Abstract
Trichloroethene (TCE) is an industrial degreasing solvent and widespread environmental contaminant. Exposure to TCE is associated with autoimmunity. The mode of action of TCE is via its oxidative metabolism, and most likely, immunotoxicity is mediated via haptenization of macromolecules and subsequent induction of immune responses. To better understand the role of protein haptenization through TCE metabolism, we immunized MRL +/+ mice with albumin adducts of various TCE reactive intermediates. Serum immunoglobulins and cytokine levels were measured to determine immune responses against haptenized albumin. We found antigen-specific IgG responses of the IgG subtypes IgG1,IgG2a, and IgG2b, with IgG1 predominating. Serum levels of G-CSF were increased in immunized mice, suggesting macrophage activation. Liver histology revealed lymphocyte infiltration in the lobules and the portal area following immunization with formyl-albumin. Our findings suggest that proteins haptenized by metabolites of TCE may act as neo-antigens that can induce humoral immune responses and T cell-mediated hepatitis.
Keywords: Trichloroethene (TCE), Albumin adducts, Autoimmunity, Autoimmune hepatitis
Introduction
Trichloroethene (trichloroethylene, TCE) is an occupational and ubiquitous environmental contaminant. TCE is also an indoor contaminant, because it is used in various household products. More than 50% of hazardous waste disposal sites are contaminated with TCE (Fay and Mumtaz, 1996), and the chemical commonly leaches into ground water. It is estimated that 34% of U.S. drinking water supplies are contaminated with TCE (ATSDR, 1997). Therefore, human exposure to TCE occurs through inhalation and contaminated drinking water. Studies have also demonstrated dermal TCE exposure and uptake (Giver et al., 2001).
Increased frequency of systemic lupus erythematosus (SLE) and other immunological disorders have been reported in populations that are chronically exposed to environmental chemicals, including TCE, through the consumption of contaminated water (Byers et al., 1988; Kilburn and Warshaw, 1992). Occupational exposure to TCE is also associated with immunological disorders (Reinl, 1957; Saihan et al., 1978; Phoon et al., 1984; Landrigan et al., 1987; Lockey et al., 1987; Flindt-Hansen and Isager, 1987; Brasington and Thorpe-Swenson, 1991; Waller et al., 1994; Goon et al., 2001).
Earlier reports from this laboratory support the hypothesis that TCE induces and exacerbates autoimmunity. We have shown that TCE and its metabolite dichloroacetyl chloride are immunogenic in MRL +/+ mice (Khan et al., 1995, 1997; Cai et al., 2006), which may occur through protein adduction. Several reports documented covalent modification of proteins by TCE metabolites in rodents (Bolt and Filser, 1977; Stott et al., 1982; Mazzullo et al., 1992). For example, in MRL +/+ mice given simultaneously TCE and diallyl sulfide, an inhibitor of the P450 2E1 enzyme known to metabolize TCE, the concentration of haptenized proteins and the degree and prevalence of autoimmune responses is markedly decreased in comparison to mice treated with TCE alone (Griffin et al., 2000). This finding indicates the importance of TCE metabolism in inducing autoimmune responses. Oxidation of TCE to trichloroethene oxide (TCEO) mediated by P450 2E1 serves as an intermediate in the formation of metabolites with the potential to haptenize proteins (Uehleke and Poplawski-Tabarelli, 1977; Miller and Guengerich, 1983).
A detailed work from the Guengerich laboratory showed that the hydrolysis of TCEO results in reactive intermediates, which have the potential to acylate proteins (Cai and Guengerich, 1999, 2000). The hydrolysis intermediate oxyacetyl chloride is capable of forming formyl protein adducts. TCEO can also rearrange to dichloroacetyl chloride (DCAC), which can acylate proteins. Formyl and dichloroacyl adducts are formed during the reaction of bovine serum albumin with TCEO, with formyl adducts surpassing dichloroacyl adducts by 20 to 50-fold (Cai and Guegerich, 2000).
To test the hypothesis that protein adducts of reactive intermediates of TCE are immunogenic and contribute to the induction of autoimmune responses, we generated TCEO, formyl, and dichloroacyl adducts of albumin. Following immunization of MRL +/+ mice with these haptenized proteins, we measured humoral responses and serum cytokine levels, and analyzed liver pathology. Our results indicate induction of autoimmunity in MRL +/+ mice by haptenized albumin, and suggest that formyl albumin may also contribute to autoimmune hepatitis.
Material and methods
Chemicals and reagents
Mouse albumin (Sigma, St. Louis, MO) was used as a carrier protein for the preparation of haptenized protein conjugates. Freund’s adjuvant was from Sigma. S-ethyl trifluorothioacetate (purity ~99%) was purchased from Aldrich (St. Louis, MO). Trichloroethene oxide, S-ethyldichlorothioacetate, and acetic formic anhydride were synthesized and characterized by nuclear magnetic resonance (NMR) spectroscopy using a Varian Mercury Plus 300 MHz spectrometer with tetramethylsilane as an internal standard. Mass spectra were obtained using a DESTR matrix assisted laser desorption ionization (MALDI) –time of flight (TOF) mass spectrometer with 3,5-dimethoxy-4-hydroxycinnamic acid as a matrix.
Preparation of haptenized protein conjugates
An excess of each hapten was added to 15 mg of mouse albumin in 3 ml NaHCO3 (pH 8.5) over a period of 30 min while stirring at room temperature. Stirring was continued for one hour. A sample of the solution (50μl) was removed to measure protein concentration (Bradford, 1976) and free amino groups (Habeeb, 1996). The remaining solution was dialyzed against phosphate buffer (0.1 M, pH 7.2) and lyophilized. Molecular weights and the number of modified amino groups are summarized in Table 1.
Table 1.
Molecular weights and numbers of modified amino acids in albumin adducts
| Protein | Molecular weight (MALDI-TOF) | Number of modified amino groups (TNBS assay) |
|---|---|---|
| albumin | 65,935 | 0 |
| TCEO-albumin | 66,997 | 34 |
| dichloroacyl-albumin | 69,149 | 41 |
| formyl-albumin | 67,250 | 48 |
| trifluoroacyl-albumin | 67,216 | 40 |
Animals and immunization
Six-week-old female MRL +/+ mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were acclimatized in UTMB’s humidity- and temperature-controlled animal care facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, for one week. At the start of the experiments, the average weight was ~28 g. Lab chow and drinking water were provided ad libitum. All experiments were performed in accordance to the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch. Mice were randomly divided into groups (5 mice each) representing groups treated with PBS, albumin, or dichloroacyl, formyl, trifluoracetyl or TCEO-adducted albumin. PBS-treated and albumin-immunized groups served as negative controls, and mice immunized with trifluoroacetyl-albumin represented a positive control (Kenna et al., 1993; Christen et al., 1994). Mice were immunized subcutaneously by injection of a total of 50 μg antigen in 200 μl of PBS and complete Freund’s adjuvant (CFA; 1:1, v/v) into three sites in the back. After two and four weeks, booster injections were given in the same manner except that incomplete Freund’s adjuvant was substituted for CFA. Mice were sacrificed nine days after the second booster immunization. Blood, liver and kidneys were collected. The serum was isolated and stored in small aliquots at −80°C untill further analysis.
Enzyme-linked immunosorbent assay (ELISA) for chemical-specific immunoglobulins
96-well plates (Costar, Cambridge, MA) were coated with 100 μl of antigen (5 μg/ml) in 0.05 M carbonate-bicarbonate solution (pH 9.6) for one hour at room temperature and washed with 50 mM Tris buffered saline (pH 8.0, 0.05% Tween 20). The residual binding was blocked with 50 mM Tris buffered saline (pH 8.0), containing 1% BSA for 30 min. For IgG1 measurements, 10% goat serum was added. The plates were washed again and incubated for two hours at room temperature with mouse serum (1:100 or 1:1000 for IgG1) diluted with blocking solution containing 0.05% Tween 20. After washing, the plates were incubated for one hour with goat anti-mouse HRP-conjugated antibody (IgG, IgG1, IgG2a, IgG2b, or IgM from Bethyl, Montgomery, TX) at a dilution of 1:5000 in blocking solution containing 0.05% Tween 20. After a final wash, the wells were developed with 100 μl of TMB substrate (Sigma) for 5 min. Then, 100 μl of 1 M H2SO4 was added to stop the reaction. The absorbance was read at 450 nm using a microplate reader (Bio-Rad, Hercules, CA).
Serum cytokines
The cytokines IL-1β, IL-6, GM-CSF, TNF-α G-CSF, IL-10 and the chemokine KC were measured using protein multiplex immunoassay kits as per the manufacturer’s instructions (Invitrogen, Carlsbad, CA). The fluorescence was measured using a Luminex 100 instrument (Bio-Rad).
Alanine aminotransferase and aspartate aminotransferase
Alanine aminotransferase and aspartate aminotransterase were measured using colorimetric kits from Biotron Diagnostics (Hemet, CA) and a modified protocol for small amounts of serum (12.5 μl). Absorbance was read at 540 nm.
Histopathology
Liver and kidney were fixed in 10% neutral buffered formalin. Tissue sections were stained with hematoxylin and eosin (H & E) for morphological evaluations.
Statistical analysis
The data are presented as mean ± standard error of the mean (SEM) of five samples. For the determination of statistical significance, the data were subjected to the analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc test. P-values ≤ 0.05 were considered to be statistically significant.
Results
Characterization of albumin adducts
Increases in molecular weight as determined by MALDI-TOF mass spectral analysis indicated successful adduction (Table 1). A TNBS assay was used to determine the extent of adduction, which varied from 34 to 48 amino groups in the adducts (Table 1).
Serum antibodies
To evaluate whether haptenization of albumin by TCE metabolites induced specific antibody responses, we measured albumin-specific serum IgG in PBS-injected mice or mice immunized with albumin or dichloroacyl, formyl, trifluoracetyl or TCEO-albumin adducts. Albumin-specific total serum IgG was not increased in albumin-immunized mice (Fig. 1). However, in mice immunized with any of the albumin adducts, albumin-specific total serum IgG was significantly increased (Fig. 1). Thus, IgG from mice immunized with haptenized albumin cross-reacted with un-adducted albumin. Antibodies raised against any of the adducted albumin preparations also cross-reacted with all other albumin adducts (Table 2). The cross-reactivity to haptenized albumin was always much stronger than that to un-adducted albumin (Table 2).
Fig. 1.
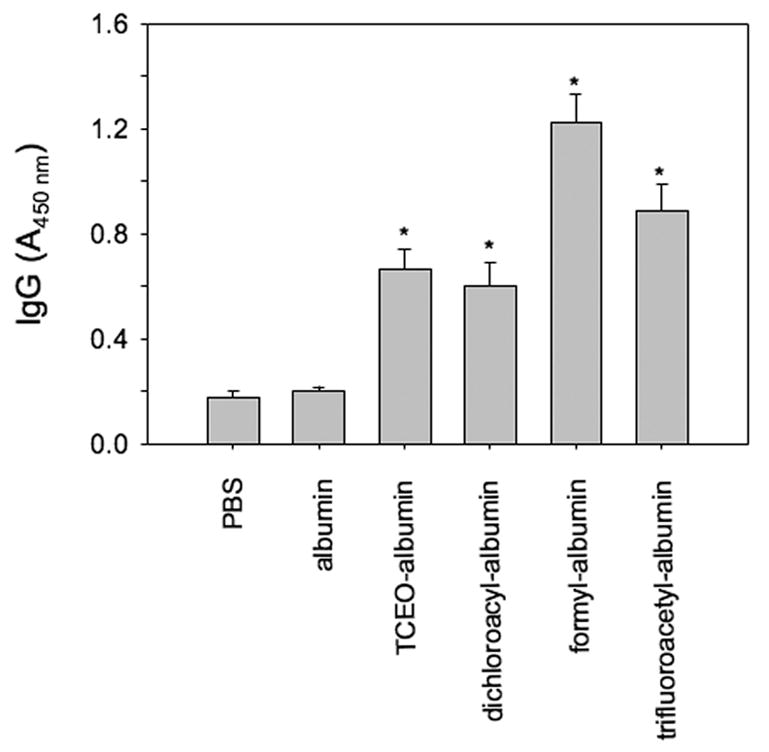
Serum concentration of albumin-specific IgG in immunized MRL +/+ mice. Control mice were injected with a mixture of PBS and adjuvant or un-adducted albumin. The immunogen (antigen used in the immunization) is indicated on the abscissa. Reactivity of immune sera (1:100 dilution) against un-adducted albumin was measured by ELISA. The data are expressed as absorbance at 450 nm. Bars represent the mean ± SEM of five mice per group tested individually. * P ≤ 0.05 compared to groups immunized with PBS or albumin.
Table 2.
Reactivity of IgG in sera from MRL +/+ mice immunized with albumin adducts
| Antiserum against | Antigen used in ELISA | ||||
|---|---|---|---|---|---|
| albumin | TCEO-albumin | dichloroacyl-albumin | formyl-albumin | trifluoroacyl-albumin | |
| TCEO-albumin | 0.27 ± 0.07* | 1.83 ± 0.16 | 1.07 ± 0.12* | 1.39 ± 0.21* | 0.99 ± 0.14* |
| dichloroacyl-albumin | 0.44 ± 0.11* | 2.04 ± 0.05 | 1.92 ± 0.04 | 1.61 ± 0.60* | 1.94 ± 0.08 |
| formyl-albumin | 0.81 ± 0.15* | 1.77 ± 0.08* | 1.45 ± 0.09* | 2.10 ± 0.04 | 1.55 ± 0.08* |
| trifluoroacyl-albumin | 0.66 ± 0.14* | 1.93 ± 0.03 | 1.81 ± 0.03 | 1.96 ± 0.05 | 1.86 ± 0.05 |
P ≤ 0.05 when compared to the immunogen (n = 5).
To determine whether isotype switching following immunization with haptenized albumin preferentially induced certain IgG subtypes, we measured serum levels of specific IgG1, IgG2a, and IgG2b (Fig. 2). For measurements of IgG1 titers, we had to dilute sera 1:1000, whereas IgG2a and IgG2b subtypes could be measured at sera dilutions of 1:100. As we observed in the case of total IgG (Table 2), IgG1 from sera of mice immunized with haptenized albumin was specific for the immunogen, but also showed cross-reactivity to the other albumin adducts, and to a lesser degree to un-adducted albumin (Fig. 2 and data not shown). Hapten-specific titers of IgG2a and IgG2b were lower than IgG1 titers, but still detectable (Fig. 2).
Fig. 2.
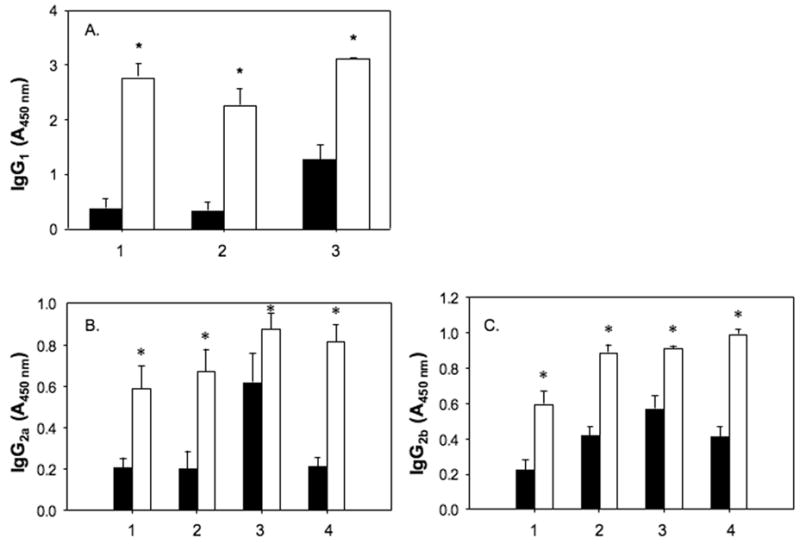
Serum concentration of immunogen-specific IgG subtypes and cross-reactivity with un-adducted albumin. Sera from mice immunized with (1) TCEO- , (2) dichloroacyl-, (3) formyl-, and (4) trifluoroacyl-albumin were tested by ELISA for IgG subtypes reactive against un-adducted albumin (black bars) and immunogen (open bars). A. Serum concentration of IgG1 specific for albumin or the haptenized albumin used as immunogen. Sera were diluted (1:1000). B. Serum concentration of IgG2a. Sera were diluted (1:100). C. Serum concentration of IgG2b. Sera were diluted (1:100). Bars represent the mean ± SEM of five mice per group tested individually. * P ≤ 0.05 compared to albumin as an antigen.
Serum cytokines
Using a multiplex cytokine immunoassay for the simultaneous detection of seven cytokines, detectable levels could only be measured for tumor necrosis factor (TNF)-α and granulocyte colony-stimulating factor (G-CSF). Serum levels of TNF-α were suppressed in mice immunized with haptenized albumin in comparison to albumin-injected mice (58% suppression for TCEO-albumin; 53% for dichloroacyl-albumin; 49% for trifluoroacetyl-albumin; and 29% for formyl-albumin). In all cases except for formyl-albumin, the suppression was statistically significant at a P-value of 0.05. Serum levels of G-CSF were only statistically significantly different from albumin-injected mice in mice immunized with trifluoroacetyl-albumin (63% increase). In all mice immunized with haptenized albumin, but not in those immunized with un-adducted albumin, G-CSF serum levels were increased compared to PBS-injected mice (77 – 126%).
Liver pathology
To determine whether immunization with haptenized albumin induced liver injury, we measured serum levels of alanine aminotransferase and aspartate aminotransferase. No significant change in the serum level of these liver enzymes was found in immunized mice (data not shown).
We also investigated pathology of the liver using histology. In mice immunized with formyl-albumin, lymphocyte infiltration was apparent in the liver lobules and the portal area (in 4 out of 5 mice) (Fig. 3). In mice immunized with trifluoroacetyl-albumin, we observed lipid deposits in hepatocytes. These lipid deposits compressed and displaced the nucleus to the periphery (data not shown). No pathological changes were observed in the other groups (data not shown). We could also not detect pathological changes in the kidneys of any of the immunized mice (data not shown).
Fig. 3.
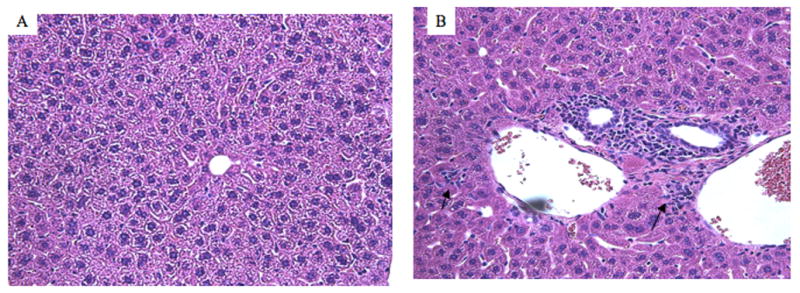
Liver histopathology of mice immunized with albumin (A) or formyl-albumin (B). Tissue sections were stained with H & E. Magnification 200x. The arrows in B indicate lymphocyte infiltration.
Discussion
It has long been recognized that environmental chemicals alter immune responses. Environmental effects on immune responses can lead to immunosuppression (Koller, 1980; Hatch et al., 1985; Moszczynski, 1997) or immunostimulation, resulting in autoimmune diseases. Chloroethenes have been implicated in inducing or accelerating autoimmunity. We and others have reported on the immunogenicity of TCE (Khan et al., 1995; Gilbert et al., 1999). We hypothesize that TCE promotes autoimmunity through a breakdown of self-tolerance by covalent haptenization of self-proteins with TCE metabolites. These newly formed protein adducts would act as neo-antigens, thus circumventing the naturally established tolerance to self-proteins.. Evidence supporting this hypothesis come from observations that T cells can recognize lipid peroxidation products of self-proteins (Wuttge et al., 1999). Further, covalent modifications of pyruvate dehydrogenase have produced a breakdown of immune tolerance in the mouse (Palmer et al., 2004)
Previously, the covalent binding of TCE metabolites to cytochrome P450 2E1 has been reported (Bolt and Filser, 1977; Stott et al., 1982; Mazzullo et al., 1992; Griffin et al., 2000). Metabolic conversion of TCE by P450 2E1 leads to TCEO, which in turn is biotransformed into DCAC and glyoxyl chloride (Uehleke and Poplawski-Tabarelli, 1977; Miller and Guengerich, 1983; Cai and Guengerich, 1999; 2000). Glyoxyl chloride forms formyl adducts with proteins, whereas DCAC forms dichloroacyl adducts. Formyl adducts are 20 to 50-fold more prevalent than dichloroacyl adducts in vitro (Cai and Guengerich, 2000). Previously, dichloroacyl adducts have been shown to be associated with pulmonary cytotoxicity (Forkert et al., 2006).
To test our hypothesis that protein adducts with TCE metabolites are immunogenic and can act as neo-antigens, we evaluated the antigenicity of TCEO, dichloroacyl, and formyl adducts of albumin. As a negative control, we used un-adducted albumin. We also prepared trifluoroacetyl adducts of albumin for comparison, because trifluoroacetyl-adducted proteins have previously been implicated in autoimmune halothane hepatitis (Kenna et al., 1993; Christen et al., 1994). Homologous albumin was used as a carrier protein to eliminate the potentially confounding effects of heterologous proteins. For example, homologous albumin adducted with acetaldehyde is not immunogenic, whereas the same adducts of heterologous albumin induce immune responses (Yokoyama et al., 1993; Shimada et al., 2002). Similarly, we observed that albumin adducts with formaldehyde also yield differential immune responses depending on homologous or heterologous origins of albumin (Li et al., 2006).
To ensure that immune responses were not due to effects of the adjuvant used in immunization, groups of mice were injected with a mixture of PBS and adjuvant as an additional control. Our results showed minimal responses in mice injected with either PBS or un-adducted albumin, confirming that autoimmunity cannot be due to responses to adjuvant or unaltered albumin. However, mice immunized with any of the adducted albumin preparations generated albumin-specific IgG (Fig. 1), indicating a robust immune response with isotype class switching. Analysis of the IgG subtypes specific for haptenized albumin revealed a preferential isotype switch to IgG1 (Fig. 2). This observation indicates that immune responses against haptenized albumin were regulated by T helper type 2 cells (Finkelman et al., 1990; Cai et al., 2006). This finding is consistent with our previous report on antibody responses in MRL +/+ mice exposed to DCAC via intraperitoneal injection and subsequent in vivo haptenization of self-proteins (Cai et al., 2006). In humans, systemic autoimmune diseases such as progressive systemic sclerosis and systemic lupus erythematosus are also associated with T helper type 2 responses (Singh et al., 1999). In contrast to our results with intraperitoneally administered DCAC, we also detected IgG2a specific for haptenized albumin, indicating that inflammatory responses occurred. This conclusion is based on the fact that interferon-γ promotes IgG2a production (Finkelman et al., 1988; Bossie and Vitetta, 1991).
Additional evidence for inflammatory responses comes from the observation that in MRL +/+ mice immunized with haptenized albumin, serum concentrations of G-CSF were increased in comparison to PBS-injected mice. This finding indicates activation of macrophages and acute inflammatory responses (Feghali and Wright, 1997). Cytokines exert their effects mainly in an autocrine and paracrine mode at the site of synthesis. Thus, it is not surprising that we could only detect two of the seven cytokines measured (TNF-α and G-CSF). The increased levels of G-CSF measured in the serum of mice nine days after the last booster immunization with haptenized albumin serum levels indicate therefore a robust immune response.
An interesting observation was that the antibody response against albumin was highest in the sera of MRL +/+ mice treated with formyl-albumin. This was accompanied by lymphocyte infiltration into the liver (autoimmune hepatitis), which occurred only in formyl-albumin treated mice. However, liver injury did not progress to affect ALT or AST serum levels. In unrelated experiments, we also observed lymphocyte infiltration into the liver in MRL +/+ mice chronically treated with TCE (unpublished results) as reported earlier (Griffin et al., 2000a). Therefore, exposure to TCE may lead to in vivo formyl haptenization of self-proteins, suggesting a potential role for formyl adducts in TCE-mediated autoimmune hepatitis.
In conclusion, our results demonstrate immunogenicity of albumin haptenized with TCE metabolites in MRL +/+ mice, suggesting that neo-antigens may be formed by self-proteins adducted in this manner. Autoimmune diseases may then occur due to the lack of T cell tolerance to haptenized self-proteins.
Acknowledgments
This publication was made possible by grant ES11584 from the National Institute of Environment Health Sciences (NIEHS), and its content are solely the responsibility of the authors and do not necessarily represent the views of the NIH or NIEHS. We gratefully acknowledge the Organic Synthesis Core at UTMB for the preparation of protein adducts and the Biomolecular Resource Facility at UTMB for their characterization by MALDI-TOF. Core facilty support by NIEHS center grant (P30ES06676) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR. Toxicological profile for trichloroethylene (update) 1997. p. 191. [Google Scholar]
- Bossie A, Vitetta ES. IFN-gamma enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: implications for the role of IFN-gamma in class switching. Cell Immunol. 1991;135:95–104. doi: 10.1016/0008-8749(91)90257-c. [DOI] [PubMed] [Google Scholar]
- Bolt HM, Filser JG. Irreversible binding of chlorinated ethylenes to macromolecules. Environ Health Persp. 1977;21:107–112. doi: 10.1289/ehp.7721107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brasington RD, Jr, Thorpe-Swenson AJ. Systemic sclerosis associated with cutaneous exposure to solvents: case report and review of the literature. Arthritis Rheum. 1991;34:631–363. doi: 10.1002/art.1780340516. [DOI] [PubMed] [Google Scholar]
- Byers VS, Levin AS, Ozonoff DM, Baldwin RW. Association between clinical symptoms and lymphocyte abnormalities in a population with chronic exposure to industrial solvent contaminated domestic water supply and a high incidence of leukemia. Cancer Immunol Immunother. 1988;27:77–81. doi: 10.1007/BF00205762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Guengerich FP. Mechanism of aqueous decomposition of trichloroethylene oxide. J Am Chem Soc. 1999;121:11656–11663. [Google Scholar]
- Cai H, Guengerich FP. Acylation of protein lysines by trichloroethylene oxide. Chem Res Toxicol. 2000;13:327–335. doi: 10.1021/tx000003p. [DOI] [PubMed] [Google Scholar]
- Cai P, König R, Khan MF, Qiu S, Kaphalia BS, Ansari GAS. Autoimmune response in MRL +/+ mice following treatment with dichloroacetyl chloride or dichloroacetic anhydride. Toxicol Appl Pharmacol. 2006;21:248–255. doi: 10.1016/j.taap.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Christen U, Quinn J, Yeaman SJ, Kenna JA, Clark JB, Gandolfi AJ, Gut J. Identification of the dihydrolipoamide acetyltransferase subunit of the human pyruvate dehydrogenase complex as an autoantigen in halothane hepatitis: Molecular mimicry of trifluoroacetyllysine by lipoic acid. Eur J Biochem. 1994;233:1035–1048. doi: 10.1111/j.1432-1033.1994.tb19082.x. [DOI] [PubMed] [Google Scholar]
- Fay RM, Mumtaz MM. Development of a priority list of chemical mixtures occurring at 1188 hazardous waste sites, using the HazDat database. Food Chem Toxicol. 1996;34:1163–1165. doi: 10.1016/s0278-6915(97)00090-2. [DOI] [PubMed] [Google Scholar]
- Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–1027. [PubMed] [Google Scholar]
- Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Flindt-Hansen H, Isager H. Scleroderma after occupational exposure to trichloroethylene and trichloroethane. Acta Dermatol Venereol. 1987;67:263–264. [PubMed] [Google Scholar]
- Forkert PG, Millen B, Lash LH, Putt DA, Ghanayem BI. Pulmonary bronchiolar cytotoxity and formation of dichloroacetyl lysine protein adducts in mice treated with trichloroethylene. J Pharmacol Exp Ther. 2006;316:520–529. doi: 10.1124/jpet.105.093062. [DOI] [PubMed] [Google Scholar]
- Gilbert KM, Griffin JM, Pumford NP. Trichloroethylene activates CD4+ T cells: potential role in an autoimmune response. Drug Metab Rev. 1999;31:901–916. doi: 10.1081/dmr-100101945. [DOI] [PubMed] [Google Scholar]
- Giver CR, Wong R, Moore DH, Pallavicini MG. Dermal benzene and trichloroethylene induce aneuploidy in immature hematopoietic subpopulations in vivo. Environ Mol Mutagen. 2001;37:185–194. doi: 10.1002/em.1027. [DOI] [PubMed] [Google Scholar]
- Goon AT, Lee LT, Tay YK, Yosipovitch G, Ng SK, Giam YC. A case of trichloroetylene hypersensitivity syndrome. Arch Dermatol. 2001;137:274–276. [PubMed] [Google Scholar]
- Griffin JM, Gilbert KM, Pumford NR. Inhibition of CYP 2E1 reverse CD4+ T cell alterations in trichloroethylene-treated MRL +/+ mice. Toxicol Sci. 2000;54:384–389. doi: 10.1093/toxsci/54.2.384. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Gilbert KM, Lamps LW, Pumford NR. CD4(+) T-cell activation and induction and induction of autoimmune hepatitis following trichloroethylene treatment in MRL +/+ mice. Toxicol Sci. 2000a;57:345–352. doi: 10.1093/toxsci/57.2.345. [DOI] [PubMed] [Google Scholar]
- Habeeb ARSA. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966;14:328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Hatch GE, Boykin E, Graham JA, Lewtas J, Pott F, Loud K, Mumford JL. Inhalable particles and pulmonary host defense: in vivo and in vitro effects of ambient air and combustion particles. Environ Res. 1985;36:67–80. doi: 10.1016/0013-9351(85)90008-8. [DOI] [PubMed] [Google Scholar]
- Kenna JG, Knight LT, Van Pet FNAM. Immunity to halothane metabolite-modified proteins in halothane hepatitis. Ann New York Acad Sci. 1993;85:646–661. doi: 10.1111/j.1749-6632.1993.tb35930.x. [DOI] [PubMed] [Google Scholar]
- Khan MF, Kaphalia BS, Prabhakar BS, Kanz MF, Ansari GAS. Trichloroethene-induced autoimmune response in female MRL +/+ mice. Toxicol Appl Pharmacol. 1995;134:155–160. doi: 10.1006/taap.1995.1179. [DOI] [PubMed] [Google Scholar]
- Khan MF, Kaphalia BS, Ansari GAS. Time-dependent autoimmune response of dichloroacetyl chloride in female MRL +/+ mice. Immunopharmacol Immunotoxicol. 1997;19:265–277. doi: 10.3109/08923979709007662. [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Warshaw RH. Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ Res. 1992;57:1–9. doi: 10.1016/s0013-9351(05)80014-3. [DOI] [PubMed] [Google Scholar]
- Koller LD. Immunotoxicology of heavy metals. Int J Immunopharmacol. 1980;2:269–279. doi: 10.1016/0192-0561(80)90027-2. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Stein GF, Kominsky JR, Ruhe RL, Watanabe AS. Common source community and industrial exposure to trichloroethylene. Arch Environ Health. 1987;42:327–332. doi: 10.1080/00039896.1987.9934354. [DOI] [PubMed] [Google Scholar]
- Li H, Wang J, König R, Ansari GAS, Khan MF. Formaldehyde-protein conjugate specific antibodies in rats exposed to formaldehyde. J Toxicol Environ Health. 2006 doi: 10.1080/15287390601172155. (in press) [DOI] [PubMed] [Google Scholar]
- Lockey JE, Kelly CR, Cannon GW, Colby TV, Aldrich V, Livingston GK. Progressive systemic sclerosis associated with exposure to trichloroethylene. J Occup Med. 1987;29:493–496. [PubMed] [Google Scholar]
- Mazzullo M, Bartoli S, Bonora B, Colacci A, Lattanzi G, Niero A, Silingardi P, Grilli S. In vivo and in vitro interaction of trichloroethylene with macromolecules from various organs of rat and mouse. Res Commun Chem Pathol Pharmacol. 1992;76:192–208. [PubMed] [Google Scholar]
- Miller RF, Guengerich FP. Metabolism of trichloroethylene in isolated hepatocytes, microsomes, and reconstituted enzyme systems containing cytochrome P-450. Cancer Res. 1983;43:1145–1152. [PubMed] [Google Scholar]
- Moszczynski P. Mercury compounds and the immune system: a review. Int J Occup Med Environ Health. 1997;10:247–258. [PubMed] [Google Scholar]
- Palmer JM, Robe AJ, Burt Ad, Kirby JA, Jones DEJ. Covalent modification as a mechanism for the breakdown of immune tolerance to pyruvate dehydrogenase complex in the mouse. Hepatology. 2004;39:1583–1592. doi: 10.1002/hep.20248. [DOI] [PubMed] [Google Scholar]
- Phoon WH, Chan MOY, Rajan VS, Tan KJ, Thirumoorthy T, Goh CL. Steven-Johnson syndrome associated with occupational exposure to trichloroethylene. Contact Dermatitis. 1984;10:270–276. doi: 10.1111/j.1600-0536.1984.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Reinl W. Scleroderma caused by trichloroethylene? Bull Hyg. 1957;32:678–679. [PubMed] [Google Scholar]
- Saihan EM, Burton JL, Heaton KW. A new syndrome with pigmentation, scleroderma, gynecomastia, Raynaud's phenomenon and peripheral neuropathy. Br J Dermatol. 1978;99:437–440. doi: 10.1111/j.1365-2133.1978.tb06184.x. [DOI] [PubMed] [Google Scholar]
- Shimada S, Yamauchi M, Takamatsu M, Uetake S, Ohata M, Saito S. Experimental studies on the relationship between immune responses and liver damage induced by ethanol after immunization with homologous acetaldehyde adducts. Alcohol Clin Exp Res. 2002;26:86S–89S. doi: 10.1097/01.ALC.0000026983.26366.D0. [DOI] [PubMed] [Google Scholar]
- Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999;20:147–161. doi: 10.1007/BF02786470. [DOI] [PubMed] [Google Scholar]
- Stott WT, Quast JF, Watanabe PG. The pharmacokinetics and macromolecular interactions of trichloroethylene in mice and rats. Toxicol Appl Pharmacol. 1982;62:137–151. doi: 10.1016/0041-008x(82)90110-7. [DOI] [PubMed] [Google Scholar]
- Uehleke H, Poplawski-Tabarelli S. Irreversible binding of 14C-labeled trichloroethylene to mice liver constituents in vivo and in vitro. Arch Toxicol. 1977;37:289–294. doi: 10.1007/BF00330820. [DOI] [PubMed] [Google Scholar]
- Waller PA, Daniel C, Cupps T, Metcalf JS, Richard MS, Leroy EC. Fascitis (not scleroderma) following prolonged exposure to organic solvents (trichloroethylene) J Rheumatol. 1994;21:1567–1570. [PubMed] [Google Scholar]
- Wuttge DM, Bruzelius M, Stemme S. T-cell recognition of lipid peroxidation products breaks tolerance to self proteins. Immunology. 1999;98:273–279. doi: 10.1046/j.1365-2567.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Ishii H, Nagata S, Kato S, Kamegaya K, Tsuchiya M. Experimental hepatitis induced by ethanol after immunization with acetaldehyde adducts. Hepatology. 1993;17:14–19. [PubMed] [Google Scholar]


