Abstract
The differentiation of Drosophila blood cells relies on a functional hierarchy between the GATA protein, Serpent (Srp), and multiple lineage-specific transcription factors, such as the AML1-like protein, Lozenge (Lz). Two major branches of Drosophila hematopoiesis give rise to plasmatocytes/macrophages and crystal cells. Serrate signaling through the Notch pathway is critical in the regulation of Lz expression and the specification of crystal cell precursors, thus providing a key distinction between the two lineages. The expression of Serrate marks a discrete cluster of cells in the lymph gland, a signaling center, with functional similarities to stromal signaling in mammalian hematopoiesis.
Keywords: Serrate, Notch, Su(H), crystal cell, Drosophila, hematopoiesis
Runx1, also known as Acute Myeloid Leukemia 1 (AML1), is essential for the development of blood cells arising from all lineages of definitive hematopoiesis in mice (Okuda et al. 1996; Wang et al. 1996). In humans, AML1 is the most frequent target for translocations resulting in acute myeloid leukemias (Werner et al. 1999). The family of GATA transcription factors is also used reiteratively in multiple stages of blood development (Orkin 1998), and a cofactor for the GATA proteins, FOG1, is required for erythropoiesis and differentiation of megakaryocytes (Tsang et al. 1998). In studying the regulation of such transcription factors, in vitro differentiation assays have suggested that multiple signaling pathways are coordinately involved in hematopoiesis (Van Den Berg et al. 1998; Yoshida et al. 1998; Milner and Bigas 1999). Unfortunately, there is a paucity of in vivo loss-of-function data involving signaling pathways that control hematopoiesis in mammals due to difficulties such as pleiotropy, redundancy, and early lethality.
There are significant differences with regard to the variety and function of Drosophila hemocytes relative to mammalian blood cells, however, several molecular aspects of early hematopoiesis and immunity have been evolutionarily conserved (Rehorn et al. 1996; Dearolf 1998; Hoffmann et al. 1999; Lebestky et al. 2000). Two major classes of Drosophila hemocytes are plasmatocytes, which can function as macrophages that phagocytose invading pathogens and debris from apoptotic cells (Tepass et al. 1994), and crystal cells that are involved in the melanization of pathogens (Rizki et al. 1980). serpent (srp), a Drosophila GATA homolog, is expressed in all hemocyte precursors and is required for the development of both classes of hemocytes (Rehorn et al. 1996; Lebestky et al. 2000). lozenge (lz) encodes an AML1/Runt domain family transcription factor (Daga et al. 1996) that is expressed in the crystal cell precursors and is required for the specification of this lineage (Rizki and Rizki 1981; Lebestky et al. 2000). glial cells missing (gcm), a novel transcription factor expressed exclusively in plasmatocytes in the embryo (Bernardoni et al. 1997), is required for their development. Its possible role in larval hematopoiesis is less clear. Srp is essential for the expression of both lz and gcm (Bernardoni et al. 1997; Lebestky et al. 2000), creating a hierarchy of transcription factors controlling the two major branches of hematopoiesis (Lebestky et al. 2000). u-shaped (ush), a FOG homolog, also functions in Drosophila hematopoiesis (Fossett et al. 2001). The molecular similarities between Srp/Ush/Lz and GATA/FOG/AML1 suggest that aspects of molecular mechanisms of blood development are shared between mammals and Drosophila. Previous studies in Drosophila have shown a role for JAK/STAT and Toll pathways in the proliferation of hemocytes and immunity (Dearolf 1998; Mathey-Prevot and Perrimon 1998; Qiu et al. 1998). However, no signaling pathway was known to specify a commitment that distinguishes between lineages.
In this study, we show that localized Notch signaling causes an important early distinction between the crystal cell and plasmatocyte lineages. The Notch pathway controls binary cell fate decisions among undetermined precursor cells in a multitude of developmental systems (Artavanis-Tsakonas et al. 1999). We also find that a signaling center expressing the ligand Serrate is important for differentiation and proliferation of larval hemocytes in Drosophila.
Results and Discussion
Notch and Su(H) are required for hemocyte proliferation and development
Morphological and molecular differentiation of hemocytes can be monitored in the larval lymph gland (Shrestha and Gateff 1982). Srp is expressed in all hemocyte progenitors, whereas the expression of Lz is largely restricted to a small subset of hemocytes in the first pair (anterior-most) lobes of the lymph gland (Fig. 1A). In the Notch temperature-sensitive allele, Nts1, Lz expression is eliminated from the lymph gland at the nonpermissive temperature (Fig. 1B). Lz+ cells are also missing in the lymph glands of Su(H)SF8/Su(H)AR9 larvae (Fig. 1C). As Su(H) is the transcription factor that activates Notch target genes (for review, see Weinmaster 2000), the canonical Notch/Su(H) pathway is important for Lz expression in crystal cell precursors.
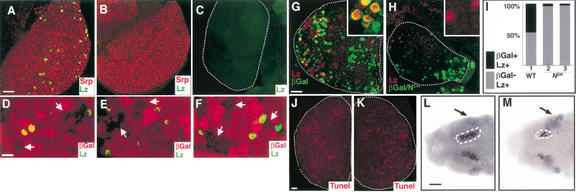
Figure 1.
Notch signaling in hematopoiesis. (A) Wild-type first lymph gland lobe from a third instar larva raised at 29°C and stained with antibodies against Lz (green) and Serpent (red). Lz is expressed in a distinct subset of Srp+ hemocytes. (B) Nts1 raised at 29°C and stained as in A. Lz protein is not expressed, but Srp (red) expression remains unaffected. (C) Su(H)SF8/Su(H)AR9 lymph gland stained with Lz antibody. Lz protein is not expressed. (D–F) hsp70-flp/FRT clones (arrows) of Notch pathway members are marked by the absence of βGal expression (red). Lz expressing cells (green) are excluded from mutant clones of N55e11 (D), Nts1 (E), and Su(H)Δ47 (F). (G) hsp70-flp; Ay-Gal4, UAS-βGal/+. In this control “flp-out” experiment (Ito et al. 1997), βGal-expressing clones were induced in the first larval instar and analyzed in the third instar larval lymph gland. Nuclear βGal (green) marks cells in which Gal4 is expressed. Many examples of colocalization of Lz and βGal can be seen (inset). (H) hsp70-flp; Ay-Gal4, UAS-βGal/UAS-NDN. In this genotype, βGal+ cells (green) also express NDN. Lz (red) and NDN (green) are mutually exclusive (inset), suggesting a requirement for Notch in the development of Lz+ cells. Additionally, the lymph gland size is reduced relative to wild-type controls. (I) Cell counts from the Notch clonal analysis described as in G and H. For wild-type clones (histogram 1), 20 lymph gland lobes were counted and of 1652 total Lz+ cells, 768 were also βGal+. NDN clones were either counted from equivalent number of lymph gland lobes (histogram 2) or equivalent number of Lz+ cells (histogram 3). From 20 lymph gland lobes counted (histogram 2), of 153 total Lz+ cells, 148 did not express βGal and, therefore, NDN. Similarly, of 1370 total Lz+ cells counted from 68 lymph gland lobes (histogram 3), only 55 expressed βGal. In either case, ∼96% of the Lz+ cells did not express βGal. (J) hsp70-flp; Ay-Gal4, UAS-βGal/+. Experimental conditions are the same as in G. Tunel staining (red) marks apoptotic cells. (K) hsp70-flp; Ay-Gal4, UAS-βGal/UAS-NDN. Experimental conditions are the same as in H. Tunel staining (red) is similar to wild type (J). (L) Dorsal view of a wild-type stage 12 embryo. Anterior is to the left. Expression of Lz protein is observed in crystal cell precursors (circled) of the head mesoderm. Additional ectodermal expression of Lz (arrow) is unrelated to its role in hematopoiesis (Lebestky et al. 2000). Cell counts were performed for 30 bilateral clusters of Lz+ cells. (M) Dorsal view of a N55e11 stage 12 embryo. Fewer Lz expressing crystal cell precursors (circled) are seen. In contrast, Lz expression in the ectoderm is expanded (arrow), due to the neurogenic phenotype of Notch, unrelated to its role in hematopoiesis. In C, G–H, and J–K, the dotted line marks the outline of the lymph gland lobe. Bars: A–C,G–H,J–M, 25 μm; D–F, 10 μm.
Using the FLP/FRT system (Golic 1991), we generated mutant clones of N55e11 (Fig. 1D), Nts1 (Fig. 1E), and Su(H)Δ47 (Fig. 1F) in the lymph gland. Clones were marked by the absence of βGal expression and were always small, reflecting an early requirement for the Notch pathway in cell proliferation. Importantly, Lz+ cells were always excluded from the mutant clones. Approximately 200 Lz+ cells were counted for each genotype. We also generated positively marked cells within the lymph glands that express an extracellular, dominant negative form of Notch (NDN; Rebay et al. 1993). As in the loss-of-function clones, marked βGal+ cells expressing NDN do not coexpress Lz (Fig. 1G,H). Therefore, Lz+ cells are derived preferentially from wild-type precursors, once again suggesting a requirement for Notch in the development of crystal cell precursors. Rare exceptions to this rule have been seen (Fig. 1I), presumably reflecting minor variability in the level of expression of UAS-NDN in individual βGal+ cells. Additionally, lymph glands that misexpress NDN are smaller than wild-type controls and fewer βGal+ cells are seen. This is not a result of increased apoptosis, as the fraction of cells that stain with TUNEL is not increased in NDN lymph glands compared with controls (Fig. 1J,K). Taken together, the above genetic analyses establish that Notch signaling is critical for the specification of the crystal cell lineage, but also has an early role in the proliferation of hematopoietic cells in the lymph gland, similar to the proliferative role of Notch in imaginal discs (Go et al. 1998; Artavanis-Tsakonas et al. 1999).
Notch function is also required for crystal cell development in the head mesoderm region during embryogenesis. The average number of Lz+ crystal cell precursors in each bilateral cluster is reduced significantly in N55e11 embryos [from 18 in wild-type to 9 in mutant stage 12 embryos (n = 30); Fig. 1L,M]. The residual crystal cell development in N55e11 embryos likely reflects the maternal contribution of Notch (Morel and Schweisguth 2000).
Serrate is the ligand for Notch in hematopoiesis
To identify the Notch ligand responsible for the development of crystal cells, we investigated the possible role of the two Notch ligands, Delta (Dl) and Serrate (Ser) in larval hematopoiesis. Expression of Dl protein was not detected in the lymph gland, and expression of Lz was not altered in the DlM2/DlR9 genetic background (data not shown). In contrast, Lz expression was absent in lymph glands of SerBd3/+ larvae (Fig. 2A,B), and as a consequence, significantly fewer circulating crystal cells were observed (Fig. 2C,D). As SerBd3 is a dominant-negative allele, we used a null allele, SerRx82, to generate loss-of-function clones. Mutant clones usually did not contain Lz-expressing cells (Fig. 2E). However, consistent with Ser being required in signaling cells, rare examples of Ser−/Ser− mutant cells at the edge of a clone were found to express Lz (Fig. 2F, arrowhead). These results establish Ser as a ligand for Notch in the specification of crystal cells.
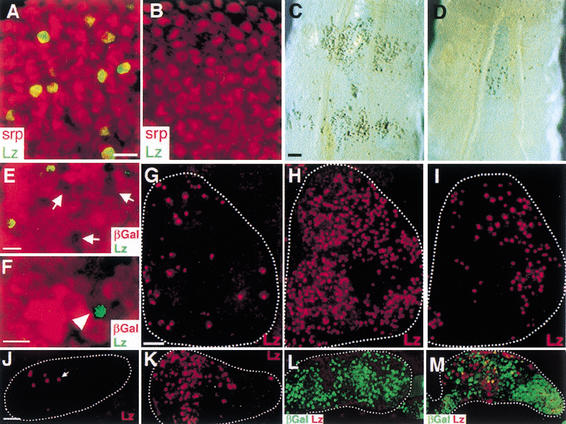
Figure 2.
Serrate/Notch signaling is necessary and sufficient for crystal cell development. (A) Wild-type first lymph gland lobe from a third instar larva. Lz protein (green) is expressed in a subset of Srp+ hemocyte precursors (red). (B) SerBd3/+. Lz protein is absent, but Srp (red) is unaffected. (C) Whole-mount third instar Bc/+ larva. Crystal cells in circulation and in sessile populations along the dorsal body wall are visible due to precocious melanization in the Black cells (Bc/+) genotype. (D) Bc/+; SerBd3/+. Mature crystal cell development is largely suppressed by the dominant Ser mutation. (E–F) hsp70-flp/FRT clones of SerRx82. Clones (arrow) are marked by the absence of βGal (red) expression. Lz+ cells (green) are generally excluded from mutant clones (E). However, rare examples (4 such cells seen of 92 Lz+ cells counted) of Ser−/Ser− cells at the edge of a mutant clone were found to express Lz (F, arrowhead). (G–M) Ectopic development of crystal cells upon misexpression of Nact or Ser. (G) Wild-type lymph gland stained for Lz protein (red). (H) hsp70-Gal4/UAS-Nact. Transient misexpression of Nact during the third larval instar causes a dramatic increase in the number of Lz+ cells. (I) hsp70-Gal4/+; UAS-Ser/+. Similar misexpression of Ser causes a more moderate increase in Lz+ cells. (J) Wild-type second lobe of the third instar lymph gland contains very few Lz+ cells (arrow). (K) hsp70-Gal4/UAS-Nact second lobe. A large number of Lz+ cells are seen upon ectopic activation of the Notch pathway. (L–M) Sustained misexpression of Ser can create ectopic signaling centers. (L) Second lobe from hsp70-flp; Ay-Gal4, UAS-βGal/+ lymph gland is outlined. Random flp-out clones express βGal (green), but no Lz+ cells are seen (red). (M) hsp70-flp; Ay-Gal4, UAS-βGal/+; UAS-Ser/+. In this genotype, βGal+ cells (green) also express Ser, causing ectopic development of Lz+ cells (red). Bars: A,B,E,F, 10 μm; C,D, 100 μm; G–M, 25 μm.
To investigate the sufficiency of the Notch pathway in crystal cell development, we used the Gal4/UAS system (Brand and Perrimon 1993) to ectopically express an activated form of Notch, Nact (Fortini et al. 1993), or Ser in the lymph glands (Fig. 2G–M). The number of Lz+ cells was dramatically increased in hsp70-Gal4/UAS-Nact lymph glands (Fig. 2G,H). A more modest increase in Lz+ cells was seen when Ser was overexpressed in hsp70-Gal4/UAS-Ser larvae (Fig. 2I). Strikingly, misexpression of Nact caused Lz+ cells to appear in the second lobe (Fig. 2K), where they are rarely seen in wild type (Fig. 2J). To create small clones of cells with sustained misexpression of Ser, we used the AyGal4 system (Ito et al. 1997). In contrast to the βGal control (Fig. 2L), the misexpression of Ser resulted in a dramatic increase in the number of crystal cell precursors differentiating in the second lobe (Fig. 2M). Thus, the misexpression of Ser can create ectopic signaling centers, which then induce Lz expression.
Serrate defines a signaling center
Expression of Ser was monitored using an antibody raised against the Ser protein and using a Ser-βGal reporter (Bachmann and Knust 1998; Fig. 3). Many Ser-expressing cells were found to be clustered at the posterior end. Additionally, scattered Ser+ cells were also seen throughout the gland (Fig. 3A). A high level of Ser-βGal expression is also observed in the cells at the posterior tip, immediately adjacent to a pericardial cell (Rugendorff et al. 1994; Fig. 3B–D). Given the unique pattern of Ser expression in this discrete cluster of cells, we designate this region as the Posterior Signaling Center (PSC). A number of Ser+ cells that are adjacent to the PSC appear to have broken off from the cluster (Fig. 3A,F, inset), suggesting that Ser+ cells may all be derived from the PSC and actively migrate into the body of the lymph gland to seed differentiation of the crystal cells. Future real-time cell imaging will be necessary to definitely establish cell migration patterns.
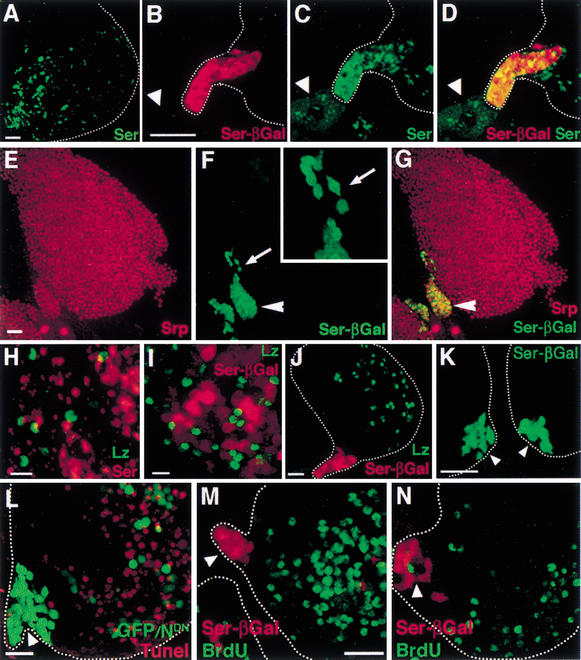
Figure 3.
Serrate expression defines a signaling center. (A) Ser protein-expressing cells are seen concentrated near the posterior end of the lymph gland (lower left corner). Additionally, dispersed cells throughout the gland also express Ser protein. (B–D) Identical flattened confocal images of the PSC region of Ser-βGal lymph gland showing Ser-βgal expression (red, B), Ser protein expression (green, C), and merged image (D). Ser protein expression and Ser-βGal colocalize in the Posterior Signaling Center (PSC) adjacent to a pericardial cell (arrowhead). (E–G) Identical flattened confocal images of a first lobe, stained for Srp (red, E), Ser-βGal (green, F), and merged (G). Ser-βGal-expressing cells colocalize with Srp in the PSC (arrowhead). (F, inset) Ser+ cells that appear to be migrating from the PSC (arrow). (H–I) The expression patterns of Lz protein (green) and Ser+ cells marked by either Ser protein (red, H), or Ser-βGal (red, I) are mutually exclusive. Multiple examples of Lz+ cells in close proximity to βGal+ cells can be seen. Visualization of the lower level of Ser-βGal expression in cells inside the lobe requires increased laser power on the confocal microscope, used here but not in E–G. (J) Expression of Lz (green) in the lymph gland is spatially distinct from the PSC marked by high-level expression of Ser-βGal (red). (K) Nts1; Ser-βGal/+. Ser expression (arrowheads) in the PSC is unaffected in the Nts1 mutant. Two bilaterally symmetric anterior lobes (arrowheads) are shown in this panel. (L) hsp70-flp; Ay-Gal4, UAS-GFP/UAS-NDN. Experimental conditions are the same as in Figure 1H. Cells expressing GFP (green) also express NDN and are preferentially observed in the PSC region (arrowhead). Apoptotic cells (red) are not observed in the PSC region. (M,N) Flattened confocal images of Ser-βGal expression (red) marking the PSC and BrdU incorporation (green) in a first lobe of third instar lymph gland. Ser-βGal and BrdU are mutually exclusive in the PSC region (arrowhead). BrdU was incorporated for either 1 h in vitro (M), or for 18 h in vivo (N). Bars: A–G,J–N, 25 μm; H–I, 10 μm.
Ser+ cells are seen at the PSC, but many are also found inside the gland. In either case, there is no overlap between cells that express Lz and Ser (Fig. 3H,I). As Ser function is critical for the development of Lz+ cells, these results suggest that cell–cell interaction between the two subpopulations is needed for crystal cell development. Consistent with this notion, many examples of Lz+ cells that are immediately adjacent to Ser+ cells were seen (Fig. 3H,I), suggestive of an inductive relationship between these two subpopulations. When Ser mutant clones were generated in the lymph gland (Fig. 2E,F), we frequently found lymph gland lobes that entirely lack Lz+ cells, although the entire gland was not mutant. This further supports the hypothesis that Ser+ cells are clustered initially to form a signaling center and later disperse and participate in the induction of Lz+ cells.
As with all the other cells in the lymph gland, the PSC cells express Serpent (GATA; Fig. 3E–G). The expression of Ser is the first observed indication that distinguishes the PSC cells from the remainder of the Srp+ progenitors within the lymph gland. Additionally, the PSC displays a number of interesting characteristics that distinguish this region from the rest of the lymph gland. First, although Ser is required for the expression of Lz, the PSC is spatially distant from the region in which the majority of crystal cells differentiate (Fig. 3J). Second, Ser expression in the PSC is unaffected in Nts1 mutants raised at the nonpermissive temperature (Fig. 3K). Also, in creating clones of NDN in the lymph gland (Fig. 1H), the majority of cells that express NDN are found in the PSC region, and these cells do not apoptose compared with cells in the rest of the lymph gland (Fig. 3L). These results suggest that although the Ser+ cells of the PSC signal through the Notch pathway, they themselves are refractory to the Notch signal and do not require Notch for their own development. Finally, the Ser+ cells of the PSC do not incorporate BrdU administered in vitro for 1 h (Fig. 3M) or through overnight (18 h) feeding of third instar larvae (Fig. 3N), unlike the majority of cells within the lymph gland that actively proliferate in the third instar (Fig. 3M,N). Thus, the Ser+ cells of the PSC represent a distinct signaling cell population that rarely proliferates, but is important for proliferation of hemocyte precursors and for their differentiation into crystal cells.
We next investigated the temporal relationship between Notch signaling and Lz expression. Ser is expressed robustly in the second instar larval lymph gland (Fig. 4A), however, very few Lz+ cells, if any, are seen at this stage (Lebestky et al. 2000). Whereas Lz is spatially restricted to the first lobe of the lymph gland, Ser is also seen in further posterior lobes (Fig. 4B). Consistent with these temporal and spatial relationships, expression of Ser is independent of lz (Fig. 4C). The expression of a 12XSu(H)–βGal reporter line (Go et al. 1998) can be used as a read-out for Notch signaling. Upon activation of the Notch pathway, the Su(H) protein causes expression of βGal. In the first lobe of the lymph gland, a small number of cells that receive Notch signal are marked by βGal expression (Fig. 4D). This expression of βGal is dependent on Notch (Fig. 4E), but is unaffected in lz null mutants (Fig. 4F). This further substantiates the fact that Notch activation is upstream of Lz function. When lymph glands expressing 12XSu(H)–βGal were stained with the α-Lz antibody (Fig. 4G–I), many examples of βGal+ cells that also express Lz protein are seen (Fig. 4I), further supporting that signaling by Notch is important for cells to become Lz+. However, cells that express βGal, but not Lz, and those that express Lz alone are also seen. The simplest explanation for this pattern is that a cell receiving a Notch signal is a prohemocyte. As a cell initiates Lz expression, it seems refractory to the Notch signal but the perdurance of the stable βGal protein is still evident. Finally, as this cell initiates a program for crystal cell differentiation, it continues to express Lz. Mature crystal cells in circulation have been shown to be Lz+ (Lebestky et al. 2000). Whether Drosophila hemocytes undergo maturation as in mammals, can be tested once suitable markers are identified.
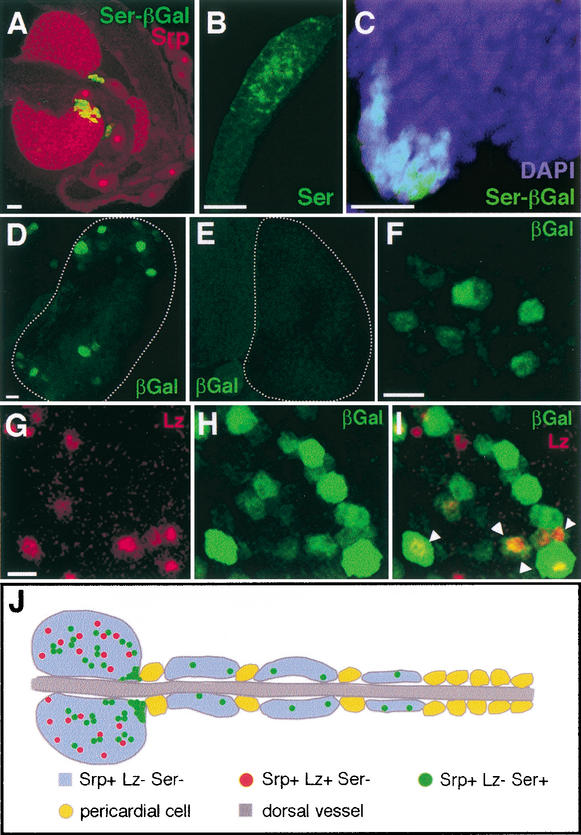
Figure 4.
Activation of the Notch pathway precedes Lz expression. (A) Ser-βGal (green) and Srp (red) expression in second instar larval lymph glands. (B) Ser protein expression is also observed within the smaller posterior lymph gland of a third instar larva. Lz is rarely expressed (Fig. 2J). (C) lzr15; Ser-βGal/+. Ser expression (green) is unaffected in a lz null mutant background. DAPI (blue) marks nuclei within the lymph gland. (D–E) Nts1; 12XSu(H)–βGal/+ first lobe from third instar lymph gland. The 12XSu(H)–βGal transgene marks cells receiving the Notch signal (Go et al. 1998). (E) When raised at 29°C, the nonpermissive temperature, βGal is no longer expressed. (F) lzr15; 12XSu(H)–βGal/+. βGal expression (green) is maintained in lz null mutant background. (G–I) 12XSu(H)–βGal. Identical confocal images of a third instar lymph gland stained for Lz protein (red, G), βGal (green, H), and merged (I). (I) Arrowheads point to cells that coexpress βGal and Lz (see text for details). (J) Schematic representation of third instar larval lymph gland. Four to six pairs of lymph gland lobes along the dorsal vessel (heart) are separated by pericardial cells. Srp is expressed in hemocyte precursors in lymph gland lobes and also in pericardial cells. Lz is expressed in crystal cell precursors in the first lobe and rarely, if at all, in the further posterior lobes. Ser is expressed at the PSC and also inside the first lobes and other posterior lobes. Bars: A–E, 25 μm; F–I, 10 μm.
Previous studies have shown that a hierarchy of transcription factors is responsible for the differentiation of two independent classes of hemocytes (Lebestky et al. 2000). Results presented here demonstrate that Notch signaling among Srp+ hemocyte precursors directly or indirectly regulates the expression of Lz. This signaling creates a molecular distinction between the two major branches of hematopoietic lineage and results in the differentiation of crystal cells (Fig. 4J).
The expression and function of Ser in the lymph gland marks a putative signaling center that highlights certain developmental and molecular similarities between hematopoietic tissues of Drosophila and mammals. The existence of discrete hematopoietic microenvironments within the bone marrow, in which stromal cells influence the proliferation and differentiation of the HSCs has been proposed in many mammalian studies (Bianco et al. 2001). In fact, stromal signaling via the mammalian Ser homolog, Jagged1, has been implicated in the expansion and self-renewal of HSCs (Varnum-Finney et al. 1998). However, in vivo identification and characterization of such signaling microenvironments within the bone marrow is difficult to achieve. Although Drosophila hemocytes are quite distinct from mammalian blood cells, spatial relationships with regards to signaling pathways in the Drosophila lymph gland will lead to a better understanding of how similar patterning events affect HSC populations in the marrow.
In mice, AML1/Runx1 is expressed in the embryonic dorsal aorta in hematopoietic clusters associated with an endothelial layer (North et al. 1999). The Notch ligand, Jagged1, is also expressed in the embryonic aorta at this stage (Loomes et al. 1999), but any possible role it may have in the generation of hematopoietic clusters has not been investigated. Furthermore, independent studies have implicated both Notch and AML1 in the progressive commitment and maturation of T cells (Aster and Pear 2001; Reizis and Leder 2002). The regulatory relationship between Notch and lz in Drosophila suggests similar interactions may exist between the Notch pathway and AML1 in mammalian hematopoiesis. Investigating such regulatory relationships is especially valuable, as AML1 is critically needed for all definitive hematopoiesis and is also the most frequent target in acute myeloid leukemias (Downing et al. 2000).
Materials and methods
Flp-out clones expressing UAS-NDN or UAS-Ser were generated using the Ay-Gal4 system (Ito et al. 1997) and marked with either UAS-nuclear βGal or UAS-GFP. To activate hsp70-flp, progeny were maintained at 18°C until the first larval instar, heat-shocked at 37°C for 40 min, and then returned to 18°C until the dissection. hsp70-Gal4/UAS-Nact or hsp70-Gal4/+ UAS-Ser/+ were maintained at 18°C until early third instar and heat-shocked twice at 37°C for 40 min, then returned to 18°C until dissection. N55e11, Nts1, Su(H)Δ47, or SerRx82 mutant clones were generated using the hsp70-FLP/FRT system (Golic 1991). Crosses were maintained at 18°C, heat-shocked for 1 h in the second instar for N55e11 and Su(H)Δ47, and in the first instar for Nts1 and SerRx82. For Figure 1, Nts1 was raised at 29°C either from first instar until third instar, or for 18 h in the early third instar before dissection in the third instar, in both cases showing loss of Lz+ cells.
Acknowledgments
We thank S. Artavanis-Tsakonas, J. Posakony, F. Schweisguth, Y. Hiromi, K. Irvine, E. Knust, and K. Matthews of the Bloomington Stock Center for providing fly stocks; R. Reuter and K. Irvine for generously providing antibodies; and Volker Hartenstein and members of the Banerjee laboratory for suggestions and comments. This work was supported by an NIH grant (RO1 HL67395) to U.B. and the U.S. Public Health Service NRSA (GMO7185) to T.L.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Note added in proof
Duvic et al. (2002) have also recently studied the role of Ser and Notch in hematopoiesis.
Footnotes
E-MAIL banerjee@mbi.ucla.edu; FAX (310) 206-9062.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1052803.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Aster JC, Pear WS. Notch signaling in leukemia. Curr Opin Hematol. 2001;8:237–244. doi: 10.1097/00062752-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Bachmann A, Knust E. Dissection of cis-regulatory elements of the Drosophila gene Serrate. Dev Genes Evol. 1998;208:346–351. doi: 10.1007/s004270050190. [DOI] [PubMed] [Google Scholar]
- Bernardoni R, Vivancos B, Giangrande A. glide/gcm is expressed and required in the scavenger cell lineage. Dev Biol. 1997;191:118–130. doi: 10.1006/dbio.1997.8702. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Daga A, Karlovich CA, Dumstrei K, Banerjee U. Patterning of cells in the Drosophila eye by Lozenge, which shares homologous domains with AML1. Genes & Dev. 1996;10:1194–1205. doi: 10.1101/gad.10.10.1194. [DOI] [PubMed] [Google Scholar]
- Dearolf CR. Fruit fly ‘leukemia’. Biochim Biophys Acta. 1998;1377:M13–M23. doi: 10.1016/s0304-419x(97)00031-0. [DOI] [PubMed] [Google Scholar]
- Downing JR, Higuchi M, Lenny N, Yeoh AE. Alterations of the AML1 transcription factor in human leukemia. Semin Cell Dev Biol. 2000;11:347–360. doi: 10.1006/scdb.2000.0183. [DOI] [PubMed] [Google Scholar]
- Duvic B, Hoffmann JA, Meister M, Royet J. Notch signaling controls lineage specification during Drosophila larval hematopoiesis. Curr Biol. 2002;12:1923–1927. doi: 10.1016/s0960-9822(02)01297-6. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Rebay I, Caron LA, Artavanis-Tsakonas S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature. 1993;365:555–557. doi: 10.1038/365555a0. [DOI] [PubMed] [Google Scholar]
- Fossett N, Tevosian SG, Gajewski K, Zhang Q, Orkin SH, Schulz RA. The Friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc Natl Acad Sci. 2001;98:7342–7347. doi: 10.1073/pnas.131215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go MJ, Eastman DS, Artavanis-Tsakonas S. Cell proliferation control by Notch signaling in Drosophila development. Development. 1998;125:2031–2040. doi: 10.1242/dev.125.11.2031. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- Loomes KM, Underkoffler LA, Morabito J, Gottlieb S, Piccoli DA, Spinner NB, Baldwin HS, Oakey RJ. The expression of Jagged1 in the developing mammalian heart correlates with. Hum Mol Genet. 1999;8:2443–2449. doi: 10.1093/hmg/8.13.2443. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B, Perrimon N. Mammalian and Drosophila blood: JAK of all trades? Cell. 1998;92:697–700. doi: 10.1016/s0092-8674(00)81396-3. [DOI] [PubMed] [Google Scholar]
- Milner LA, Bigas A. Notch as a mediator of cell fate determination in hematopoiesis: Evidence and speculation. Blood. 1999;93:2431–2448. [PubMed] [Google Scholar]
- Morel V, Schweisguth F. Repression by suppressor of hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes & Dev. 2000;14:377–388. [PMC free article] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marín-Padilla M, Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Orkin SH. Embryonic stem cells and transgenic mice in the study of hematopoiesis. Int J Dev Biol. 1998;42:927–934. [PubMed] [Google Scholar]
- Qiu P, Pan PC, Govind S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development. 1998;125:1909–1920. doi: 10.1242/dev.125.10.1909. [DOI] [PubMed] [Google Scholar]
- Rebay I, Fehon RG, Artavanis-Tsakonas S. Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell. 1993;74:319–329. doi: 10.1016/0092-8674(93)90423-n. [DOI] [PubMed] [Google Scholar]
- Rehorn KP, Thelen H, Michelson AM, Reuter R. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development. 1996;122:4023–4031. doi: 10.1242/dev.122.12.4023. [DOI] [PubMed] [Google Scholar]
- Reizis B, Leder P. Direct induction of T lymphocyte-specific gene expression by the mammalian Notch signaling pathway. Genes & Dev. 2002;16:295–300. doi: 10.1101/gad.960702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki TM, Rizki RM. Alleles of lz as suppressors of the Bc-phene in Drosophila melanogaster. Genetics. 1981;97:s90. [Google Scholar]
- Rizki TM, Rizki RM, Grell EH. A mutant affecting the crystal cells in Drosophila melanogaster. Roux Arch dev Biol. 1980;188:91–99. doi: 10.1007/BF00848799. [DOI] [PubMed] [Google Scholar]
- Rugendorff A, Younossi-Hartenstein A, Hartenstein V. Embryonic origin and differentiation of the Drosophila heart. Roux Arch Dev Biol. 1994;203:266–280. doi: 10.1007/BF00360522. [DOI] [PubMed] [Google Scholar]
- Shrestha R, Gateff E. Ultrastructure and cytochemistry of the cell types in the larval hematopoietic organ and hemolymph of Drosophila melanogaster. Dev Growth Differ. 1982;24:65–82. doi: 10.1111/j.1440-169X.1982.00065.x. [DOI] [PubMed] [Google Scholar]
- Tepass U, Fessler LI, Aziz A, Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 1994;120:1829–1837. doi: 10.1242/dev.120.7.1829. [DOI] [PubMed] [Google Scholar]
- Tsang AP, Fujiwara Y, Hom DB, Orkin SH. Failure of megakaryopoiesis and arrested erythropoiesis in mice lacking the GATA-1 transcriptional cofactor FOG. Genes & Dev. 1998;12:1176–1188. doi: 10.1101/gad.12.8.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg DJ, Sharma AK, Bruno E, Hoffman R. Role of members of the Wnt gene family in human hematopoiesis. Blood. 1998;92:3189–3202. [PubMed] [Google Scholar]
- Varnum-Finney B, Purton LE, Yu M, Brashem-Stein C, Flowers D, Staats S, Moore KA, Le Roux I, Mann R, Gray G, et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood. 1998;91:4084–4091. [PubMed] [Google Scholar]
- Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmaster G. Notch signal transduction: A real rip and more. Curr Opin Genet Dev. 2000;10:363–369. doi: 10.1016/s0959-437x(00)00097-6. [DOI] [PubMed] [Google Scholar]
- Werner MH, Shigesada K, Ito Y. Runt domains take the lead in hematopoiesis and osteogenesis. Nat Med. 1999;5:1356–1357. doi: 10.1038/70920. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takakura N, Hirashima M, Kataoka H, Tsuchida K, Nishikawa S. Hematopoietic tissues, as a playground of receptor tyrosine kinases of the PDGF-receptor family. Dev Comp Immunol. 1998;22:321–332. doi: 10.1016/s0145-305x(98)00008-1. [DOI] [PubMed] [Google Scholar]