxide synthase and promote apoptosis through nitric oxide-independent pathways in rat macrophages. J Biol Chem. 1995;270:6017–6021. doi: 10.1074/jbc.270.11.6017. [DOI] [PubMed] [Google Scholar]
- Callejas NA, Fernandez-Martinez A, Castrillo A, Bosca L, Martin-Sanz P. Selective inhibitors of cyclooxygenase-2 delay the activation of nuclear factor κ B and attenuate the expression of inflammatory genes in murine macrophages treated with lipopolysaccharide. Mol Pharmacol. 2003;63:671–677. doi: 10.1124/mol.63.3.671. [DOI] [PubMed] [Google Scholar]
- Martin AG, San Antonio B, Fresno M. Regulation of nuclear factor κB transactivation. Implication of phosphatidylinositol 3-kinase and protein kinase C zeta in c-Rel activation by tumor necrosis factor α. J Biol Chem. 2001;276:15840–15849. doi: 10.1074/jbc.M011313200. [DOI] [PubMed] [Google Scholar]
- Martin AG, Fresno M. Tumor necrosis factor-α activation of NF-κB requires the phosphorylation of Ser-471 in the transactivation domain of c-Rel. J Biol Chem. 2000;275:24383–24391. doi: 10.1074/jbc.M909396199. [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-κB recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G. Two waves of nuclear factor κB recruitment to target promoters. J Exp Med. 2001;193:1351–1359. doi: 10.1084/jem.193.12.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo A, Mojena M, Hortelano S, Bosca L. Peroxisome proliferator-activated receptor-γ-independent inhibition of macrophage activation by the non-thiazolidinedione agonist L-796,449. Comparison with the effects of 15-deoxy-Δ(12,14)-prostaglandin J(2). J Biol Chem. 2001;276:34082–34088. doi: 10.1074/jbc.M102472200. [DOI] [PubMed] [Google Scholar]
- Hortelano S, Traves PG, Zeini M, Alvarez AM, Bosca L. Sustained nitric oxide delivery delays nitric oxide-dependent apoptosis in macrophages: contribution to the physiological function of activated macrophages. J Immunol. 2003;171:6059–6064. doi: 10.4049/jimmunol.171.11.6059. [DOI] [PubMed] [Google Scholar]
- Cuenca J, Goren N, Martin-Sanz P, Bosca L. Infiltration of inflammatory cells plays an important role in matrix metalloproteinase expression and activation in the heart during sepsis. Am J Pathol. 2006;169:1567–1576. doi: 10.2353/ajpath.2006.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, O’Kirwan F, Khan N, Hannestad J, Wu KH, Elashoff D, Lawson G, Gold PW, McCann SM, Licinio J. Identification, characterization, and gene expression profiling of endotoxin-induced myocarditis. Proc Natl Acad Sci USA. 2003;100:14241–14246. doi: 10.1073/pnas.2336220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EJ, Lenzo JC, Sivamoorthy S, Mansfield JP, Cull VS, James CM. Type I IFN-β gene therapy suppresses cardiac CD8+ T-cell infiltration during autoimmune myocarditis. Immunol Cell Biol. 2004;82:119–126. doi: 10.1046/j.0818-9641.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- Tavener SA, Kubes P. Cellular and molecular mechanisms underlying LPS-associated myocyte impairment. Am J Physiol. 2006;290:H800–H806. doi: 10.1152/ajpheart.00701.2005. [DOI] [PubMed] [Google Scholar]
- Baumgarten G, Knuefermann P, Schuhmacher G, Vervolgyi V, von Rappard J, Dreiner U, Fink K, Djoufack C, Hoeft A, Grohe C, Knowlton AA, Meyer R. Toll-like receptor 4, nitric oxide, and myocardial depression in endotoxemia. Shock. 2006;25:43–49. doi: 10.1097/01.shk.0000196498.57306.a6. [DOI] [PubMed] [Google Scholar]
- Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res. 2004;95:700–707. doi: 10.1161/01.RES.0000144175.70140.8c. [DOI] [PubMed] [Google Scholar]
- Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon γ and bacterial lipopolysaccharide. J Exp Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Guerra MJM, Castrillo A, Martin-Sanz P, Bosca L. Negative regulation by protein tyrosine phosphatase of IFN-γ-dependent expression of inducible nitric oxide synthase. J Immunol. 1999;162:6776–6783. [PubMed] [Google Scholar]
- Pimentel-Muiños FX, Mazana J, Fresno M. Biphasic control of nuclear factor-κB activation by the T cell receptor complex: role of tumor necrosis factor α. Eur J Immunol. 1995;25:179–186. doi: 10.1002/eji.1830250130. [DOI] [PubMed] [Google Scholar]
- Sanjabi S, Williams KJ, Saccani S, Zhou L, Hoffmann A, Ghosh G, Gerondakis S, Natoli G, Smale ST. A c-Rel subdomain responsible for enhanced DNA-binding affinity and selective gene activation. Genes Dev. 2005;19:2138–2151. doi: 10.1101/gad.1329805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich R, Scherrer-Crosbie M, Bloch KD, Ichinose F, Nakajima H, Picard MH, Zapol WM, Quezado ZM. Congenital deficiency of nitric oxide synthase 2 protects against endotoxin-induced myocardial dysfunction in mice. Circulation. 2000;102:1440–1446. doi: 10.1161/01.cir.102.12.1440. [DOI] [PubMed] [Google Scholar]
- Petersen CA, Krumholz KA, Carmen J, Sinai AP, Burleigh BA. Trypanosoma cruzi infection and nuclear factor κB activation prevent apoptosis in cardiac cells. Infect Immun. 2006;74:1580–1587. doi: 10.1128/IAI.74.3.1580-1587.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CA, Krumholz KA, Burleigh BA. Toll-like receptor 2 regulates interleukin-1β-dependent cardiomyocyte hypertrophy triggered by Trypanosoma cruzi. Infect Immun. 2005;73:6974–6980. doi: 10.1128/IAI.73.10.6974-6980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi Y, Chan TO, Brown MA, Zhang J, DeGeorge BR, Jr, Funakoshi H, Gibson G, McTiernan CF, Kubota T, Jones WK, Feldman AM. Cardioprotection afforded by NF-κB ablation is associated with activation of Akt in mice overexpressing TNF-α. Am J Physiol. 2006;290:H590–H598. doi: 10.1152/ajpheart.00379.2005. [DOI] [PubMed] [Google Scholar]
- Planavila A, Rodriguez-Calvo R, de Arriba AF, Sanchez RM, Laguna JC, Merlos M, Vazquez-Carrera M. Inhibition of cardiac hypertrophy by triflusal (4-trifluoromethyl derivative of salicylate) and its active metabolite. Mol Pharmacol. 2006;69:1174–1181. doi: 10.1124/mol.105.016345. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhao H, Graveline AR, Buys ES, Schmidt U, Bloch KD, Rosenzweig A, Chao W. MyD88 and NOS2 are essential for Toll-like receptor 4 (TLR4)-mediated survival effect in cardiomyocytes. Am J Physiol. 2006;291:H1900–H1909. doi: 10.1152/ajpheart.00112.2006. [DOI] [PubMed] [Google Scholar]
- Millán O, Ballesta S, Castrillo A, de la Oliva J, Traves PG, Rojas JM, Bosca L. H-Ras-specific activation of NF-κB protects against stimulus-dependent apoptosis. Oncogene. 2003;22:477–483. doi: 10.1038/sj.onc.1206179. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Baetz D, Regula KM, Ens K, Shaw J, Kothari S, Yurkova N, Kirshenbaum LA. Nuclear factor-κB-mediated cell survival involves transcriptional silencing of the mitochondrial death gene BNIP3 in ventricular myocytes. Circulation. 2005;112:3777–3785. doi: 10.1161/CIRCULATIONAHA.105.573899. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide stimulates nitric oxide synthase-2 expression in murine skeletal muscle and C(2)C(12) myoblasts via Toll-like receptor-4 and c-Jun NH2-terminal kinase pathways. Am J Physiol. 2004;287:C1605–C1615. doi: 10.1152/ajpcell.00010.2004. [DOI] [PubMed] [Google Scholar]