Abstract
In studies designed to understand the roles of P2 nucleotide receptors in differentiation of T lymphocytes, we observed a transient and protein synthesis-independent enhancement of mRNA expression for the G protein-coupled P2Y2 receptor in mouse thymocytes after the addition of steroid hormone or T cell receptor (TCR) crosslinking by anti-TCR mAb. Conversely, dexamethasone-induced increases in mRNA expression for the ligand-gated ion channel P2X1 receptor was detected in rat, but not mouse, thymocytes, raising questions about the previously suggested role of P2X1 receptors in thymocyte apoptosis. Flow cytometry analysis of thymocyte subsets excluded the possibility that the observed increases in P2Y2 receptor mRNA expression were due to the enrichment of steroid-treated cells with an P2Y2 mRNA-rich thymocyte subset. Triggering of TCR-mediated intracellular signaling pathways through crosslinking of TCR or by addition of phorbol ester and Ca2+ ionophore also resulted in the up-regulation of P2Y2, but not P2X1, receptor mRNA. It is proposed that the rapid increase of P2Y2 receptor mRNA expression could be a common early event in responses of T cells to different activating stimuli. Taken together with the recently discovered ability of nucleotide receptor-initiated signaling to antagonize or enhance the effects of TCR crosslinking or steroids on thymocytes, the observed rapid up-regulation of P2Y2 receptor mRNA expression may reflect an immediate early gene response where newly expressed cell surface nucleotide receptors provide regulatory feedback signaling from extracellular ATP in the T cell differentiation process.
Keywords: receptors purinergic, gene expression, T cell receptor, steroid hormones
The specificity of the T cell antigen receptor (TCR) for major histocompatibility complex peptides is developed during the tightly regulated “positive” and “negative” selection of thymocytes that results in the death of the majority of thymocytes (1, 2). Both TCR-dependent and -independent (e.g., steroid hormone activated) biochemical pathways (3–5) appear to be involved in the selection of thymocytes, but interactions between these pathways and their distinct second messengers in T lymphocytes have not been explored sufficiently.
In recent studies, we have investigated whether receptors for extracellular ATP (ATPe) and adenosine play roles in T cell differentiation, expansion, and effector functions by contributing to TCR- and steroid hormone-mediated effects (6). The effects of both ATP and adenosine were observed to be thymocyte subset-specific and could antagonize and/or complement TCR- and steroid hormone-induced signaling, apoptosis, and thymocyte differentiation in vitro (6).
Effects of ATP and adenosine have been shown to be mediated by subtypes of P2 nucleotide and P1 purinergic receptors, respectively, that have been implicated in a wide variety of physiological responses in different cell systems (7–10). The functional role of P2 nucleotide receptors for extracellular nucleotides in lymphocytes has not been elucidated but signaling, cell-permeabilizing, and apoptotic effects of ATPe on thymocytes, T-cells, and macrophages have been documented (11–15). The presence of ATPe in biological fluids has been demonstrated and, importantly, the activation of T cells with anti-TCR mAb leads to the accumulation of ATPe (16).
Thymocyte subset-specific and apoptosis-antagonizing effects of ATP and adenosine on T cells (ref. 6 and unpublished observations) in vitro suggest a possible physiological role for nucleotide receptors in T cell development and expansion. It has been hypothesized (6) that the expression of P2 receptors in thymocytes may be regulated in response to cell differentiation or other stimuli. The working hypothesis is based on the assumption that ATP (or adenosine) released from TCR-activated T cells (13) causes feedback regulation of TCR- and/or steroid-mediated processes through activation of P2 nucleotide (or P1 purinergic) receptors (6). Accordingly, the expression of P2 receptors in thymocytes may be regulated in response to cell differentiation or other stimuli. Our attempts to test this assumption by direct estimation of expression of P2 receptors on protein level using antireceptor Ab failed, but the recent availability of cDNA encoding both G protein-coupled P2Y (17, 18) and ligand-gated ion channel P2X receptor subtypes (19, 20) has facilitated the development of a direct and quantitative method for studying nucleotide receptor expression at the mRNA level. The studies presented here support our hypothesis and indicate that the pattern of expression of the P2Y2 receptor subtype in thymocytes follows that of immediate early response genes.
MATERIALS AND METHODS
Cells.
Mouse P2Y2 receptor cDNA was transfected into human astrocytoma (1321N1) cells as described (21), and P2Y2 receptor activity was assayed by monitoring changes in the concentration of cytoplasmic free calcium, ([Ca2+]i,) upon stimulation with extracellular nucleotides (see Fig. 1A). Mouse 2B4 T helper hybridoma cells were maintained in RPMI 1640 medium supplemented with 10% (vol/vol) fetal calf serum (Biofluids, Rockville, MD). Thymocytes were isolated from 6-week-old DBA/2 mice and were resuspended in culture-media supplemented with 5% (vol/vol) fetal calf serum and kept on ice until use.
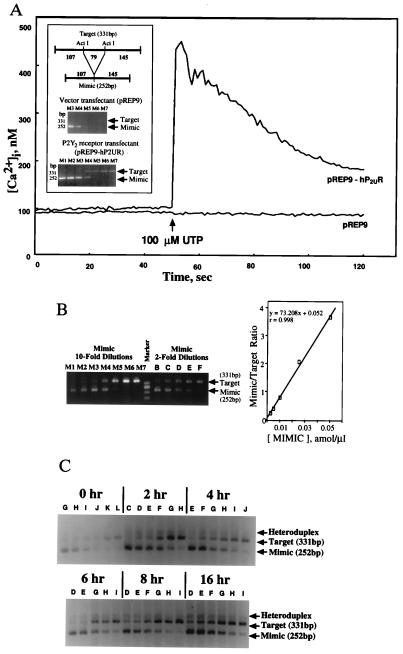
Figure 1.
Quantitative RT-PCR for P2Y2 receptor mRNA in transfectants and thymocytes. (A) UTP induces increases in [Ca2+]i in transfectants with P2Y2 receptor cDNA (pREP9-hP2UR) but not vector transfected controls (pREP9). Changes in [Ca2+]i were measured using the fluorescent Ca2+ indicator fura-2, as described (22). (Inset) Use of P2Y2 receptor transfectants as specificity controls in the development of competitive RT-PCR for P2Y2 receptors. Arrows indicate positions of target (331 bp) and mimic (252 bp) PCR products in pREP9-hP2UR and pREP9 transfectants. P2Y2 receptor mRNA was detected only in pREP9-hP2UR cells. (B) Development of competitive quantitative RT-PCR for P2Y2 receptor mRNA using pREP9-hP2UR transfectants. Equal amounts of target cDNA were amplified with different dilutions of known amounts of mimic DNA and the ratio of mimic band intensity to target band intensity was determined by densitometry, as described. Ten-fold serial dilutions of mimic were used to determine the approximate equivalent point (where mimic/target ratio = 1.0), followed by the more precise estimation with 2-fold serial dilutions of mimic. The mimic concentrations used here and the later figures were as follows: 10-fold series, M1 = 1 × 10 amol/μl, M2 = 1 amol/μl, M3 = 1 × 10−1 amol/μl, M4 = 1 × 10−2 amol/μl, M5 = 1 × 10−3 amol/μl, M6 = 1 × 10−4 amol/μl, M7 = 1 × 10−5 amol/μl; 2-fold series, A = 1 × 10−1 amol/μl, B = 5 × 10−2 amol/μl, C = 2.5 × 10−2 amol/μl, D = 1 × 10−2 amol/μl, E = 5 × 10−3 amol/μl, F = 2.5 × 10−3 amol/μl, G = 1 × 10−3 amol/μl, H = 5 × 10−4 amol/μl, I = 2.5 × 10−4 amol/μl, J = 1 × 10−4 amol/μl, K = 5 × 10−5 amol/μl, L = 2.5 × 10−5 amol/μl, M = 1 × 10−5 amol/μl. The linearity of the mimic/target ratio plot was confirmed and the concentration of the target cDNA was estimated by determining the concentration of mimic at the equivalent point. The positions of the target and mimic RT-PCR products are indicated by arrows. (C) Competitive RT-PCR for P2Y2 receptor mRNA in control, untreated thymocytes. Thymocytes were incubated at 37°C for the indicated times, RNA was extracted and quantitative RT-PCR was performed to determine levels of P2Y2 receptor mRNA expression in the absence of exogenously added apoptotic stimuli. DNA bands of mimic (252 bp), target (331 bp), and mimic-target heteroduplex are indicated by arrows.
Steroid Hormone and TCR-Triggered Activation of Thymocytes.
Thymocytes were incubated for the times indicated in the figures at 37°C in 5% CO2 with or without addition of 1 μM dexamethasone (DEX; Sigma), 50 μg/ml cycloheximide (CHX; Aldrich), DEX + CHX, or with immobilized anti-TCR-CD3 mAb as described below. For TCR activation studies, thymocytes (1 × 106) were added to 24-well plates (Costar) precoated with 250 μl of 10 μg/ml of anti-CD3 (2C11) mAb in PBS. For second messenger studies, thymocytes were incubated with 15 nM phorbol 12-myristate 13-acetate (PMA; Sigma) and/or 0.3 μM ionomycin (Calbiochem). The cells were incubated at 37°C in 5% CO2 for the times indicated in the figure legends.
Mouse T Cell P2Y2 Receptor cDNA Cloning.
A cDNA library was constructed from mouse 2B4 T cell mRNA and screened with the ORF sequence of P2Y2 receptor cDNA from murine NG-108 cells (ref. 17; kindly provided by K. Lustig and D. Julius) as a probe under high-stringency conditions. The corresponding mouse T cell P2Y2 receptor cDNA clone was isolated and sequenced using standard procedures (Sequenase Version 2.0 DNA sequence kit; United States Biochemical). The mouse T-cell P2Y2 receptor cDNA sequence, which we found shared the identical ORF sequence with that of NG108-15 cell P2Y2 receptor cDNA, was used to design PCR primers that were synthesized by Genosys (The Woodlands, TX).
Reverse Transcription PCR (RT-PCR) for P2Y2 and P2X1 Receptors.
Total RNA was prepared by the single-step method of Chomczynski and Sacchi (22) (RNA Zol B; Tel-Test, Friendswood, TX). The first strand cDNA was synthesized by SuperScript preamplification system (Life Technologies, Gaithersburg, MD) according to the manufacturer’s instruction. After RNase H treatment the reaction mixture was diluted to a final volume of 100 μl.
The mouse P2Y2 receptor sequence was amplified with primers corresponding to 74–93 and 404–389 nt of ORF of mouse T cell P2Y2 receptor cDNA yielding a 331-bp product.
The mouse P2X1 receptor sequence was amplified with primers corresponding to 413–434 and 914–895 nt of the ORF of rat vas deferens P2X1 receptor cDNA (20) yielding a 502-bp product. Mouse P2X1 cDNA has a C whereas rat P2X1 has a G nucleotide at position 903 which is situated at the 12th base of the reverse primer. This difference had no effect on PCR amplification of mouse P2X1 using this primer.
Determination of P2Y2 and P2X1 Receptor mRNA Expression by Competitive RT-PCR.
The RT-PCR product of the mouse P2Y2 receptor cDNA contained two AciI sites (see Fig. 1A Inset). Digestion of the 331-bp product by AciI gave 145-, 107-, and 79-bp fragments. The 107- and 145-bp fragments were ligated to give the 252-bp double-stranded internal standard DNA (mimic) with the same sequences as the 331-bp product on both ends. The mimics for quantitation of P2X1 receptor, β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA by RT-PCR were prepared using the PCR MIMIC Construction Kit (CLONTECH) according to the manufacturer’s instructions. The amplification efficiency of each mimic sequence did not show any significant difference from that of the corresponding target cDNA sequence (data not shown).
The competitive PCR was performed under standard conditions in a 50-μl reaction volume that included 5 μl of the diluted cDNA and 2 μl of mimic DNA at the concentrations indicated in the figure legends. In some experiments, the total reaction volume was reduced to 30 μl with the amount of each component reduced accordingly. Ten microliters of PCR product was electrophoresed on a 3% (wt/vol) NuSieve 3:1 agarose gel (FMC) and examined by ethidium bromide staining. The density of each band was determined (imagequant Version 3.3, Molecular Dynamics) from the negative picture of the gel (#55 Positive-Negative 4×5 Instant Sheet Film, Polaroid, Cambridge, MA). The PCR cycle number was chosen to enable visualization of the PCR product in the agarose gel and to minimize heteroduplex formation. Previous studies have shown that heteroduplex formation is negligible up to 25 cycles of PCR (23) and in the present studies heteroduplex formation was insignificant up to 30 cycles. The data were corrected for heteroduplex formation according to the procedure described by McCulloch et al. (24).
The densities of RT-PCR products were normalized for differences in cDNA quantity between samples using PCR quantitation of β-actin or GAPDH mRNA. Mouse β-actin cDNA specific primers (Stratagen) were used to amplify a 245-bp sequence. Primers for mouse GAPDH cDNA amplification were 290–309 and 1010–989 of ORF (25). Mimics for β-actin and GAPDH mRNA gave the PCR products of 400 bp and 548 bp, respectively.
Quantitative determination of mRNA was done by estimating the equivalent point (where mimic/target PCR product ratio equals 1.0) in a competitive RT-PCR experiment with serial dilutions of mimic. To estimate the equivalent point, 10-fold serial dilutions of mimic DNA were used in preliminary PCR experiments followed by the precise estimation with 2-fold dilutions of mimic DNA. Semiquantitative competitive RT-PCR with several mimic concentrations was performed to determine the relative changes in P2Y2 or P2X1 receptor mRNA expression normalized to β-actin or GAPDH mRNA levels, as described above. The data presented below were representative of several experiments.
Flow Cytometry Analysis.
Flow cytometric analysis of thymocyte subsets was done as described (26) and quantitation of live, apoptotic, and dead thymocytes was done according to a modified flow cytometry procedure (27). Briefly, 0.5–1 million cells were incubated in 0.2 ml media in 96-well plates (Costar) for 16–18 h or as indicated. After the incubation, the cells were gently pipetted and transferred directly into polystyrene tubes (12 × 75 mm; Falcon/Becton Dickinson Labware), and 200 μl of buffer [PBS with 2% (vol/vol) fetal calf serum and 0.05% (wt/vol) sodium azide] were added to each sample. Propidium iodide solution was added to each tube for 10 sec before analysis. Duplicate or triplicate sampled were analyzed at the same flow rate. Live, dead, and apoptotic cells were enumerated by counting cell numbers in appropriate gates using forward/side scatter dot plot in linear scale and propidium iodide staining in log scale. Flow cytometry data acquisition and analyses were done on FACScan using cellquest programs (Becton Dickinson).
Separation of Thymocyte Subsets by Cell Sorting.
To enrich for target thymocyte subsets, thymocytes from the 6-week-old DBA/2 female mice were treated for 15 min with anti-mouse CD4 and/or CD8 antibodies (clone YTS169.4; Sera Lab/Accurate Chemical) followed by a 45-min incubation with rabbit complement at 37°C. Thymocytes were stained with phycoerythrin-conjugated anti-mouse CD4 antibodies and/or fluorescein isothiocyanate-conjugated anti-mouse CD8 antibody (PharMingen) and sorted at the Flow Cytometry Facility of the National Institute of Allergy and Infectious Diseases. The sorted cells were incubated with or without DEX and CHX for 2 h, as defined in the figure legends. Total RNA extraction from thymocyte subsets and subsequent cDNA synthesis were performed as described above.
RESULTS
Cloning of P2Y2 Receptor cDNA from Mouse T Cells.
Mouse 2B4 T helper hybridoma mRNA was used to construct a cDNA library from which T cell P2Y2 receptor cDNA was isolated by screening under high stringency conditions with a 1.2-kb DNA fragment containing NG-108 cell P2Y2 receptor cDNA ORF (17) as a probe. The complete identity of the ORF of the putative mouse T cell P2Y2 receptor cDNA with that of NG108-15 cell P2Y2 receptor cDNA was confirmed by DNA sequencing.
RT-PCR Detection of P2Y2 Receptor mRNA.
Quantitative RT-PCR was employed with cell transfectants expressing the recombinant P2Y2 receptor (pREP9-hP2UR) (21). The pREP9-hP2UR cells responded to both UTP and ATP [Fig. 1A, and Erb et al. (22)] by increasing [Ca2+]i, whereas vector (pREP9)-transfected cells were unresponsive (Fig. 1A). PCR data confirmed that a P2Y2 receptor cDNA fragment of the correct size (331 bp) was amplified in pREP9-hP2UR cells but not in pREP9 cells. Quantitation of the signal indicated that 8 × 104 cDNA molecules of P2Y2 receptor/μg total RNA were expressed in pREP9-hP2UR cells (Fig. 1B).
Freshly isolated thymocytes were found to express low levels of P2Y2 receptor mRNA as determined by RT-PCR (Fig. 1C, time zero), consistent with the inability of UTP to increase [Ca2+]i in thymocytes (28). However, up-regulation of P2Y2 receptor mRNA was observed after long-term (up to 16 h) incubation of thymocytes in the absence of either growth factors or P2Y2 receptor ligands (Fig. 1C), conditions that lead to “spontaneous” apoptosis in thymocytes.
Glucocorticoid-Induced Changes in P2Y2 Receptor mRNA Levels in Thymocytes.
Steroid hormone- and TCR-induced apoptosis is considered to be important in negative and positive selection of thymocytes. It has been suggested that direct effects of steroids or TCR ligands can lead to thymocyte death, whereas steroids and TCR ligands together induce antagonistic signaling pathways that promote thymocyte survival (4). We hypothesized that signaling through P2 nucleotide receptors can contribute to these processes (6), and therefore examined whether changes in expression levels of P2Y2 or P2X1 receptor mRNA occurred after stimulation of thymocytes with steroids (Figs. 2 and 3) and TCR ligands (see Fig. 5).
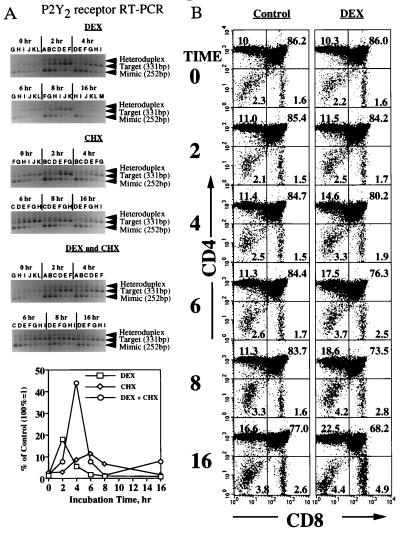
Figure 2.
Time course of DEX-induced changes in P2Y2 receptor mRNA expression and thymocyte subset composition. (A) Quantitative RT-PCR measurements of P2Y2 receptor mRNA levels in female DBA/2 mouse thymocytes. Thymocytes were incubated for the indicated lengths of time with DEX (1 μM), CHX (50 μg/ml), or DEX and CHX. At each time point P2Y2 receptor mRNA levels were determined by quantitative RT-PCR. DNA bands for mimic (252 bp), target (331 bp), and mimic-target heteroduplex are indicated by arrows. Mimic concentrations were described in Fig. 1. The data presented represent P2Y2 receptor mRNA levels in thymocytes relative to untreated thymocytes after correction for heteroduplex formation and normalization to levels of β-actin mRNA expression determined by semiquantitative RT-PCR (not shown), as described. (B) Flow cytometry analysis of DEX-mediated time course change in thymocyte subset composition. Thymocytes were incubated in the presence or absence of DEX for 0, 2, 4, 6, 8, or 16 h, and the proportion of different thymocyte subsets was determined by staining with anti-CD4 or anti-CD8 mAb, as described (26). Apoptotic cells were enumerated by propidium iodide staining and by forward and side scatter parameters. The percentage of each subset was shown.
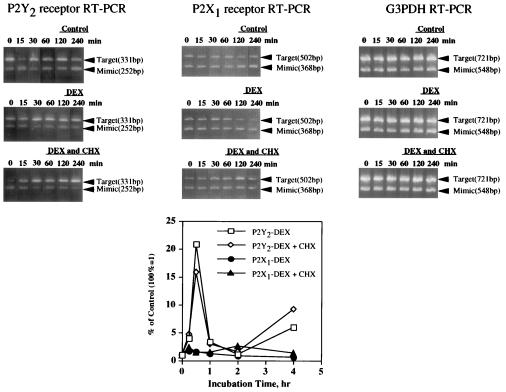
Figure 3.
Time course of DEX-induced changes in P2Y2 or P2X1 receptor mRNA expression. Semiquantitative RT-PCR measurements of P2Y2 or P2X1 receptor mRNA levels in male DBA/2 mouse thymocytes in response to DEX, CHX, or DEX and CHX. Experiments were performed as in Figs. 1C and 2A except that the data were normalized to GAPDH (G3PDH) mRNA expression, as described. Mimic concentrations shown here was G = 1 × 10−3 amol/μl for P2Y2 or P2X1 and M2 = 1 amol/μl for GAPDH.
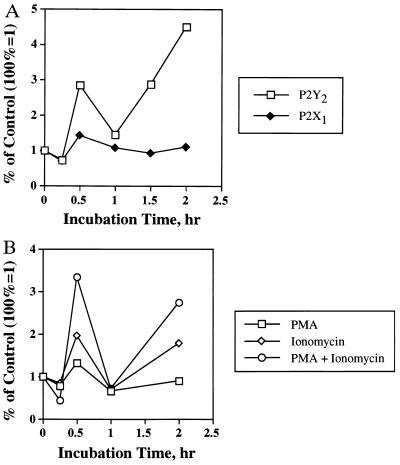
Figure 5.
TCR- or second messenger-mediated changes in P2Y2 or P2X1 receptor mRNA expression. (A) TCR-mediated changes in P2Y2 or P2X1 receptor mRNA expression. Thymocytes from 6-week-old male or female DBA/2 mice were incubated with immobilized anti-CD3 (2C11) mAb (10 μg/ml) for indicated lengths of time. P2Y2 or P2X1 receptor mRNA levels were determined by quantitative or semiquantitative RT-PCR. The RT-PCR results were corrected for heteroduplex formation and normalized to β-actin mRNA expression determined by semiquantitative RT-PCR, as described. Data shown here is a representative result from male mice. (B) Second messenger-mediated changes in P2Y2 receptor mRNA expression. Thymocytes from 6-week-old male DBA/2 mice were incubated for indicated lengths of time with 15 nM PMA and/or 0.3 μM ionomycin. P2Y2 receptor and GAPDH mRNA levels were determined by semiquantitative RT-PCR. The RT-PCR results were corrected for heteroduplex formation and normalized to GAPDH mRNA expression, as described.
The synthetic glucocorticoid DEX caused the transient up-regulation of P2Y2 receptor mRNA expression in thymocytes (Fig. 2A). The levels of P2Y2 receptor mRNA expression were normalized by correcting for DEX-induced changes in the expression of the “housekeeping” genes for β-actin or GAPDH. Normalization was important because the treatment of cells with DEX can induce DNA degradation associated with apoptosis (4). In parallel experiments, time-course flow cytometry indicated that DEX incubations as long as 4 h did not cause apoptosis-related changes in forward and light scatter and in propidium iodide staining of thymocytes (Fig. 2B). These results do not exclude the possibility that apoptosis-related DNA degradation occurred in the absence of early morphological apoptotic changes detectable by flow cytometry. To correct for possible apoptosis-related DNA degradation, we analyzed each RNA sample by semiquantitative RT-PCR for changes in levels of β-actin or GAPDH mRNA expression (Fig. 3, and data not shown) and for PCR heteroduplex formation (Figs. 1C, 2A, and 4). Expression levels of P2Y2 or P2X1 receptor mRNA were normalized to mRNA levels of β-actin (Figs. 2A and 4) or GAPDH (Figs. 3 and 5). Consistent with previous reports that GAPDH mRNA levels serve as a better internal control than β-actin mRNA levels (29), we found that GAPDH mRNA levels were more stable than β-actin mRNA levels in thymocytes incubated for up to 4 h with DEX (data not shown). β-Actin mRNA degradation was especially pronounced between 4 and 16 h after DEX addition, whereas no changes were detected in GAPDH mRNA expression after the first 4 h (Fig. 3, and data not shown).
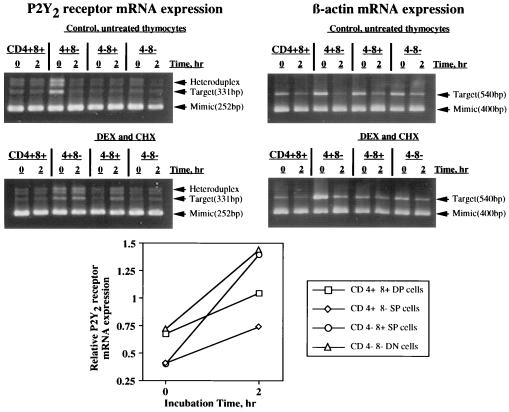
Figure 4.
Semiquantitative RT-PCR measurements of P2Y2 receptor mRNA levels in purified thymocyte subsets in response to DEX + CHX. CD4−CD8− (double negative, DN), CD4+CD8− (4+ SP, single positive), CD4−CD8+ (8+ SP), and CD4+CD8+ (double positive, DP) thymocyte subsets were separated by flow cytometry sorting and incubated in the presence or absence of DEX and CHX for 2 h followed by determination of P2Y2 receptor and β-actin mRNA change by competitive RT-PCR. The number and purity, respectively, of the thymocyte subsets were as follows: DP, 5.0 × 106, 99.3% pure; DN, 3.75 × 106, 99.5% pure; 4+ SP, 5.0 × 106, 99.5% pure, 8+ SP, 2.5 × 106, 99.2% pure. The data are corrected for heteroduplex formation and normalized for β-actin mRNA expression, as described.
The addition of DEX resulted in a strong but transient increase in P2Y2 receptor mRNA expression (Figs. 2A and 3) that was highly reproducible, although the time at which peak mRNA levels were obtained varied from 0.5 h to 6 h among thymocyte preparations from different individual mice.
The addition of the protein synthesis inhibitor CHX did not prevent DEX-induced up-regulation of P2Y2 receptor mRNA expression in thymocytes (Figs. 2A and 3). This suggests that the expression of P2Y2 receptor mRNA does not depend on protein synthesis and may be regulated by constitutively expressed transcription factors.
In contrast to findings with P2Y2 receptors, no significant changes were found in expression of P2X1 receptors mRNA in DEX-treated male mouse thymocytes (Fig. 3). This result differs from findings that DEX induces up-regulation in the expression of the RP-2 gene, a P2X1 receptor cDNA homolog, in rat thymocytes (30). Accordingly, dot blot analysis of P2X1 receptor mRNA expression confirmed that DEX-induced up-regulation of P2X1 receptor mRNA expression in rat thymocytes, but not in mouse thymocytes (data not shown), suggesting that these effects are species-specific. No data on P2Y2 receptor expression were reported so far.
Flow cytometry analysis of thymocytes using CD4 and CD8 surface markers was performed to determine whether DEX causes time-dependent changes in the proportion of thymocytes at different stages of cell differentiation. Results indicated that during the first 4 h of incubation (Fig. 2B), DEX caused no significant changes in the relative numbers of thymocyte subsets including CD4−CD8− double negative (DN), CD4+CD8+ double positive (DP), and more differentiated CD4+CD8− and CD4−CD8+ single positive (SP) thymocytes (2). These results exclude the possibility that the up-regulation of P2Y2 receptor mRNA in response to DEX was due to the enrichment of the total thymocyte population with an apoptosis-resistant, P2Y2 receptor mRNA-rich thymocyte subset.
P2Y2 receptor mRNA expression was detected in all highly purified thymocyte subsets immediately after separation by flow cytometry and P2Y2 receptor expression increased with time of incubation both in the presence and absence of DEX (Fig. 4), and also in control samples where sorted thymocytes were incubated alone.
Up-Regulation of P2Y2 Receptor mRNA Expression in Thymocytes in Response to Activation of TCR-Triggered Biochemical Signaling Pathways.
The possibility that changes in P2Y2 receptor mRNA expression in thymocytes was due to receptor-mediated signaling events was further explored. The rapid, transient, and protein synthesis-independent nature of the increases in P2Y2 mRNA expression seen in DEX-treated thymocytes (Figs. 2A and 3) suggests that P2Y2 receptors may belong to the immediate early genes family. This hypothesis is consistent with results indicating that increases in P2Y2 receptor mRNA occur in response to the activation of cell surface receptors (e.g., TCR) in thymocytes (Fig. 5A).
Activation of TCR on thymocytes with anti-CD3 (2C11) mAb caused a rapid (≈30 min) and transient increase in P2Y2, but not P2X1, receptor mRNA expression (Fig. 5A) that was not as pronounced as the response in DEX-treated thymocytes (Figs. 2A and 3). In addition, TCR activation leads to a secondary increase in P2Y2 receptor mRNA expression that had not peaked after 2 h of incubation of thymocytes with 2C11 mAb (Fig. 5A).
The TCR-mediated increase in P2Y2 receptor mRNA expression in thymocytes could be observed in response to receptor-independent activation of intracellular signaling pathways. The simultaneous addition to thymocytes of ionomycin to increase [Ca2+]i and PMA to activate protein kinase C (31) increased levels of P2Y2 receptor mRNA (Fig. 5B). Similar to the effect of TCR activation, ionomycin and PMA caused a biphasic increase in P2Y2 receptor mRNA expression with a transient peak after 30 min, followed by a secondary increase after 2 h (Fig. 5B). These results suggest that TCR-mediated signaling in thymocytes can up-regulate P2Y2 receptor expression.
DISCUSSION
The studies described above relate to the hypothesis that TCR-driven differentiation, expansion, and effector functions of T cells are affected by extracellular ATP- and adenosine-mediated signaling through P2 and P1 receptors, respectively (6). This hypothesis is supported by our recent demonstration that ATPe and adenosine acting through purinergic receptors can antagonize the apoptotic effects of DEX and T cell antigens, respectively, whereas ATPe and adenosine can induce apoptosis in T cells when added alone (ref. 6, and unpublished results).
The strong correlation found between nucleotide receptor-mediated signaling and the apoptosis-inducing effects of ATP and adenosine led to the expectation that the expression of nucleotide receptors should be tightly regulated by other apoptotic stimuli (e.g., TCR ligands and glucocorticoids) that have been implicated in thymocyte differentiation (6). Here we report that steroid hormones and TCR ligands, as well as agents such as ionomycin and PMA that directly activate intracellular signaling pathways, cause a rapid, transient, and protein synthesis-independent up-regulation of P2Y2 receptor mRNA expression in thymocytes (Figs. 2, 3, and 5). The effects of ionomycin and PMA are similar to those of T cell antigens that activate phospholipase C through TCR (31, 32), and ATP that activate phospholipase C through G protein-coupled P2Y receptors (33). The possibility that enrichment of a P2Y2 receptor mRNA-rich thymocyte subset due to the apoptotic death of P2Y2 receptor mRNA-poor thymocytes was excluded in experiments where individual thymocyte subsets were identified and quantitated by flow cytometry and immunostaining with anti-CD4 and anti-CD8 mAb. It was found that, in thymocytes treated with DEX and CHX for up to 4 h the increases in P2Y2 receptor mRNA expression did not correlate with changes in thymocyte subtypes (Fig. 2B) or with the number of live cells (data not shown).
The addition of the protein synthesis inhibitor cycloheximide did not decrease, and in some experiments increased, P2Y2 receptor mRNA expression (Figs. 2A and 3), suggesting that P2Y2 receptor gene transcription factors are constitutively expressed and regulated posttranslationally.
It was also found that no significant changes in the levels of P2X1 receptor mRNA were induced by DEX and CHX in mouse thymocytes (Fig. 2B). Owens et al. (30) have reported that DEX induces the protein synthesis-independent up-regulation of P2X1 receptor mRNA in rat thymocytes, similar to the effects of DEX on P2Y2 receptor mRNA expression in mouse thymocytes. The differences between the P2 receptor mRNA subtypes that are up-regulated in mice and rats in response to DEX may reflect similar functions for these molecularly distinct receptors in these closely related species. Because both rat and mouse thymocytes undergo DEX-induced apoptosis, but only rat thymocytes have increased expression of P2X1 receptor mRNA (Fig. 3) our data and the recent report (38) seem to refute the previously hypothesized role for P2X1 receptors in mediating apoptosis of thymocytes (19, 20).
Comparison of the time courses of DEX-induced increases in P2Y2 receptor mRNA expression with the apoptotic changes induced by DEX in thymocytes (Figs. 2 and 3, and unpublished results) strongly suggests that the transient up-regulation of P2Y2 receptor mRNA is a very early event that precedes the appearance of detectable apoptotic changes and cell death. The variability in the time course of P2Y2 receptor mRNA expression in DEX-treated thymocytes that was observed between individual mice may reflect the sensitivity of P2Y2 receptor mRNA expression to the hormonal status of animals at the time of thymocyte isolation and/or to the presence of extracellular signaling molecules in the incubation media.
The regulation of P2Y2 receptor mRNA expression shares common features with the regulation of the expression of immediate early genes (34): (i) expression of P2Y2 receptor mRNA is transcriptionally activated within 30 min after exogenous stimuli are applied, and (ii) expression of both immediate early genes (35) and P2Y2 receptor mRNA (Fig. 3) does not require de novo protein synthesis.
As with all immediate early genes, the expression of P2Y2 receptor mRNA is expected to be controlled by preexisting transcriptional factors. Also, the relatively short duration of the transient increase in P2Y2 receptor mRNA levels (Figs. 2, 3, 4, 5) is consistent with the time course of responses regulated by protein phosphorylation (36). Indeed, P2Y2 receptor mRNA expression in thymocytes is likely regulated by activation of T cell antigen receptors (Fig. 5A) that mobilize intracellular Ca2+, and by protein kinase C that can be activated by addition of phorbol esters (Fig. 5B, and ref. 37).
Taken together, these data point to a role for P2Y2 nucleotide receptor expression in the feedback regulation of apoptotic responses in activated T cells. We speculate that, in response to activating stimuli, thymocytes up-regulate P2Y2 and possibly other P2 nucleotide and P1 purinergic receptors, making the cells susceptible to the effects of extracellular ATP and adenosine. The ensuing P2/P1 receptor-mediated signaling events may regulate the expression of other genes as has been shown for other immediate early gene products (34). Responses to signals transmitted by activation of newly expressed nucleotide receptors may be additive or antagonistic to the effects of a primary ligand (e.g., TCR ligands or steroid hormones), thereby serving in the feedback regulation of T lymphocyte responses. Signaling through P2 receptors is predicted to be additive or synergistic to TCR-mediated responses in thymocytes, whereas signaling through A2A receptors, the dominant P1 adenosine receptor subtype in mouse thymocytes, is predicted to antagonize the effects of TCR activation (ref. 6; S.A., M.K. and M.V.S., unpublished data; M.K., S. Huang, and M.V.S., unpublished observations). Future studies are needed to determine whether ecto-ATPases and adenosine deaminases that may be differentially expressed in individual thymocyte subsets (6) play a role in the feedback regulation of TCR and steroid receptor-mediated responses by nucleotide receptors.
Acknowledgments
We are grateful to Dr.G. Buell (Glaxo, Geneva) for kindly providing sequence information for the mouse P2X1 receptor cDNA prior to publication. Anti-CD4 mAb and rabbit complement were kindly provided by Dr. H. Kojima (National Institute of Allergy and Infectious Diseases).
Footnotes
Abbreviations: TCR, T cell antigen receptor; DEX, dexamethasone; ATPe, extracellular ATP; CHX, cycloheximide; PMA, phorbol 12-myristate 13-acetate; RT-PCR, reverse transcription PCR; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
References
- 1.Surch C D, Sprent J. Nature (London) 1994;372:100–103. [Google Scholar]
- 2.Robey E, Fowlkes B J. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Scollay R. Nature (London) 1994;372:44–45. doi: 10.1038/372044a0. [DOI] [PubMed] [Google Scholar]
- 4.Vacchio M S, Papadopoulos V, Ashwell J D. J Exp Med. 1994;179:1835–46. doi: 10.1084/jem.179.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McConkey D J, Nicotera P, Orrenius S. Immunol Rev. 1994;142:343–63. doi: 10.1111/j.1600-065x.1994.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 6.Apasov S, Koshiba M, Redegeld F, Sitkovsky M. Immunol Rev. 1995;146:5–19. doi: 10.1111/j.1600-065x.1995.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 7.Abbracchio M P, Burnstock G. Pharmacol Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 8.Dubyak G R, El-Moatassim C. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 9.Boarder M R, Weisman G A, Turner J T, Wilkinson G F. Trends Pharmacol Sci. 1995;16:133–139. doi: 10.1016/s0165-6147(00)89001-x. [DOI] [PubMed] [Google Scholar]
- 10.Ramkumar V, Pierson G, Stiles G L. Prog Drug Res. 1988;32:195–247. doi: 10.1007/978-3-0348-9154-7_7. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Krishnaraj R, Kemp R G. J Immunol. 1985;135:3403–3410. [PubMed] [Google Scholar]
- 12.Zheng L M, Zychlinsky A, Liu C C, Ojcius D M, Young J D. J Cell Biol. 1991;112:279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filippini A, Taffs R E, Agui T, Sitkovsky M V. J Biol Chem. 1990;265:334–340. [PubMed] [Google Scholar]
- 14.Zanovello P, Bronte V, Rosato A, Pizzo P, DiVirgilio F. J Immunol. 1990;145:1545–1550. [PubMed] [Google Scholar]
- 15.Steinberg T, Newman A S, Swanson J A, Silverstein S C. J Biol Chem. 1987;262:8884–8888. [PubMed] [Google Scholar]
- 16.Filippini A, Taffs R E, Sitkovsky M V. Proc Natl Acad Sci USA. 1990;87:8267–8271. doi: 10.1073/pnas.87.21.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lustig K D, Shiau A K, Brake A J, Julius D. Proc Natl Acad Sci USA. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb T E, Simon J, Krishek B J, Bateson A N, Smart T G, King B F, Burnstock G, Barnard E A. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- 19.Brake A, Wagenbach M J, Julius D. Nature (London) 1994;371:519–524. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 20.Valera S, Hussy N, Evans R J, Adami N, North R A, Surprenant A, Buell G. Nature (London) 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 21.Erb L, Garrad R, Wang Y, Quinn T, Turner J T, Weisman G A. J Biol Chem. 1995;270:4185–4188. doi: 10.1074/jbc.270.9.4185. [DOI] [PubMed] [Google Scholar]
- 22.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.Schneeberger C, Speiser P, Kury F, Zeillinger R. PCR Methods Applic. 1995;4:234–238. doi: 10.1101/gr.4.4.234. [DOI] [PubMed] [Google Scholar]
- 24.McCulloch R, Choong S, Hurley D. PCR Methods Applic. 1995;4:219–226. doi: 10.1101/gr.4.4.219. [DOI] [PubMed] [Google Scholar]
- 25.Sabath D E, Broome H E, Prystowsky M B. Gene. 1990;91:185–191. doi: 10.1016/0378-1119(90)90087-8. [DOI] [PubMed] [Google Scholar]
- 26.Apasov S, Sitkovsky M. Proc Natl Acad Sci USA. 1993;90:2837–2841. doi: 10.1073/pnas.90.7.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz M A, Lassota P, Traganos F. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 28.Chused T, Apasov S, Sitkovsky M. J Immunol. 1996;157:1371–1380. [PubMed] [Google Scholar]
- 29.Inghirami G, Grignani F, Sternas L, Lombardi L, Knowles D M, Dall-Favera R. Science. 1990;250:682–686. doi: 10.1126/science.2237417. [DOI] [PubMed] [Google Scholar]
- 30.Owens G P, Hahn W E, Cohen J J. Mol Cell Biol. 1991;11:4177–4188. doi: 10.1128/mcb.11.8.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park D J, Rho H W, Rhee S G. Proc Natl Acad Sci USA. 1991;88:5453–5456. doi: 10.1073/pnas.88.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wingett D, Long A, Kelleher D, Magnuson N S. J Immunol. 1996;156:549–57. [PubMed] [Google Scholar]
- 33.Cowen D S, Baker B, Dubyak G R. J Biol Chem. 1990;265:16181–16189. [PubMed] [Google Scholar]
- 34.Hughes P, Dragunow M. Pharmacol Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- 35.Cochran B H, Reffel A C, Stiles C D. Cell. 1983;33:939–948. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- 36.Hughes P, Dragunow M. Pharmacol Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- 37.Berrebi G, Takayama H, Sitkovsky M V. Proc Natl Acad Sci USA. 1987;84:1364–1368. doi: 10.1073/pnas.84.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang S, Kull B, Fredholm B B, Orrenius S. Immunol Lett. 1996;49:197–201. doi: 10.1016/0165-2478(96)02507-2. [DOI] [PubMed] [Google Scholar]