Abstract
Senescence may function as a two-edged sword that brings unexpected consequences to organisms. Here we provide evidence to support this theory by showing that the absence of the Brca1 full-length isoform causes senescence in mutant embryos and cultured cells as well as aging and tumorigenesis in adult mice. Haploid loss of p53 overcame embryonic senescence but failed to prevent the adult mutant mice from prematurely aging, which included decreased life span, reduced body fat deposition, osteoporosis, skin atrophy, and decreased wound healing. We further demonstrate that mutant cells that escaped senescence had undergone clonal selection for faster proliferation and extensive genetic/molecular alterations, including overexpression of cyclin D1 and cyclin A and loss of p53. These observations provide the first in vivo evidence that links cell senescence to aging due to impaired function of Brca1 at the expense of tumorigenesis.
Keywords: p53, p21, senescence, aging, tumorigenesis
Senescence is considered the final phenotypic state displayed by cells in response to several distinct cellular physiologic processes, including DNA replication (Hayflick 1965), oncogene activation (Serrano et al. 1997; Zhu et al. 1998; Zindy et al. 1998), and oxidative damage (Chen et al. 1995; von Zglinicki et al. 1995). This physiologic response appears to trigger growth inhibition via activation of the tumor suppressor p53, which consequently up-regulates cyclin-dependent kinase (CDK) inhibitors p21, p19, and p16 (Brown et al. 1997; Lundberg et al. 2000). Senescent cells have a large, flat morphology and high acidic β-galactosidase enzymatic activity and are accompanied by profound growth defects (Dimri et al. 1995). Because senescence prevents cell proliferation, it is considered as a mechanism to suppress tumorigenesis (for review, see Campisi 2001). On the other hand, senescence may render cells resistant to apoptosis (Sasaki et al. 2001) and may also allow cells to undergo clonal selection for mutations, as it was shown recently that high-frequency genomic changes were detected in cultured human mammary epithelial cells when they emerged from senescence (Romanov et al. 2001). The accumulated DNA damage could lead to the inactivation of p53, and/or p16, rendering growth advantages to the cells. This, in turn, would allow further cellular changes, leading to immortalization and malignant transformation. Thus, it has been hypothesized that senescence may protect organisms from cancers while young, but promote carcinogenesis in later life (for reviews, see Wynford-Thomas 1999; Campisi 2000, 2001; Fossel 2000; Reddel 2000).
Most combined familial breast and ovarian cancers and ∼40% of familial breast cancer cases have been linked to mutations in the breast-cancer-associated gene 1 (BRCA1; Miki et al. 1994; Alberg and Helzlsouer 1997; Brody and Biesecker 1998; Paterson 1998; Rahman and Stratton 1998). In mouse, loss-of-function mutations of Brca1 result in embryonic lethality that is accompanied by pleiotropic effects, including growth retardation, apoptosis, defective DNA damage repair, centrosome amplification, loss of G2/M cell cycle checkpoint, and genetic instability (Deng and Scott 2000; Scully and Livingston 2000; Zheng et al. 2000; Venkitaraman 2002). In mutant mice where Brca1 is specifically disrupted in mammary epithelial cells, tumorigenesis requires long latency and is also associated with retarded ductal development, apoptosis, and chromosome abnormality (Xu et al. 1999a). Although these observations indicate that BRCA1 may play important roles in multiple biological processes, it raises questions about potential mechanisms through which BRCA1 represses tumor formation. In particular, how can BRCA1 mutations result in breast cancer if mutant cells fail to grow?
Investigations on the genetic interactions between Brca1 and p53 revealed that pleiotrophic phenotypes in Brca1 mutant mice are, at least in part, caused by deleterious effects of p53 activation. It has been shown that Brca1-null mutant embryos died at embryonic day 7–8 (E7–E8), and the embryonic lethality could be rescued partially by p53 or p21 deficiency (Hakem et al. 1997; Ludwig et al. 1997; Shen et al. 1998). Studying mutant embryos carrying a hypomorphic mutation of Brca1 (Brca1Δ11/Δ11), we showed that haploid loss of p53 could completely rescue embryonic lethality (Xu et al. 2001). The absence of p53 was found to relax cell cycle control and decrease apoptosis. Therefore the mutant cells could survive and proliferate, leading to tumorigenesis (Xu et al. 2001). Consistently, it was found that BRCA1 familial breast cancer tumors contain significantly higher rates of p53 mutations than do sporadic cancers (Schuyer and Berns 1999).
Activation of p53 also causes premature aging, which is characterized by the age-related deterioration of physiological functions necessary for the survival of an organism (Donehower 2002; Tyner et al. 2002). Aging happens to all organisms and, because of its complexity, very little is known about the underlying molecular mechanism. Recent studies indicate that proteins involved in DNA damage repair play an essential role in life-span determination, as a number of mouse mutants carrying targeted disruption of genes involving in DNA damage repair exhibit premature aging (Vogel et al. 1999; DePinho 2000; Kuro-o 2001; Mohaghegh and Hickson 2001; de Boer et al. 2002). Considering a role of BRCA1 in DNA damage repair and its extensive interactions with p53 (Scully et al. 1997; Gowen et al. 1998; Moynahan et al. 1999; Xu et al. 2001; Zhong et al. 2002), we hypothesize that Brca1 may also play a role in aging. Because human BRCA1 homozygous mutation carriers are not viable (Kuschel et al. 2001) and Brca1-null mutations in mouse result in embryonic lethality (for review, see Deng 2002), a link between BRCA1 and organismal aging processes has not been established. The survival of Brca1Δ11/Δ11 p53+/− mice provides an opportunity to address this issue.
In this study, we show that the Brca1Δ11 deficiency triggers physiologic and pathologic responses, which are manifested as p53-dependent, p21-associated senescence at the cellular level and premature aging at the organismal level. We demonstrate that senescence, although limiting Brca1Δ11/Δ11 cell proliferation, allows the cells to undergo clonal expansion and immortalization at the expense of genetic stability. The immortalized mutant cells also exhibit overexpression of cyclin A and cyclin D1, loss of p53, increased proliferation, and ultimately undergo malignant transformation.
Results
Brca1Δ11/Δ11 p53+/− mice exhibit aging phenotypes
Brca1Δ11/Δ11 p53+/− mice survive to adulthood, and female mice develop mammary tumors, ovarian tumors, and/or lymphoma before 12 mo of age (Xu et al. 2001). As Brca1Δ11/Δ11 p53+/− male mice usually do not develop mammary tumors, we have followed a cohort of mutant male mice and found that the majority of them also died within 1 yr of age. Our analysis indicated that ∼30% of mice developed lymphoma and died before they were 7 mo old (Bachelier et al. 2003). Starting from 8 mo of age, the majority of mutant mice, although tumor-free, exhibited a variety of phenotypes usually found in aging mice and died from aging-related mortality (Table 1). Compared with littermates or age-matched control (p53+/−) mice, Brca1Δ11/Δ11 p53+/− mice were smaller (Fig. 1A) and had lower body weights (Fig. 1B). The differences became more profound with increasing ages of animals (Fig. 1C,D). We found that ∼80% of mutant mice exhibited kyphosis at varying severity (Fig. 1E). All the mutant mice were thin with very little fat (Fig. 1F). By radiographic analysis, 70% of the mutant mice exhibited osteoporosis, as reflected by decreased bone radiodensity (Fig. 1G). Histologic analysis confirmed a decrease in the thickness of cortical bone (Fig. 1J) and bone loss in trabecular bones (Fig. 1K) relative to controls (Fig. 1H,I).
Table 1.
Aging-related changes and tumorigenesis in p53+/−and Brca111Δ/11Δ p53+/− mice
| Phenotypes
|
p53+/−
|
Brca111Δ/11Δ p53+/−
|
Number of mice affected/analyzed
|
|---|---|---|---|
| Aging-related changes | |||
| Life span | >12 mo | 6–12 mo | 45a |
| Kyphosis | normal | pronounced after 8 mo | 15/18 |
| Osteoporosis | normal | pronounced after 8 mo | 18/25 |
| Wound healing | normal | decreased after 8 mo | 4/4 |
| Body weight | normal | greatly reduced after 6 mo | 28/30 |
| Dermal thickness | normal | reduced after 8 mo | 12/14 |
| Thickness of adipose layer of skin | normal | reduced after 8 mo | 14/14 |
| Adipose tissue | normal | reduced after 6 mo | 7/7 |
| Hair regeneration | normal | reduced after 8 mo | 4/6 |
| Muscle atrophy | normal | reduced after 6 mo | 7/7 |
| Anaesthetic stress tolerance | normal | reduced | 3/8 |
| Death of unknown reason | normal | 7–11 mo | 10 |
| Tumorigenesis in female mice | |||
| Mammary tumor | 0/(11) | 6–12 mo | 13/19 |
| Other types of tumor | 1/(11) | 7–10 mo | 6/19b |
Braca1 mutant mice (31 males and 14 females) that were >6 mo were followed for aging. All the males did not have an obvious tumor.
These tumors occurred at bone (2), brain (1), liver (1), lung (1), thymus (1), and spleen (2).
Figure 1.
Aging-related phenotypes in Brca1Δ11/Δ11 p53+/− mice. (A) Photograph of 3-month-old p53+/− (all p53+/− mice are wild type for Brca1 and are simply referred to as Wt) and Brca1Δ11/Δ11 p53+/− (Mt) male mice. (B) Body weights of p53+/− and Brca1Δ11/Δ11 p53+/− mice (including both male and female, n = 65). Photograph of 8-month-old p53+/− (C) and Brca1Δ11/Δ11 p53+/− (D) female mice. (E) X-Ray radiograph of an 8-month-old Brca1Δ11/Δ11 p53+/− male mouse showing kyphosis (5 out of 18 mice examined exhibited this phenotype). (F) Photograph of 8-month-old female mouse, showing that all examined mutant mice (n > 10) have less fat (arrows). (G) Long-bone radiograph of 9-month-old p53+/− and Brca1Δ11/Δ11 p53+/− mice; 18 out of 25 mutant mice examined showed decreased bone density. (H–K) Haematoxylin-eosin staining section of 10-month-old p53+/− (H,I) and Brca1Δ11/Δ11 p53+/− (J,K) female mice. The mutant mice exhibited decreased bone density (G), thinner cortical bone (J), and reduced trabecular bones (K). Most mice were tumor-free at the time when they were analyzed.
Reduced dermal thickness and subcutaneous adipose tissue is often associated with human aging. Histologic examinations of skin of 8-mo and older mutant mice revealed a reduction in the thickness of dermis and fat layer compared with age-matched control mice (Fig. 2A,B). No obvious skin changes were found in mutant mice at younger age (data not shown). Some older mutant mice also displayed unhealthy hair growth patterns (data not shown) and chronic skin lesions, perhaps caused by unhealed wounds from fighting (data not shown). These abnormalities are usually observed in naturally or genetically engineered aging mice (Chuttani and Gilchrest 1995; Kuro-o et al. 1997; Rudolph et al. 1999; Vogel et al. 1999; Tyner et al. 2002). Aging is also associated with decreased hair regeneration and a reduced capacity to respond to stresses such as wound healing. To see if this is the case, we performed hair regeneration and wound-healing studies. We found that the mutant mice showed impaired hair regeneration (data not shown) and delayed wound repair compared with control mice (Fig. 2G). Histologic examinations of the wounds revealed a reduction in re-epithelialization from the wound edge in mutant mice (Fig. 2F) compared with control mice (Fig. 2E). During the wounding experiment, some older mutant mice died, suggesting that the mutant mice had a reduced ability to tolerate the stress. Some aging mice (six males and four females) were also found dead without any apparent cause that would result in death (Table 1). In addition to the targeted organ analysis, three male mutant mice at 6.5, 8, and 9.5 mo old were subjected to necropsy study. This analysis revealed more extensive abnormalities in multiorgan/tissue systems in the 9.5-month-old animal than the younger ones (Table 2). This observation is consistent with a view that aging is characterized by a progressive decline of functions of many organs, ultimately leading to death.
Figure 2.
Skin-aging phenotypes in Brca1Δ11/Δ11 p53+/− mice. (A,B) Haematoxylin-eosin-stained section of 8-month-old p53+/− (A) and Brca1Δ11/Δ11 p53+/− (B) mice. The thickness of the dermal layer (d) and subcutaneous fat (f) is reduced in mutant mice. (C,D) Quantitative measurement of thickness of dermal layer (C) and fat layer (D; n = 14). (E,F) Haematoxylin-eosin-stained section at day 4 postwounding in 8-month-old p53+/− (E) and Brca1Δ11/Δ11 p53+/− (F) female mice. Epithelialization is visible in control mice, but this process is delayed in mutant mice (arrow in F‘). (G) Comparison of wound healing in 11-month-old p53+/− and Brca1Δ11/Δ11 p53+/− male mice (data were summarized from 12 wounds in 4 mutant mice and 12 wounds in 4 control mice). (H) Western blot analysis of p53 expression in 1-month-old (1M) and 6-month-old (6M) p53+/− and Brca1Δ11/Δ11 p53+/− mice. Protein extracts (50 μg/lane) from spleen were used for the assay. Three mutant and three control mice are shown at each time point.
Table 2.
Necropsy of Brca1 mutant mice at 6.5, 8, and 9.5 mo
| Organ tissuesa
|
Phenotypes
|
9.5 mo
|
8 mo
|
6.5 mo
|
|---|---|---|---|---|
| Mandibular, lymph node | lymphoid hyperplasia, mild | + | + | + |
| Adrenal | adenoma, cortex, unilateral | + | ||
| Thyroid | follicular dilatation, C cell hyperplasia | + | ||
| Esophagus | hyperplasia, hyperkeratosis | + | ||
| Heart | chronic cardiomyopathy | + | + | |
| Liver | hepatocellular necrosis inflammation | + | + | |
| Colon | goblet cell hyperplasia | + | + | + |
| Spleen | marginal zone hyperplasia, decreased extramedullary hematopoiesis | + | ||
| Stomach, glandular | eosinophilic cytoplasmic globules | + | ||
| Stomach, nonglandular | hyperplasia, hyperkeratosis | + | ||
| Ileum | GALT hyperplasia | + | + | |
| Cecum | proliferative typhlitis | + | ||
| Kidney | mineralization, minimal, multipfocal | + | + | |
| Mesenteric, lymph node | lymphoid hyperplasia | + | + | + |
| Testis | tubular degeneration | + | ||
| Epididymis | hypospermia | + | + | + |
| Eye | retina degeneration | + | ||
| Bone marrow | granulacytic hyperplasia | + | ||
| Inguinal, lymph node | pigment, lymphoid hyperplasia | + | ||
| Bulbourethral gland | cystic hyperplasia | + | + | |
| Nasal structures | eosinophilic cytoplasmic globules respiratory and olfactory epithelium inflammation | + | + |
All three mice were alive at time of sacrificing. The 9.5-month-old mouse showed visible signs of aging, as judged by body size, weight, fur appearance, and movement.
Organ/tissue systems examined include brain, salivary gland, pancreas, trachea, pituitary, thymus, jejunum, liver, gall bladder, lung, duodenum, rectum, skin, seminal vesicles, coagulating gland, urinary bladder, prostate, kidney, eyes, Harderian glands, femur–bone, femur–bone marrow, vertebra, spinal cord, and tongue. Only the ones with abnormalities are listed.
We also examined female Brca1Δ11/Δ11 p53+/− mice and found that 13 out of 14 mice examined exhibited similar aging phenotypes when they were >8 mo old. Of note, we found that Brca1Δ11/Δ11 p53+/− female mice were prone to tumorigenesis. In addition to mammary tumor formation, which is specific to females, 6 out of 19 developed tumors in other organs, including lung, liver, spleen, bone, thymus, and brain (Table 1). The high incidence of tumorigenesis raises a possibility that premature aging in the Brca1 mutant female mice is secondary to tumor-associated cachexia. However, our analysis of these tumors indicated that five tumors (except for one brain tumor) were small (<1 cm in diameter), whereas the animals already showed pronounced aging phenotypes. In addition, our study of Brca1Co/Co MMTV-Crep53+/− female mice, all of which developed mammary tumors within 1 yr of age (Xu et al. 1999b), did not detect premature aging. These observations provide compelling evidence that impaired Brca1 function also caused premature aging of female mice.
Recently, it was shown that activation of p53 results in premature aging in mouse (Tyner et al. 2002). Because the genetic instability associated with the Brca1 mutation could cause p53 activation, we were interested in determining p53 levels in Brca1Δ11/Δ11 p53+/− mice. Western blots on proteins extracted from the spleen of 1-month-old and 6-month-old mice detected higher levels of p53 in Brca1Δ11/Δ11 p53+/− than in p53+/− mice (Fig. 2H). This observation suggests that p53 activation plays a role in premature aging of Brca1Δ11/Δ11 p53+/− mice.
Brca1Δ11/Δ11 embryos show senescence and senescence-like growth defects
We next investigated whether the embryonic lethality of Brca1Δ11/Δ11 embryos is associated with senescence, a pivotal event in aging (Fossel 2000). We found that mutant embryos exhibit acidic β-galactosidase activity, a characteristic marker of senescence (Dimri et al. 1995). Very strong X-gal staining patterns were found in mutant embryos at E16–E18 (Fig. 3A), especially in the tail and limbs (Fig. 3B,C), whereas weak staining was observed at E12–E15, and no staining was detected at E10–E11 (data not shown). These results indicate that the premature senescence occurred at late stages of embryonic development.
Figure 3.
Senescence phenotypes in Brca1Δ11/Δ11 embryos. (A–C) Photograph of acidic β-galactosidase staining of Brca1Δ11/Δ11 (Mt) and Brca1+/+ wild-type (Wt) E18 embryos. Strong staining was found in the tail (B) and limbs (C) of all tested mutant embryos (n = 6) that were E16.5 and older. (D,E) BrdU incorporation of Brca1Δ11/Δ11 (D) and wild-type (E) E16 embryos in thoracic region. Mutant embryos contained fewer BrdU-positive cells than wild-type embryos. The boxed areas in D and E are shown in D‘ and E‘. To provide a quantitative comparison, BrdU+ cells in comparable areas equivalent to the size shown in D‘ and E‘ in wild-type (n = 4) and mutant (n = 4) embryos were counted and subjected to the T-test. The average number of BrdU+ cells was 118 ± 11.9 cells/area and 88.5 ± 12.2 cells/area in wild-type and mutant, respectively (p ≤ 0.017).
We demonstrated previously that the Brca1Δ11/Δ11 embryos exhibited widespread apoptosis with significantly increased intensity in the central nervous system (Xu et al. 2001). This pattern of cell death is complementary to that revealed by acidic β-galactosidase activity assays, which exhibited the highest levels on the ventral side of the mutant embryos and the lowest in the central nervous system. It is conceivable that senescence may represent a protection mechanism that puts cells into a quiescent state to avoid acute death. Therefore, we predict that the senescence that occurred in Brca1Δ11/Δ11 embryos might be related to the premature aging observed in adult Brca1Δ11/Δ11 p53+/− mice.
Senescence is characterized in vitro by a spontaneous decline in growth rate and culmination of a terminal arrest in the G1 phase of the cell cycle (Noda et al. 1994; Atadja et al. 1995; Alcorta et al. 1996; Hara et al. 1996; Reznikoff et al. 1996; Serrano et al. 1997). Therefore, we investigated whether proliferation is affected in Brca1Δ11/Δ11 embryos by BrdU incorporation assays. Examination of E16.5 embryos revealed significantly lower incorporation of BrdU in Brca1Δ11/Δ11 embryos than in their littermates (Fig. 3D,E). Decreased cell proliferation, but less obvious than in E16.5 embryos, was also found in mutant embryos of E10.5–E13.5 (data not shown). These results suggested that the premature senescence observed in Brca1Δ11/Δ11 embryos might result in the reduction of embryonic growth and could be responsible for the embryonic lethality arising from developmental defects.
Brca1Δ11/Δ11 MEF cells display premature senescence and senescence-like growth defect
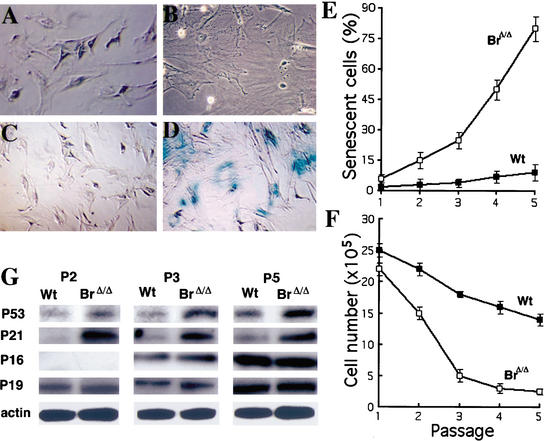
To study the molecular mechanisms underlying senescence in Brca1Δ11/Δ11 embryos and aging in Brca1Δ11/Δ11 p53+/− adult mice, we generated and characterized Brca1Δ11/Δ11 MEF cells and monitored their replicative capacity. Our analysis confirmed our previous finding that Brca1Δ11/Δ11 MEFs exhibited a stronger p53-mediated G1/S cell cycle checkpoint and proliferated significantly slower than wild-type controls (Xu et al. 2001). We also found that many Brca1Δ11/Δ11 MEF cells displayed a flattened and enlarged senescence phenotypic morphology at early passages (Fig. 4A,B). With increases in passages, increasing number of cells displayed this morphology.
Figure 4.
Senescence phenotypes in Brca1Δ11/Δ11 MEF cells. Morphology (A,B) and X-gal staining (C,D) of passage 4 MEF cells. The mutant MEF cells show flattened and enlarged morphology (B) and strong staining of X-gal (D). (E) Percentage of senescent cells at each passages in Brca1+/+ MEF cells (closed squares) and Brca1Δ11/Δ11 MEF cells (open squares). (F) Proliferation analysis of wild-type MEF cells (closed squares) and Brca1Δ11/Δ11 MEF cells (open squares). (G) Expression analysis of p53, p21, p16, and p19, at passage 2 (P2), passage 3 (P3), and passage 5 (P5) cells.
To determine whether the flattened and enlarged MEF cells were in senescence, we checked the activity of acidic β-galactosidase in these cells. Consistent with what was observed in embryos, Brca1Δ11/Δ11 MEFs exhibited significantly stronger activity of senescence-associated β-galactosidase than did control cells (Fig. 4C,D). Similar results were obtained in adult mutant skin fibroblasts (data not shown). Quantitative analysis indicated that the increased percentage of cells in senescence (Fig. 4E) was inversely correlated with their growth rate as reflected by a dramatic decline in cell numbers (Fig. 4F). These results indicate that the Brca1Δ11 mutation induces premature senescence and senescence-related growth defects in mutant cells and embryos. In addition, the Brca1Δ11/Δ11 MEF cells also exhibited sensitivity to γ-irradiation and oxygen toxicity-induced senescence (data not shown).
It has been shown that senescence is accompanied by the accumulation of p53, p21, p16, and/or p19 (Noda et al. 1994; Atadja et al. 1995; Alcorta et al. 1996; Hara et al. 1996; Reznikoff et al. 1996). To characterize the nature of the senescence and senescence-like growth arrest of Brca1Δ11/Δ11 MEF cells, we examined the expression of these proteins in MEF cells from passages 1 through 5 by Western blot. The expression of p53 and p21 gradually increased in both wild-type and Brca1 mutant MEF cells with each passage (Fig. 4G). Notably, in Brca1 mutant cells, the increases of p53 and p21 were higher than those of wild-type cells, which is consistent with previous studies of p53 and p21 expression in Brca1Δ11/Δ11 embryos (Xu et al. 2001). In contrast, expression of p16 and p19 was similar between mutant and wild-type cells, although the proteins increased with each passage (Fig. 4G). These observations suggest that the accumulation of p53 and/or its downstream mediator p21 may be responsible for the Brca1-associated premature senescence.
Senescence of Brca1Δ11/Δ11 embryos and MEF cells is p53-dependent and partially p21-mediated
Next, we investigated whether p53 and p21 were indeed involved in the senescence observed in Brca1Δ11/Δ11 embryos and MEF cells using a genetic approach. By crossing Brca1+/Δ11 mice with p53−/− or p21−/− mice, we generated embryos and MEF cells with full ranges of combinations of Brca1 and p53 or Brca1 and p21 mutations. Our analysis revealed that Brca1Δ11/Δ11 embryos exhibited low levels of activity of acidic β-galactosidase in either p53+/− or p53−/− genetic backgrounds (data not shown). However, in cultured MEFs, only complete loss of p53 could overcome senescence (Fig. 5B,E) and growth defects (Fig. 5F). Brca1Δ11/Δ11 p53+/− MEFs still displayed senescence, slow proliferation, and high levels of activity of acidic β-galactosidase, although to lesser extents than Brca1Δ11/Δ11 p53+/+ MEFs (Fig. 5E,F; data not shown). Although these observations indicate that the activation of p53 is indeed a primary cause for the senescence of Brca1Δ11/Δ11 embryos and MEFs, they also point out a differential requirement of p53 dosages for the induction of senescence in vivo and in vitro.
Figure 5.
Effects of p53 and p21 on senescence of Brca1Δ11/Δ11 embryos and MEF cells. (A,B) Morphology and X-gal staining of passage 4 Brca1Δ11/Δ11 MEF cells (A) and Brca1Δ11/Δ11 p53−/− MEF cells (B). The Brca1Δ11/Δ11 MEF cells showed a senescence-like morphology, and strong X-gal staining. (C,D) Photograph of X-gal staining of E18 embryos (C), and postnatal (P) day 1 pups (D). All Brca1Δ11/Δ11 p21−/− pups died within 24 h after birth. (E) Percentages of senescent MEF cells at passage 1 (P1), passage 3 (P3), and passage 5 (P5). (F) Cell proliferation of MEF cells at passages 1–5.
P21 is a downstream mediator of p53 in cell cycle regulation (Brugarolas et al. 1995; Deng et al. 1995). Our analysis revealed that absence of p21 could rescue the senescence-like phenotype observed in Brca1Δ11/Δ11 embryos as manifested by the absence of obvious activity of acidic β-galactosidase in Brca1Δ11/Δ11 p21−/− embryos (Fig. 5C). The Brca1Δ11/Δ11 p21−/− embryos also survived significantly longer than Brca1Δ11/Δ11 p21+/+ embryos; however, they all died within 24 h after birth for reasons presently unknown (Table 3; Fig. 5D). Because Brca1Δ11/Δ11p53−/− and Brca1Δ11/Δ11 p53+/− embryos survive to adulthood, the prenatal lethality of Brca1Δ11/Δ11 p21−/− embryos suggests that although the absence of p21 rescues the embryonic lethality caused by the Brca1Δ11 mutation, the postnatal development of Brca1Δ11/Δ11 mice may involve other p53-mediated pathways that are p21-independent.
Table 3.
Offspring obtained from BrΔ/+ p21+/− × BrΔ/+ p21+/− crosses
| Ages
|
Total no.
|
BrΔ/+ p21−/−
|
Br+/+ p21−/−
|
BrΔ/Δ p21−/−
|
BrΔ/+ p21+/−
|
Br+/+ p21+/−
|
BrΔ/Δ p21+/−
|
BrΔ/+ p21+/+
|
Br+/+ p21+/+
|
BrΔ/Δ p21+/+
|
|---|---|---|---|---|---|---|---|---|---|---|
| E16 | 14 | 1 | 2 | 3 | 4 | 1 | 1 | 1 | 1 | 0 |
| E18 | 12 | 1 | 2 | 2 | 4 | 3 | 1 | 0 | 0 | |
| P1 | 71 | 13 | 7 | 6 + 1a | 20 | 9 | 1a | 8 | 6 | 0 |
| Predicted no. at P1b | 71 | 9 | 4.5 | 4.5 | 18 | 9 | 9 | 9 | 4.5 | 4.5 |
These two embryos (1 BrΔ/Δ p21−/−and 1 BrΔ/Δ p21+/−) were born dead. All six other BrΔ/Δ p21−/−embryos died within 24 h after birth. No live BrΔ/Δ p21+/−embryos found at birth suggests that loss of only one copy of p21 does not have an obvious effect on survival of BrΔ/Δ embryos.
Brca1 and p21 are located on mouse chromosomes 11 and 17, respectively. The predicted number is based on the Mendelien ratio.
Notably, we found that the absence of p21 in cultured MEF cells could only partially rescue senescence and growth defects at early passages (1∼3; Fig. 5E,F). At later passages (4 and 5), the growth of Brca1Δ11/Δ11 p21−/− MEF cells significantly decreased, and showed senescence-like morphology and acidic β-galactosidase activity at extents similar to those of Brca1Δ11/Δ11 p53+/− MEF cells. These observations provide evidence for the existence of a culture shock-induced senescence that cannot be overcome by the loss of p21 or haploid loss of p53.
Immortalized Brca1Δ11/Δ11 MEF cells exhibited faster proliferation and more extensive genetic/molecular alterations than immortalized controls
Our data so far demonstrated that the Brca1Δ11 mutation results in premature aging at organism levels and cellular senescence characterized by decreased cell proliferation in mutant embryos and cultured MEF cells. This observation seems contradictory to the tumor-suppressor functions of Brca1. To provide further insights into this phenomenon, we studied the growth properties of Brca1Δ11/Δ11 MEF cells. Our attempts to pass Brca1Δ11/Δ11 MEF cells by the 3T3 protocol failed to produce immortalized cells because of their profound senescence phenotype, whereas wild-type MEF cells could routinely be immortalized by this protocol. However, when Brca1Δ11/Δ11 MEF cells were left in culture dishes for 2 mo, some foci (colonies) started to form. We picked a dozen individual colonies and pooled the remaining colonies (n > 20) from both Brca1Δ11/Δ11 and wild-type MEFs. We found that most colonies and the pooled cells could continue to grow and became immortalized. We next analyzed the proliferation capacity of these cells. Opposite to what was observed in primary MEF cells (Fig. 5F), the immortalized Brca1Δ11/Δ11 cells grew faster than wild-type controls that were immortalized in the same fashion (Fig. 6A). For long-term study, we monitored the growth properties of one individually picked colony and one pooled cell line from Brca1Δ11/Δ11 and control cells for >2 yr by continuously passing them every 3–4 d. Our data indicated that Brca1Δ11/Δ11 MEF cells exhibited significantly increased proliferative capacity compared with controls when assayed at multiple time points (Fig. 6A).
Figure 6.
Transformation and tumorigenesis of immortalized Brca1Δ11/Δ11 MEF cells. (A) Cell proliferation analysis of immortalized Brca1+/+ (Wt) and Brca1Δ11/Δ11 (Mt) MEF cells at early (E, 5–10 passages), mediate (M, ∼50 passages), and later (L, >150 passages) passages after immortalization. (B) Expression of cyclin A (CyA), cyclin D1 (CyD1), and p53 in MEF cells at early and late passages. (C) p53 activity as reflected by p21 induction upon γ-irradiation of immortalized Brca1Δ11/Δ11 MEF cells at early passages. (D,E) Chromosome spreads of immortalized Wt control (D) and Brca1Δ11/Δ11 (E) MEF cells. Note that only parts of spreads are shown in order to highlight the short chromosomes (arrows) found in mutant cells. (F–K) Soft agar colony formation assay of immortalized Brca1Δ11/Δ11 (F,H,J) and control (G,I,K) MEF cells at early (F,G), mediate (H,I), and late (J,K) passages, respectively. (L,M) Tumorigenicity assay of MEF cells at mediate passages in nude mice. Nude mice were tumor-free even 3 mo after injection of wild-type MEF cells (L), whereas tumors (arrows) were observed 3 wk after injection of Brca1Δ11/Δ11 MEF cells (M).
Next, we examined expression of several cell cycle-related proteins by Western blots. We found that expression of cyclin D1 and cyclin A went up in both wild-type and Brca1Δ11/Δ11 MEF cells at late passages, and the increase was greater in mutant cells than in wild-type cells (Fig. 6B). This is consistent with the observation that Brca1 mutant cells grew significantly faster than wild-type cells. Cells from all the colonies analyzed at late passages were negative for p53, and 2 of 3 colonies at early passages contained detectable p53 (Fig. 6B). To test if the detected p53 was functional, we irradiated cells from 10 mutant colonies of early passages (less than passage 10 after cloning) to examine their p53 functions. We found that only one colony (Fig. 6C, Brca1Δ11/Δ11-2) had functional p53, as indicated by p53 and p21 induction upon irradiation, but all others had lost the wild-type p53 function (Fig. 6C; data not shown). This observation suggests that, in principle, escaping senescence by Brca1Δ11/Δ11 cells requires loss of p53 function. It was recently shown that frequent genomic changes occurred in cultured human mammary epithelial cells immerging from senescence (Romanov et al. 2001). We prepared and analyzed chromosome spreads from immortalized mutant and control cells. We found that 90% of mutant MEF cells had >80 chromosomes, whereas only 40% of the control cells had >80 chromosomes (data not shown). About 30% of mutant MEF cells also contained many short chromosomes (Fig. 6E), which were rarely observed in wild-type MEF cells (Fig. 6D). These observations may suggest that Brca1Δ11/Δ11 MEF cells escape senescence through clonal selection of cells, which contain more extensive genetic and molecular alterations than control cells.
Malignant transformation of immortalized Brca1Δ11/Δ11 MEFs
The immortalized mutant MEF cells also showed loss of contact inhibition, reduced cell sizes and cell adhesion, as well as decreased response to apoptotic stimulation, including serum-free culture, H2O2, and doxorubicin treatment, which are hallmarks of transformed cells (data not shown). To further investigate the tumorigenicity of immortalized mutant MEF cells, we analyzed their foci formation properties in soft agar and tumorigenesis in nude mice. The mutant MEF cells at late passages exhibited malignant features as reflected by foci formation in soft agar (Fig. 6H,J) and tumorigenesis in nude mice (Fig. 6M). In contrast, matched control MEFs showed much fewer and smaller colonies (Fig. 6I,K) and failed to form tumors in nude mice (Fig. 6L). These observations indicate that immortalized Brca1 mutant MEFs have greater potential to become malignantly transformed than controls.
Discussion
In this study, we demonstrated that the impaired function of Brca1 in mouse causes senescence in mutant MEFs and embryos as well as premature aging in adult animals. We further demonstrated that the involvement of Brca1 in senescence and aging is dependent on wild-type functions of tumor suppressor p53. As the absence of p21, which mediates a p53-dependent G1–S cell cycle checkpoint (Brugarolas et al. 1995; Deng et al. 1995), could also rescue senescence in Brca1Δ11/Δ11 embryos, we conclude that senescence is most likely caused by the activation of a p53-dependent cell cycle checkpoint that prevents cell proliferation.
Senescence is a phenotypic state that a cell adapts in response to a variety of stimuli, including DNA damage (Chen et al. 1995; von Zglinicki et al. 1995), telomere shortening (Harley et al. 1990; Allsopp et al. 1992), activation of oncogenes (Serrano et al. 1997; Zhu et al. 1998; Zindy et al. 1998), and the inappropriate activation of mitogenic signals (Lin et al. 1998). Consistently, expression changes of many genes involved in some of these processes, including Ras, Raf, E2F1, MEK, p53, p16, Rb, and telomerase, resulted in senescence in mutant MEF cells (for review, see Campisi 2001). Several previous investigations demonstrated that the absence of Brca1 resulted in defective DNA damage repair, abnormal G2–M cell cycle checkpoint, and centrosome duplication, all of which are essential for maintaining genetic stability (for review, see Deng and Brodie 2000). Thus, the cell senescence observed in Brca1Δ11/Δ11 embryos and MEF cells is most likely triggered by genetic instability. The extensive chromosome alterations, both numeric and structural, found in Brca1Δ11/Δ11 embryos and MEF cells (Xu et al. 1999b; this study) are consistent with this claim. Notably, we found that the increased expression of p53 and p21 was detected in E14.5 embryos (Xu et al. 2001) and P2 MEF cells, whereas high activity of β-galactosidase only became obvious in E16.5 embryos and P3 MEF cells. Because increased levels of p53 and p21 are detected at stages prior to the onset of senescence, it is conceivable that p53 activation due to the accumulation of DNA damage, which in turn activates a p21-mediated cell cycle checkpoint, is a primary cause for the observed premature senescence.
Despite the fact that the removal of only one p53 allele enables Brca1 mutant embryos to bypass senescence and survive to adulthood, the Brca1Δ11/Δ11 MEFs, however, require the removal of both p53 alleles to escape senescence, indicating a stronger activation of the p53 checkpoint in vitro than in vivo. Although this may reflect an intrinsic differential requirement of p53 dosage for the initiation of senescence, a number of factors need to be considered. The senescence observed in Brca1Δ11/Δ11 embryos is mainly triggered by genetic instability due to the absence of Brca1, whereas senescence occurring in cultured Brca1Δ11/Δ11 MEFs may also be triggered by physiologic and environmental changes. An acute response of cells to this “culture shock” (Sherr and DePinho 2000) is the alteration of gene expression, including the up-regulation of p16, p19, p21, and p53 (Fig. 4G). This may provide a possible explanation for why p53 haploinsufficiency or absence of p21 fails to overcome senescence of Brca1Δ11/Δ11 MEFs in culture.
Although p53 haploinsufficiency enables Brca1Δ11/Δ11 p53+/− embryos to bypass senescence and survive to adulthood, it does not prevent them from prematurely aging, which is characterized by a progressive decline of functions of many organs, ultimately leading to aging-related diseases, tumorigenesis, and death. This is perhaps because of the fact that such a rescue is established on the relaxed G1–S checkpoint and attenuated apoptosis, which never corrects any deficiency caused by the Brca1 mutation. Therefore, the “rescue” enables mutant cells to propagate without being properly checked. Because of the important roles of Brca1 in DNA damage repair (Scully et al. 1997; Gowen et al. 1998; Moynahan et al. 1999), the proliferation of Brca1Δ11/Δ11 p53+/− cells is expected to accumulate more and more DNA damage during the development and growth of the mutant animals. Thus, analogous to what was observed in the cultured Brca1Δ11/Δ11 p53+/− MEF cells, we hypothesize that the remaining wild-type allele of p53 in Brca1Δ11/Δ11 p53+/− mice could be activated by the accumulation of DNA damage when they reach a threshold, leading to the initiation of cell senescence in vivo. When the number of senescent cells in organs reaches a critical level, these organs cannot function properly, which eventually results in a decreased life span. This explanation is consistent with many recent investigations, which demonstrated that mutations of genes that are involved in DNA damage repair could result in premature aging in both human and mouse (Vogel et al. 1999; DePinho 2000; Fossel 2000; Mohaghegh and Hickson 2001; de Boer et al. 2002).
Cell senescence has been considered as a mechanism to repress tumorigenesis, as it irreversibly arrests the growth of cells at risk of neoplastic transformation (Campisi 2001). An intriguing finding here is that Brca1 mutant MEFs exhibit both premature senescence and greater potential for malignant transformation. We found that mutant cells always underwent senescence earlier than controls, and were also more difficult to immortalize using the 3T3 procedure. However, once immortalized, the mutant cells proliferated faster and gradually exhibited many features often observed in malignantly transformed cells, that is, loss of contact inhibition, anchorage-independent growth (formation of colonies in soft agar), and tumorigenesis in nude mice. Concomitant to these phenotypic changes at the cellular level, mutant cells showed extensive genetic and molecular alterations as manifested by chromosomal changes and increased expression of cyclin A and cyclin D1 as well as the loss of p53. These observations suggest that cells escaping senescence may have gone through a selective process for mutations that promote cell proliferation. Such mutations, as they promote cell proliferation, are often associated with malignant transformation. This may account for the reason that both premature cell senescence and tumorigenesis occur in the same type of cells.
Of note, the phenotypes exhibited by Brca1Δ11/Δ11 p53+/− mice are reminiscent of those observed in the late-generation (i.e., G6) telomerase knockout mice (Rudolph et al. 1999). This suggests that there is nothing unique about telomere malfunction that contributes to organismal aging, but that instability produced by many mechanisms can produce a similar phenotype. In the light of our finding, we propose that the genomic instability caused by the absence of Brca1 activates p53, which initiates multiple p53-dependent processes, including cell cycle arrest, senescence, and apoptosis, that result in embryonic lethality in mutant embryos (Fig. 7). This physiologic response of p53 activation in Brca1 mutant MEF cells and mice should, in theory, prevent tumorigenesis as demonstrated recently by an activating mutation of p53, which results in aging and decreases in tumor formation (Tyner et al. 2002). However, the genetic instability triggered by the absence of Brca1 could mutate p53 and other genes and eventually lead to tumorigenesis. p53 mutation is emphasized in this model as we found that 9 out of 10 Brca1 mutant colonies emerging from senescence have lost p53 function, suggesting that this protein is a primary target during the selection process for growth advantages. Thus, our study provides an excellent example for the hypothesis that senescence could serve as a two-edged sword that suppresses tumorigenesis early in life but may have deleterious effects later, leading to aging and tumor formation (Wynford-Thomas 1999; Campisi 2000; Fossel 2000; Reddel 2000; Sharpless and DePinho 2002). The Brca1Δ11/Δ11 p53+/− mice should serve as a good animal model for future aging and tumorigenesis studies.
Figure 7.
A working model summarizing how the Brca1 deficiency causes cell senescence, premature aging, and tumorigenesis.
Materials and methods
Mice, MEF cells, immortalization, and analysis
Brca1Δ11/Δ11 embryos, Brca1Δ11/Δ11 p21−/− embryos, and Brca1Δ11/Δ11 p53+/− mice were generated as described (Xu et al. 2001). The genetic background of these mice is 50% FVB, 25% 129, and 25% Black Swiss. MEF cells were derived from E14.5 embryos generated from intercrosses of Brca1+/Δ11 p53+/− mice. Because of difficulties in immortalizing Brca1Δ11/Δ11 MEF cells by the regular 3T3 protocol, we seeded 1 × 106 cells in a 10-cm dish and left them untouched (except for the medium change every 3 d) for 2 mo. We then picked individual colonies and passed them sequentially through 24-well, 12-well, 6-well, and 10-cm plates. For proliferation analysis, 5 × 104 wild-type and Brca1Δ11/Δ11 MEF cells were plated on 6 wells in DMEM supplemented with 10% FBS. Cell numbers were counted every day for up to 6 d.
Cell senescence analysis
We plated 5 × 104 MEF cells in a 1-well FALCON culture slide (Becton Dickinson) for 3 d, and then processed them for acidic β-galactosidase activity as described (Dimri et al. 1995). Staining for embryos was carried out at 37°C for 6–8 h.
Immunoblot analysis
Western blot analysis was accomplished according to standard procedures using ECL detection (Amersham). The following primary antibodies were used: p53 (Ab-7, Oncogene); p21WAF1 (Ab-6, Oncogene); p16 (M-156, Santa Cruz); p19ARF (NOVUS); Cyclin D1 (C-20, Santa Cruz); and Cyclin A (H-432, Santa Cruz). Horseradish peroxidase-conjugated donkey anti-rabbit or sheep anti-mouse antibodies (Amersham) were used as secondary antibodies.
Wound-healing experiments
Mice were anaesthetized with methoxyfluorane, and the dorsum was shaved and cleaned with alcohol. Four equidistant 1-cm full-thickness incisional wounds were made through the skin and panniculus carnosus muscle. Wounded skin specimens were collected at day 4 postwounding and were bisected for histology.
Bone X-ray imaging and histologic analysis
Mice were killed by CO2. The skin and the viscera were removed. Photographs were taken in an X-ray machine (Faxitron X-ray). We used an X-ray dose of 15 kV for 100 sec. For histologic analysis, bones were fixed in 4% paraformaldehyde at 4°C overnight and decalcified in 0.5 M EDTA/PBS. Decalcified tissues were dehydrated, embedded in wax, and sectioned using standard procedures.
BrdU labeling of embryos
BrdU labeling of the cells in the S phase of the cell cycle was performed as described. BrdU (100 mg/g of body weight) was injected i.p. into pregnant females at E16.5. The females were killed 2 h after injection; the embryos were fixed in 4% paraformaldehyde at 4°C overnight and processed for immunohistochemistry. The tissues were stained with BrdU staining bulk kit (ZYMED).
Colony formation in soft agar and tumorigenicity assay
For colony formation, 5 × 104 immortalized wild-type and Brca1Δ11/Δ11 MEF cells were suspended in 0.3% agar (in DMEM supplemented with 10% FBS) and plated on 6-well plates containing a solidified bottom layer (0.5% agar in growth medium). For the tumorigenicity assay, 2 × 106 immortalized wild-type and Brca1Δ11/Δ11 MEF cells were injected into 6-week-old female nude nice for 3 wk to 3 mo.
Acknowledgments
We thank R. Bachelier, W. Qiao, C. Li, R. Wang, X. Wang, and X. Xu for their technical assistance and critical discussion of this work.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL chuxiad@bdg10.niddk.nih.gov; FAX (301) 480-1135.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1050003.
References
- Alberg AJ, Helzlsouer KJ. Epidemiology, prevention, and early detection of breast cancer. Curr Opin Oncol. 1997;9:505–511. doi: 10.1097/00001622-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16INK4a in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atadja P, Wong H, Garkavtsev I, Veillette C, Riabowol K. Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci. 1995;92:8348–8352. doi: 10.1073/pnas.92.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelier, R., Xu, X., Wang, X., Li, W., Naramura, M., Gu, H., and Deng, C.X. 2003. Normal lymphocyte development and thymic lymphoma formation in Brca1 exon 11-deficient mice. Oncogene (In Press). [DOI] [PubMed]
- Brody LC, Biesecker BB. Breast cancer susceptibility genes. BRCA1 and BRCA2. Medicine (Baltimore) 1998;77:208–226. doi: 10.1097/00005792-199805000-00006. [DOI] [PubMed] [Google Scholar]
- Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cancer, aging and cellular senescence. In Vivo. 2000;14:183–188. [PubMed] [Google Scholar]
- ————— Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuttani A, Gilchrest BA. Aging. In: Masoro EJ, editor. Handbook of physiology. 1995. , Section 11, pp. 309–324. Oxford Univ. Press, New York. [Google Scholar]
- de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GT, van Leeuwen W, Themmen AP, et al. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- Deng CX. Tumor formation in Brca1 conditional mutant mice. Environ Mol Mutagen. 2002;39:171–177. doi: 10.1002/em.10069. [DOI] [PubMed] [Google Scholar]
- Deng CX, Brodie SG. Roles of BRCA1 and its interacting proteins. Bioessays. 2000;22:728–737. doi: 10.1002/1521-1878(200008)22:8<728::AID-BIES6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Deng CX, Scott F. Role of the tumor suppressor gene Brca1 in genetic stability and mammary gland tumor formation. Oncogene. 2000;19:1059–1064. doi: 10.1038/sj.onc.1203269. [DOI] [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA. Does p53 affect organismal aging? J Cell Physiol. 2002;192:23–33. doi: 10.1002/jcp.10104. [DOI] [PubMed] [Google Scholar]
- Fossel M. Cell senescence in human aging: A review of the theory. In Vivo. 2000;14:29–34. [PubMed] [Google Scholar]
- Gowen LC, Avrutskaya AV, Latour AM, Koller BH, Leadon SA. BRCA1 required for transcription-coupled repair of oxidative DNA damage. Science. 1998;281:1009–1012. doi: 10.1126/science.281.5379.1009. [DOI] [PubMed] [Google Scholar]
- Hakem R, de la Pompa JL, Elia A, Potter J, Mak TW. Partial rescue of Brca1 (5–6) early embryonic lethality by p53 or p21 null mutation. Nat Genet. 1997;16:298–302. doi: 10.1038/ng0797-298. [DOI] [PubMed] [Google Scholar]
- Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Disease model: Human aging. Trends Mol Med. 2001;7:179–181. doi: 10.1016/s1471-4914(01)01921-9. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kuschel B, Gayther SA, Easton DF, Ponder BA, Pharoah PD. Apparent human BRCA1 knockout caused by mispriming during polymerase chain reaction: Implications for genetic testing. Genes Chromosomes Cancer. 2001;31:96–98. doi: 10.1002/gcc.1122. [DOI] [PubMed] [Google Scholar]
- Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes & Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T, Chapman DL, Papaioannou VE, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: Lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes & Dev. 1997;11:1226–1241. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- Lundberg AS, Hahn WC, Gupta P, Weinberg RA. Genes involved in senescence and immortalization. Curr Opin Cell Biol. 2000;12:705–709. doi: 10.1016/s0955-0674(00)00155-1. [DOI] [PubMed] [Google Scholar]
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Mohaghegh P, Hickson ID. DNA helicase deficiencies associated with cancer predisposition and premature ageing disorders. Hum Mol Genet. 2001;10:741–746. doi: 10.1093/hmg/10.7.741. [DOI] [PubMed] [Google Scholar]
- Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Paterson JW. BRCA1: A review of structure and putative functions. Dis Markers. 1998;13:261–274. doi: 10.1155/1998/298530. [DOI] [PubMed] [Google Scholar]
- Rahman N, Stratton MR. The genetics of breast cancer susceptibility. Annu Rev Genet. 1998;32:95–121. doi: 10.1146/annurev.genet.32.1.95. [DOI] [PubMed] [Google Scholar]
- Reddel RR. The role of senescence and immortalization in carcinogenesis. Carcinogenesis. 2000;21:477–484. doi: 10.1093/carcin/21.3.477. [DOI] [PubMed] [Google Scholar]
- Reznikoff CA, Yeager TR, Belair CD, Savelieva E, Puthenveettil JA, Stadler WM. Elevated p16 at senescence and loss of p16 at immortalization in human papillomavirus 16 E6, but not E7, transformed human uroepithelial cells. Cancer Res. 1996;56:2886–2890. [PubMed] [Google Scholar]
- Romanov SR, Kozakiewicz BK, Holst CR, Stampfer MR, Haupt LM, Tlsty TD. Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature. 2001;409:633–637. doi: 10.1038/35054579. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Kumazaki T, Takano H, Nishiyama M, Mitsui Y. Senescent cells are resistant to death despite low Bcl-2 level. Mech Ageing Dev. 2001;122:1695–1706. doi: 10.1016/s0047-6374(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Schuyer M, Berns EM. Is TP53 dysfunction required for BRCA1-associated carcinogenesis? Mol Cell Endocrinol. 1999;155:143–152. doi: 10.1016/s0303-7207(99)00117-3. [DOI] [PubMed] [Google Scholar]
- Scully R, Livingston DM. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature. 2000;408:429–432. doi: 10.1038/35044000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston DM. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. p53: Good cop/bad cop. Cell. 2002;110:9–12. doi: 10.1016/s0092-8674(02)00818-8. [DOI] [PubMed] [Google Scholar]
- Shen SX, Weaver Z, Xu X, Li C, Weinstein M, Chen L, Guan XY, Ried T, Deng CX. A targeted disruption of the murine Brca1 gene causes γ-irradiation hypersensitivity and genetic instability. Oncogene. 1998;17:3115–3124. doi: 10.1038/sj.onc.1202243. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, DePinho RA. Cellular senescence: Mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Vogel H, Lim DS, Karsenty G, Finegold M, Hasty P. Deletion of Ku86 causes early onset of senescence in mice. Proc Natl Acad Sci. 1999;96:10770–10775. doi: 10.1073/pnas.96.19.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: A model for senescence? Exp Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D. Cellular senescence and cancer. J Pathol. 1999;187:100–111. doi: 10.1002/(SICI)1096-9896(199901)187:1<100::AID-PATH236>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999a;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- Xu X, Weaver Z, Linke SP, Li C, Gotay J, Wang XW, Harris CC, Ried T, Deng CX. Centrosome amplification and a defective G2–M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999b;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- Xu X, Qiao W, Linke SP, Cao L, Li WM, Furth PA, Harris CC, Deng CX. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat Genet. 2001;28:266–271. doi: 10.1038/90108. [DOI] [PubMed] [Google Scholar]
- Zheng L, Li S, Boyer TG, Lee WH. Lessons learned from BRCA1 and BRCA2. Oncogene. 2000;19:6159–6175. doi: 10.1038/sj.onc.1203968. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Boyer TG, Chen PL, Lee WH. Deficient nonhomologous end-joining activity in cell-free extracts from Brca1-null fibroblasts. Cancer Res. 2002;62:3966–3970. [PubMed] [Google Scholar]
- Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes & Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, Roussel MF. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes & Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]