Abstract
The thylakoid membrane, located inside the chloroplast, requires proteins transported across it for plastid biogenesis and functional photosynthetic electron transport. The chloroplast Tat translocator found on thylakoids transports proteins from the plastid stroma to the thylakoid lumen. Previous studies have shown that the chloroplast Tat pathway is independent of NTP hydrolysis as an energy source and instead depends on the thylakoid transmembrane proton gradient to power protein translocation. Because of its localization on the same membrane as the proton motive force–dependent F0F1 ATPase, we believed that the chloroplast Tat pathway also made use of the thylakoid electric potential for transporting substrates. By adjusting the rate of photosynthetic proton pumping and by utilizing ionophores, we show that the chloroplast Tat pathway can also utilize the transmembrane electric potential for protein transport. Our findings indicate that the chloroplast Tat pathway is likely dependent on the total protonmotive force (PMF) as an energy source. As a protonmotive-dependent device, certain predictions can be made about structural features expected to be found in the Tat translocon, specifically, the presence of a proton well, a device in the membrane that converts electrical potential into chemical potential.
INTRODUCTION
The twin arginine protein translocation (Tat, or in chloroplasts, cpTat) pathway derives its name from a nearly invariant Arg-Arg motif found in the signal peptides of substrates for this pathway (1). The Tat pathway, originally discovered in plants (2–6) and subsequently found in prokaryotes and archaea (7), has a number of unusual properties. In both thylakoids and bacteria it transports fully folded proteins across the membrane in an NTP-independent manner (3,4,8–11). The Tat transport process has been shown in thylakoids to not compromise the ionic integrity of the bilayer (12). A recent investigation into the expenditure of Gibbs free energy for the transport of a cpTat substrate concluded that the energetic cost to transport the protein was greater than that to synthesize it (13). The mechanism underlying transport on the Tat pathway in both chloroplasts and bacteria is currently unknown.
A large body of work concerning the cpTat pathway has shown that transport is dependent solely on the thylakoid transmembrane pH gradient (ΔpH) as an energy source (3,4,14), although this has been disputed by two recent publications concerning cpTat transport in vivo (15–17). A long-standing unanswered question with significant mechanistic ramifications is whether a transmembrane electric potential (Δψ), which in addition to the ΔpH comprises the total protonmotive force (PMF), can contribute to the transport driving force. Previous attempts to demonstrate such an effect with the electrogenic ionophore valinomycin have not been successful (3,4). This is not necessarily surprising given the observation, seemingly not well known, that under some circumstances the Δψ in isolated thylakoids can develop in the presence of valinomycin under steady-state illumination (18,19).
It is thought that isolated thylakoids generally partition most of their steady-state PMF as a ΔpH with only a moderate contribution from the Δψ (20). To test the hypothesis that the Δψ could contribute to the energetic driving force for cpTat protein transport, we sought to establish conditions under which the membrane potential would constitute a larger proportion of the total PMF than previous experiments had allowed. We reasoned that if the ΔpH established by photosynthetic electron transport decreased without similarly affecting the membrane potential, then the remaining steady-state PMF would be carried increasingly by the Δψ. Under such conditions, provided that the Δψ contributed to the driving force for cpTat transport, the collapse of the Δψ by valinomycin would be expected to alter the rate of protein transport.
We show in this study that by manipulating the thylakoid PMF either by attenuating the rate of photosynthetic proton pumping or by the addition of ionophores, we can demonstrate a dependence on Δψ as a driving force for transport on the cpTat pathway. Our experiments not only have ramifications for the mechanism of cpTat protein transport but point to a resolution of the question of whether the energetics of this pathway is different in vivo and in vitro.
MATERIALS AND METHODS
Isolated thylakoids were prepared from 9- to 12-day-old peas (Pisum sativum var. Little Marvels) as described previously (13). Thylakoids were stored on ice in a buffer consisting of 50 mM K+-Tricine at pH 8.0, 330 mM sorbitol, and 5 mM MgCl2, which also served as the protein transport reaction buffer.
The cpTat substrate used in all experiments was maize [35S]iOE17, which was prepared by in vitro transcription and translation in rabbit reticulocyte lysate from a cDNA clone as described (13).
In the experiment of Fig. 1, samples contained 2 μl of in vitro translated iOE17, 20 μM methyl viologen as a terminal electron acceptor, thylakoids at 20 μg chlorophyll and, depending on the treatment, 1 μM valinomycin, 300 nM nigericin, or 1 μl of ethanol as a loading control; the remaining reaction volume was brought to 60 μl with reaction buffer. Protein translocation was initiated by placing the samples in heat-filtered white light (45 μE/m2·s) for 6 min, a period of time determined to be within the linear regime of protein transport (data not shown). Reactions were stopped by diluting the samples 10-fold with ice-cold reaction buffer and centrifuging in a microfuge for 5 min to pellet the thylakoids. Protein transport was determined by SDS-PAGE and subsequent phosphor screen autoradiography and is reported as percentage transport of substrate protein added.
FIGURE 1.
Valinomycin decreases the rate of cpTat transport at low values of ΔpH. (A) The import data of four ionophore treatments replicated nine times are shown, as well as triplicates of 10% substrate loading standards used for quantification purposes interspersed with a nonradioactive molecular weight marker (empty lanes). The average percentage transport of each treatment as well as the standard errors and t-tests are presented graphically (B). The two pairs of columns labeled Control and Nigericin show the percentage transport of iOE17 Tat substrate under high-light conditions in the absence and presence of 300 nM of nigericin. Light shaded bars are untreated reactions, and solid bars are reactions that contain 1 μM valinomycin.
Protein transport in the experiments of Figs. 2–4 was measured at a lower chlorophyll concentration and larger volume to permit simultaneous spectroscopic determination of thylakoid energetic parameters. For measurements of the transmembrane ΔpH by 9-aminoacridine (9-AA) fluorescence quenching (21), reactions contained thylakoids at 20 μg chlorophyll, 20 μM methyl viologen, 20 μM 9-AA (λex = 420, λem = 480), 2 μl of in vitro translated iOE17, and, depending on the treatment, 1 μM valinomycin and 1 μl of ethanol as a loading control. Nigericin was added to achieve concentrations between 0.2 nM and 6 nM in a 1 μl load to titrate the chemical potential. The total reaction volume was brought to 500 μl with reaction buffer. During the light titration experiments, the actinic beam was attenuated by neutral density filters. The ΔpH was calculated as previously discussed (13,21). The reaction took place in a stirred cuvette over which a mild air current was blown to cycle O2 into the reaction mixture. Reactions were stopped by diluting the samples twofold with ice-cold reaction buffer and immediately centrifuging in a microfuge for 5 min to pellet the thylakoids. The extent of protein transport was determined by SDS-PAGE and subsequent phosphor screen autoradiography.
FIGURE 2.
ECS of carotenoids indicates the electric potential in thylakoids. The ECS values presented are the deflection of carotenoid absorbance from a dark baseline to a steady-state level achieved during a 1-min actinic illumination period. (A) Effect of valinomycin when the ΔpH was lowered using increasing nigericin concentrations. (B) Effect of valinomycin when the ΔpH was lowered using decreasing actinic light intensities. In both panels the two paired columns represent the ECS in the absence (solid bars) or presence (light shaded bars) of 1 μM valinomycin. The standard error is based on three to four replicates of fourfold signal-averaged ECS measurements. (C–F) Selected paired traces of ECS measurements of which the steady state magnitude was used in creating the above graphs. Of the paired traces, in all cases, the steady-state electric potential–indicating ECS signal that developed without a rapid spike of electrochromism at the onset of illumination was the treatment containing 1 μM valinomycin. (C, D) The two paired ECS traces were each performed at an actinic intensity of 20 μE s−1·m−2, but (C) without nigericin and (D) with 1.5 nM nigericin. (E, F) The two paired ECS traces consist of experiments performed with actinic light intensities of 20 and 10 μE s−1·m−2, respectively.
FIGURE 3.
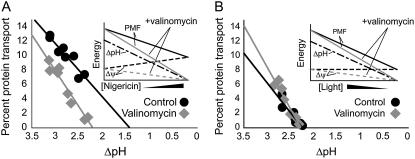
Protein transport as a function of ΔpH attenuated with nigericin (A) or actinic light intensity (B). Protein transport is plotted against ΔpH determined by 9-AA fluorescence quenching in the absence (solid circles) or presence of 1 μM valinomycin (light shaded diamonds). Insets represent models of the components of the PMF in a thylakoid during a ΔpH titration with either nigericin (A) or actinic intensity (B). Solid lines represent the total PMF, which is the sum of the ΔpH and the Δψ; dashed lines represent the Δψ; dot-dashed line represents the ΔpH; solid and light shaded lines represent titrations in the presence or absence of valinomycin, respectively.
FIGURE 4.
Protein transport during a 1-min reaction is affected by Δψ when the ΔpH is titrated with light. The attenuated thylakoid ΔpH was set by nigericin addition (A) or by low actinic light intensity (B). Averages and standard errors of six replicate treatments are shown without (solid circles) and with (light shaded squares) 1 μM valinomycin, respectively, in otherwise identical conditions and (B) at a lower light intensity to reset the ΔpH in the presence of 1 μM valinomycin (light shaded triangle) to that without valinomycin.
The electric potential–indicating electrochromic absorbance shift of carotenoids in the thylakoid membrane (ECS) was determined using a NoFOSpec instrument described previously (22). The data shown are the averages of three or four repetitions of signal-averaged (n = 4) ECS measurements. Each individual sample contained thylakoids at 40 μg chlorophyll/ml, 20 μM methyl viologen, reaction buffer to 500 μl, and, depending on the treatment, 1 μM valinomycin, nigericin between 0.3 and 15 nM, and ethanol as a loading control. The nigericin concentrations and actinic intensities used to determine the ECS were verified to achieve similar values of ΔpH as in the protein transport experiments by 9-AA fluorescence quenching.
RESULTS
To test the hypothesis that we could diminish the thylakoid ΔpH without similarly affecting the Δψ, the transport of iOE17, a well-characterized cpTat substrate, was probed under conditions of high and low inferred values of ΔpH, the latter parameter adjusted by application of the nonelectrogenic protonophore nigericin (Fig. 1). Shown are the polyacrylamide gels of nine replicate imports for each ionophore treatment (Fig. 1 A). The bands labeled mOE17 that correspond to translocated and N-terminally processed cpTat substrate were quantified by densitometry based on the 10% load standards. In the graph representing the average and standard error of each treatment (Fig. 1 B), it can be seen that in the absence of nigericin, a modest reduction in the protein transport rate was affected by valinomycin, but the change was not statistically significant (two-tailed t-test, P = 0.4). However, in the presence of a nonsaturating concentration of nigericin (300 nM), valinomycin reduced the rate of cpTat transport by a level that was clearly statistically significant (two-tailed t-test, P < 0.01). That valinomycin has an effect on cpTat pathway transport indicates that the electric potential can contribute to the driving force for the cpTat transport process.
In the experiment described above, the changes in both ΔpH and Δψ were inferred on the basis of addition of ionophores. To provide more quantitative support for the idea that the electric potential can contribute to the driving force for cpTat transport, we monitored the magnitude of the steady-state Δψ in isolated thylakoids. Carotenoids, accessory pigments located in the thylakoid membrane, exhibit an ECS that is linearly related to the transmembrane electric potential (23,24). We sought to measure the steady-state ECS of thylakoids during 1 min of actinic illumination under conditions designed to attenuate the different components of the PMF (Fig. 2). We first determined the response of the ECS signal when the actinic intensity was constant and the passive proton flux through the thylakoid membrane was increased by the addition of increasing nigericin concentrations (Fig. 2 A). Nigericin addition alone actually promoted an increase of the Δψ, likely as a result of decreased proton backpressure or photosynthetic control on electron transport (25). Under the same conditions, but with 1 μM valinomycin present in the reaction, the steady-state electric potential was dissipated with increasing nigericin concentrations. It can be seen (Fig. 2 B) that the ECS does not appreciably decrease when the actinic light is limited (shaded bars). The reduction of the ECS by the inclusion of 1 μM valinomycin (solid bars) in the reaction medium is imperceptible at high actinic intensities but increases as actinic light is limited.
Component ECS traces are shown to illustrate the electric potential changes we observed (Fig. 2, C–F). The steady-state levels of the ECS traces were used to plot the bar graphs above the traces (Fig. 2, A and B). In each paired trace, the reaction run without valinomycin exhibits a spike of electric potential occurring at the onset of actinic illumination; the reactions exhibiting a slower initial rate of electric potential change are those run with valinomycin. The characteristic thylakoid electric potential spike at the onset of actinic illumination is present at high-light conditions (Fig. 2, C–E), but with lower-actinic-intensity illumination, the spike is subdued even though the ECS signal still develops rapidly (Fig. 2 F). In every case, valinomycin has the effect of muting the initial electric potential spike. However, when the actinic intensity is low (Fig. 2 F), or there is additional potassium ion flux into the membrane via nigericin (Fig. 2 D), valinomycin also depresses the total electric potential that the thylakoids are able to develop compared to high-light controls (Fig. 2, C and E, respectively).
With the information from Fig. 2 concerning the response of the thylakoid electric potential to the presence of valinomycin under changing PMF conditions brought about either by nigericin addition or by actinic light intensity attenuation, protein transport experiments were designed to determine semiquantitatively the dependence of the cpTat pathway on Δψ as a function of ΔpH. To guide our experimental design, we constructed models of response of the thylakoid steady-state PMF to changes in actinic light intensity or to nigericin titrations (Fig. 3, insets). Because nigericin titration resulted in an increase in the steady-state electric potential, whereas actinic light intensity attenuation had no effect on this parameter, we modeled the thylakoid Δψ response to those titrants as rising and level, respectively. Because the addition of valinomycin dissipates the steady-state electric potential both at low actinic intensity and at high nigericin concentrations (as demonstrated in Fig. 2), both of which result in a reduction of the total PMF driving force, we predicted that the greatest effect of valinomycin on protein transport would be observed under these conditions.
In Fig. 3, we show the results of cpTat protein transport experiments carried out using 6 min of actinic illumination and in which the ΔpH was decreased by two different methods. In the first, the ΔpH was controlled by including nigericin, which increases the passive proton permeability of the membrane (as in the experiment of Fig. 1) and, coupled to reduced feedback on photosynthetic electron transport, causes an increase in Δψ (Fig. 2 A). Under these conditions valinomycin addition had a clear impact on the protein transport rate (Fig. 3 A). This experiment demonstrates that valinomycin has an unmistakable effect on cpTat protein transport when measured under conditions in which the electric potential constitutes a significant portion of the total PMF. When the second method was used, the ΔpH was controlled by altering the light intensity used to drive the photosynthetic electron transport chain, which affects the rate of proton deposition into the thylakoid lumen (Fig. 3 B). Contrary to our expectations, the rate of translocation of iOE17 into the thylakoid lumen was not affected by the addition of valinomycin.
We considered that the ECS signal at 1 min, as determined in Fig. 2, may not be an accurate reflection of the Δψ during the 6-min protein transport experiment reported in Fig. 3. In an attempt to reconcile our Δψ measurements with protein transport data, we decreased the duration of the cpTat transport experiments to 1 min. Fig. 4 shows that in the presence of 100 nM nigericin and at low actinic light intensity, valinomycin effected a significant decrease in cpTat transport during a 1-min experiment, even though the ΔpH was approximately the same for both samples (compare circle and square at the same abscissa value in Fig. 4 A). This confirms our previous results seen in Figs. 1 and 3 A and indicates that the thylakoid Δψ can contribute to the driving force for cpTat transport.
Under identical low-actinic-illumination conditions, but without nigericin (Fig. 4 B), valinomycin addition (square) resulted in a similar rate of protein transport compared to the control (circle). However, valinomycin had the effect of increasing the ΔpH across the thylakoid membrane. This result suggests that valinomycin caused an increase in the rate of proton pumping by relieving the electrical backpressure on the electron transport chain, an observation that to our knowledge has not been reported before. We can expect that the decrease in Δψ by valinomycin addition was approximately energetically equivalent to the increase in ΔpH. When the intensity of actinic illumination was adjusted so that the valinomycin-treated samples maintained a ΔpH roughly similar to that of the control samples (triangle), a decrease in import by valinomycin was apparent (two-tailed t-test, n = 6, P < 0.01). This demonstrates that, in addition to its effect on the cpTat pathway when the PMF is controlled by nigericin, valinomycin also affects the rate of protein transport under conditions in which the PMF is controlled by the light-dependent photosynthetic proton pumping rate, a result not apparent in our earlier 6-min experiment (Fig. 3 B).
DISCUSSION
The data reported in this communication demonstrate that, in addition to the ΔpH established across the thylakoid membrane in the light, the transmembrane Δψ can also contribute to the energetics of protein transport on the cpTat pathway. We suspect that one reason this was not previously detected is that the prior transport assays were carried out at high light intensities for relatively long periods of time. These conditions would make electric potential effects difficult to observe for two possible reasons. First, when the photosynthetic electron transport chain is operating at the higher end of its capacity, the steady-state electric potential across the thylakoid membrane contributes a relatively small proportion of the total thylakoid PMF. Under these conditions, loss of the electric potential on valinomycin addition would affect cpTat transport subtly and could be overlooked.
Second, the effect of valinomycin addition on thylakoid electric potential during long periods of actinic illumination is different from the effect known for short illumination periods. At the onset of actinic illumination, the valinomycin-induced rate of potassium flux across the membrane is sufficiently high to quickly dissipate the electric potential caused by charge separation in the reaction centers or by initial proton pumping by the electron transport chain. However, as light-driven proton flux into the thylakoid lumen continues, the rate of valinomycin-dependent potassium efflux decreases, as is evidenced by the buildup of the electric potential (Fig. 2). This reduction in valinomycin activity is likely caused by depletion of potassium ions from the finite volume of the thylakoid lumen. By using 10 μl/mg chlorophyll as an estimate of the thylakoid lumen volume (26) and by assuming that potassium is equilibrated across the membrane in dark-adapted thylakoids, that most internal potassium is not bound, and that proton pumping occurs at a modest rate of 120 μmol per mg chlorophyll per hour, one can calculate that the potassium store of the lumen could be depleted by electrogenic compensation for proton pumping within seconds after the onset of illumination. Thus, under continuous illumination, the potassium ion gradient established by valinomycin would approach the Nernst equilibrium set by the electric potential resulting from light-dependent proton pumping. In contrast to this, the electric potential was diminished under all light regimes when valinomycin was applied in conjugation with nigericin, a H+/K+ antiporter, which would serve to replenish the lumen potassium concentration during proton gradient dissipation.
Valinomycin seems to have a similar effect on the rate of development of electric potential as does low actinic intensity. Comparing the ECS trace obtained in high light in the presence of valinomycin (Fig. 2 E, lower trace) to that in a low light control (Fig. 2 F, upper trace), it can be seen that neither condition allows for the electric potential to develop as rapidly as in high-light conditions in the absence of electrogenic ionophores (Fig. 2, C–E, upper traces). The similarity between the traces obtained under those two conditions must result from comparable ratios of electric potential accumulation and dissipation, even though the absolute ion flux rates in the two cases should be quite different.
The dual energy utilization of the cpTat pathway suggests that it is likely a PMF-utilizing device, as are the reversible H+-ATPases located on the thylakoid and other energy-transducing membranes. If true, then protein transport studies such as this one may also lead to information pertaining to thylakoid energetics. For instance, the difference in cpTat transport between valinomycin-treated and control samples as a function of ΔpH (expressed in mV) could be used as a means to estimate in vitro the magnitude of the Δψ under steady-state illumination and to calibrate the ECS signal. Specifically, the data in Fig. 4 B show that valinomycin caused an increase in the ΔpH of ∼0.5 units without significantly changing the driving force for iOE17 transport (the PMF), which suggests that the original steady-state Δψ was ∼30 mV under the conditions of that experiment.
Our findings bear directly on the question of whether the energetics of cpTat protein transport are different in vitro and in vivo (15–17). In the first of the studies leading to this suggestion, mutants of Chlamydomonas reinhardtii that are unable to generate a pH gradient were seen to accumulate cpTat substrates in the thylakoid lumen. However, these algae were able to generate a light-induced electric field (27), and our finding that the Δψ can contribute to the energetics of cpTat protein transport explains how they were able to transport cpTat substrates. The second study questioning whether the ΔpH is the driving force for cpTat protein transport utilized tobacco protoplasts poisoned with ionophores to dissipate the PMF. However, the Δψ was not measured in these experiments, and we show in Fig. 2 that an electric field is in fact generated in the presence of valinomycin during continuous illumination, an observation that has been made previously (18,19). The existence of a small PMF in those experiments, even in the presence of nigericin and valinomycin, is inferred from the ability of the cells to carry out limited protein synthesis, a process depending on ATP generation. Thus, we believe our experiments demonstrate that a driving force for cpTat transport was available in all the experiments in these two studies and that there is no need to invoke a different energetics for this pathway in vitro and in vivo (17).
It has long been recognized that although the ΔpH and Δψ are thermodynamically equivalent, they are not mechanistically equivalent, and thus, work obtained from each energy source need not lead to the same rates of a given chemiosmotic reaction. In response to this, Mitchell proposed that the H+-ATPases operate via a proton well wherein the electric potential is converted into a ΔpH within the membrane and that the real chemical work is performed only by the latter (28). Such a proton well is realized in the structure of the F0/CF0/EF0 portions of the H+-ATPases in which protons have an unrestricted path through the F0 subunit for travel from the p to the n side of the membrane before encountering the F1 subunit (29,30). Our finding that the cpTat translocon is likely a PMF-utilizing device leads to a similar prediction: that its structure will also contain a pathway allowing free passage of protons into the membrane, wherein the electric potential will be converted to a local pH gradient within the translocon. The positive net charge on the Tat–directing signal peptides and the basic nature of some cpTat passenger proteins would appear to rule out electrophoresis as a transport driving force, making the thermodynamically equivalent proton trap (which converts the ΔpH into a Δψ (28)) a less likely alternative. Experiments designed to detect the presence of such a proton well in the cpTat translocon are currently under way.
Acknowledgments
We thank D. Kramer and J. Cruz for assistance with the NoFOSpec and E. Leaver for discussions.
This project was funded by a grant from the Dept. of Energy to S.M.T.
Editor: Mark Girvin.
References
- 1.Berks, B. C. 1996. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 22:393–404. [DOI] [PubMed] [Google Scholar]
- 2.Voelker, R., and A. Barkan. 1995. Two nuclear mutations disrupt distinct pathways for targeting proteins to the chloroplast thylakoid. EMBO J. 14:3905–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cline, K., W. F. Ettinger, and S. M. Theg. 1992. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J. Biol. Chem. 267:2688–2696. [PubMed] [Google Scholar]
- 4.Mould, R., and C. Robinson. 1991. A proton gradient is required for the transport of two lumenal oxygen-evolving proteins across the thylakoid membrane. J. Biol. Chem. 266:12189–12193. [PubMed] [Google Scholar]
- 5.Cline, K., R. Henry, C. Li, and J. Yuan. 1993. Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J. 12:4105–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Settles, A. M., A. Yonetani, A. Baron, D. R. Bush, K. Cline, and R. Martienssen. 1997. Sec-independent protein translocation by the maize hcf106 protein. Science. 278:1467–1470. [DOI] [PubMed] [Google Scholar]
- 7.Pohlschroder, M., E. Hartmann, N. J. Hand, K. Dilks, and A. Haddad. 2005. Diversity and evolution of protein translocation. Annu. Rev. Microbiol. 59:91–111. [DOI] [PubMed] [Google Scholar]
- 8.Thomas, J. D., R. A. Daniel, J. Errington, and C. Robinson. 2001. Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli. Mol. Microbiol. 39:47–52. [DOI] [PubMed] [Google Scholar]
- 9.Santini, C. L., B. Ize, A. Chanal, M. Muller, G. Giordano, and L. F. Wu. 1998. A novel sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J. 17:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark, S. A., and S. M. Theg. 1997. A folded protein can be transported across the chloroplast envelope and thylakoid membranes. Mol. Biol. Cell. 8:923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creighton, A. M., A. Hulford, A. Mant, D. Robinson, and C. Robinson. 1995. A monomeric, tightly folded stromal intermediate on the delta pH-dependent thylakoidal protein transport pathway. J. Biol. Chem. 270:1663–1669. [DOI] [PubMed] [Google Scholar]
- 12.Teter, S. A., and S. M. Theg. 1998. Energy-transducing thylakoid membranes remain highly impermeable to ions during protein translocation. Proc. Natl. Acad. Sci. USA. 95:1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alder, N. N., and S. M. Theg. 2003. Energetics of protein transport across biological membranes: A study of the thylakoid DeltapH-dependent/cpTat pathway. Cell. 112:231–242. [DOI] [PubMed] [Google Scholar]
- 14.Klosgen, R. B., I. W. Brock, R. G. Herrmann, and C. Robinson. 1992. Proton gradient-driven import of the 16 kDa oxygen-evolving complex protein as the full precursor protein by isolated thylakoids. Plant Mol. Biol. 18:1031–1034. [DOI] [PubMed] [Google Scholar]
- 15.Finazzi, G., C. Chasen, F. A. Wollman, and C. de Vitry. 2003. Thylakoid targeting of Tat passenger proteins shows no delta pH dependence in vivo. EMBO J. 22:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Cola, A., S. Bailey, and C. Robinson. 2005. The thylakoid delta pH/delta psi are not required for the initial stages of Tat-dependent protein transport in tobacco protoplasts. J. Biol. Chem. 280:41165–41170. [DOI] [PubMed] [Google Scholar]
- 17.Theg, S. M., K. Cline, G. Finazzi, and F. A. Wollman. 2005. The energetics of the chloroplast Tat protein transport pathway revisited. Trends Plant Sci. 10:153–154. [DOI] [PubMed] [Google Scholar]
- 18.Admon, A., Y. Shahak, and M. Avron. 1982. Adenosine triphosphate-generated transmembrane electric potential in chloroplasts. Biochim. Biophys. Acta. 681:405–411. [Google Scholar]
- 19.Thorne, S. W., G. Horvath, A. Kahn, and N. K. Boardman. 1975. Light-dependent absorption and selective scattering changes at 518 nm in chloroplast thylakoid membranes. Proc. Natl. Acad. Sci. USA. 72:3858–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry, S., and B. Rumberg. 1996. H+/ATP coupling ratio at the unmodulated CF0CF1-ATP synthase determined by proton flux measurements. Biochim. Biophys. Acta. 1276:51–56. [Google Scholar]
- 21.Schuldiner, S., H. Rottenberg, and M. Avron. 1972. Determination of delta pH in chloroplasts: 2. Fluorescent amines as a probe for the determination of delta pH in chloroplasts. Eur. J. Biochem. 25:64–70. [DOI] [PubMed] [Google Scholar]
- 22.Sacksteder, C. A., M. E. Jacoby, and D. M. Kramer. 2001. A portable, non-focusing optics spectrophotometer (NoFOSpec) for measurements of steady-state absorbance changes in intact plants. Photosynth. Res. 70:231–240. [DOI] [PubMed] [Google Scholar]
- 23.Witt, H. T., and A. Zickler. 1974. Vectorial electron flow across the thylakoid membrane. Further evidence by kinetic measurements with an electrochromic and electrical method. FEBS Lett. 39:205–208. [DOI] [PubMed] [Google Scholar]
- 24.Junge, W., and H. T. Witt. 1968. On the ion transport system of photosynthesis—investigations on a molecular level. Z. Naturforsch. B. 23:244–254. [DOI] [PubMed] [Google Scholar]
- 25.Giersch, C. 1981. Stimulation of photophosphorylation by low concentrations of uncoupling amines. Biophys. Biochem. Res. Commun. 100:666–674. [DOI] [PubMed] [Google Scholar]
- 26.Ort, D. R., and R. A. Dilley. 1976. Photophosphorylation as a function of illumination time I. Effects of permeant cations and permeant anions. Biochim. Biophys. Acta. 449:95–107. [DOI] [PubMed] [Google Scholar]
- 27.Lemaire, C., F.-A. Wollman, and P. Bennoun. 1988. Restoration of phototrophic growth in a mutant of Chlamydomonas reinhardtii in which the chloroplast atpB gene of the ATP synthase has a deletion: An example of mitochondria-dependent photosynthesis. Proc. Natl. Acad. Sci. USA. 85:1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell, P. 1968. Chemiosmotic Coupling and Energy Transduction. Glynn Research, Bodmin, UK.
- 29.Senior, A. E., S. Nadanaciva, and J. Weber. 2002. The molecular mechanism of ATP synthesis by F1F0-ATP synthase. Biochim. Biophys. Acta. 1553:188–211. [DOI] [PubMed] [Google Scholar]
- 30.Cramer, W. A., and D. B. Knaff. 1990. Energy Transduction in Biological Membranes: A Textbook of Bioenergetics. Springer-Verlag, New York.