Abstract
The T-box transcription factor Tpit was identified as a cell-specific factor for expression of the pituitary proopiomelanocortin (POMC) gene. Expression of this factor is exclusively restricted to the pituitary POMC-expressing lineages, the corticotrophs and melanotrophs. We have now determined the role of this factor in pituitary cell differentiation. Tpit is a positive regulator for late POMC cell differentiation and POMC expression, but it is not essential for lineage commitment. The pituitary intermediate lobe normally contains only Tpit-expressing melanotrophs. Inactivation of the Tpit gene results in almost complete loss of POMC-expressing cells in this tissue, which now has a large number of gonadotrophs and a few clusters of Pit-1-independent thyrotrophs. The role of Tpit as a negative regulator of gonadotroph differentiation was confirmed in transgenic gain-of-function experiments. One mechanism to account for the negative role of Tpit in differentiation may be trans-repression between Tpit and the gonadotroph-restricted factor SF1. These data suggest that antagonism between Tpit and SF1 may play a role in establishment of POMC and gonadotroph lineages and that these lineages may arise from common precursors.
Keywords: T-box, pituitary development, transcription factor, POMC, gonadotropin, trans-repression, Tbx19
The pituitary gland is a very convenient model to study mechanisms of cellular differentiation. It has been particularly informative through identification of regulatory factors and their molecular mechanisms of action on organogenesis, as well as on cell differentiation and gene transcription. The pituitary gland is of dual embryonic origin and arises through intimate association of neural and oral roof ectoderm (Sheng and Westphal 1999). The mature pituitary gland of rodents is ultimately composed of three lobes. The posterior lobe, containing axonal projections emanating from the hypothalamus, is derived from neural ectoderm. The anterior and intermediate lobes are derived from a midline invagination of the oral ectoderm, Rathke's pouch, and contain the six hormone-secreting cell types: thyrotrophs producing thyrotropin (TSH), somatotrophs producing growth hormone (GH), lactotrophs producing prolactin (PRL), gonadotrophs producing gonadotropins (LH, FSH), melanotrophs producing α-melanotropin (αMSH), and corticotrophs producing adrenocorticotropin (ACTH). ACTH and αMSH are both processed from the same precursor, proopiomelanocortin (POMC). There are thus two separate lineages expressing the unique POMC gene; this expression is differentially controlled in each lineage (Drouin et al. 1990). Whereas the melanotrophs constitute all the secreting cells of the intermediate lobe (IL), the corticotrophs represent about 5% of anterior lobe (AL) cells in the adult rodent. Despite intensive investigation and identification of a number of cell-restricted transcription factors that play essential roles in specific lineages, the precursor/progeny relationships between these lineages are not yet clear.
During organogenesis, the developing pituitary maintains intimate contact with neural tissues of the ventral diencephalon, which produce signaling molecules important for pituitary differentiation and proliferation (Daikoku et al. 1982; Takuma et al. 1998). Bone morphogenic protein 4 (BMP4) and fibroblast growth factor 8 (FGF8) are expressed sequentially in the ventral diencephalon directly overlying Rathke's pouch (Ericson et al. 1998; Treier et al. 1998). BMP4 expression is detected as early as embryonic day 8.5 (E8.5) and precedes that of FGF8. These signals were shown to be important for the initial inductive phase of pituitary development and proliferation. In addition, sonic hedgehog (Shh) is expressed throughout the oral ectoderm except in Rathke's pouch, and was shown to be important for pituitary proliferation and patterning (Treier et al. 2001). These signaling molecules appear to influence expression of transcription factors essential for pituitary lineage differentiation, but their specific contribution to the differentiation process remains unclear.
Various cell-restricted transcription factors have been implicated in pituitary cell differentiation. The somatolactotroph and thyrotroph lineages require Prop1 and Pit-1 for their differentiation (Bodner et al. 1988; Ingraham et al. 1988; Sornson et al. 1996). In gonadotrophs, GATA-2 and SF1 play positive roles in activation of gonadotroph-specific genes, and they are required for terminal differentiation (Ingraham et al. 1994; Steger et al. 1994; Dasen et al. 1999; Zhao et al. 2001). Some factors may also play negative roles in the differentiation process. At high levels of expression in the presumptive gonadotrophs, GATA-2 may inhibit Pit-1 expression but not at lower levels in thyrotrophs, where both Pit-1 and GATA-2 are coexpressed and important for activation of thyrotroph-specific genes (Dasen et al. 1999). Pit-1 may also have a negative role in thyrotrophs, where it prevents GATA-2 binding to gonadotroph-specific promoters (Dasen et al. 1999). These experiments have suggested mutually antagonistic roles for GATA-2 and Pit-1 in the gonadotroph and thyrotroph lineages, but it is not yet clear whether these two lineages arise from a common and unique precursor pool, because in mice deficient for these factors, the fate of these lineages have not been observed to change.
The relationship of POMC-expressing lineages with other pituitary cell types is still unclear, particularly because knockout of genes such as Lhx3 and Pitx2, involved in early pituitary organogenesis, prevents differentiation of all lineages except corticotrophs (Sheng et al. 1996; Gage et al. 1999; Lin et al. 1999). Two transcription factors have been identified thus far and were shown to be restricted to corticotrophs and/or melanotrophs in the pituitary: NeuroD1 in corticotroph (Poulin et al. 1997, 2000) and Tpit (Tbx19) in both POMC lineages (Lamolet et al. 2001). Because all pituitary cells appear to have a common origin in Rathke's pouch, relationships must exist between the different lineages, and some regulatory genes must play crucial roles in cell fate decisions. The present work reveals a positive role of Tpit in the POMC lineage as well as a negative role of the same factor to prevent gonadotroph and Pit-1-independent thyrotroph differentiation. Indeed, intermediate lobe cells destined to become melanotrophs mostly differentiate into gonadotrophs in Tpit-deficient mice. These findings implicate Tpit as a major regulatory gene for establishment of cell fate between POMC and gonadotroph lineages.
Results
The Tpit transcription factor is a T-box factor cloned for its interaction with Pitx1 on the POMC promoter (Lamolet et al. 2001). Its expression is restricted to the POMC lineages of the pituitary. It is sufficient for POMC gene activation in undifferentiated pituitary cells in gain-of-function transgenic mice, suggesting a role of Tpit in POMC cell differentiation. To better understand the role of Tpit during pituitary development, we produced Tpit-null mice by deleting most of Tpit's T-box coding sequences. LacZ coding sequences were fused in-frame with the remaining Tpit coding sequences (Fig. 1A). Using this targeting vector, two independent mouse mutant lines were derived (Fig. 1B). Both lines were bred with Balb/c and 129sv mice and, in each case, homozygous mutant mice were viable and fertile. Similar results were obtained in both genetic backgrounds (data not shown). Absence of pituitary Tpit expression was confirmed in Tpit−/− mice by immunohistochemistry (Fig. 1C). In Tpit+/− mice, lacZ-expressing cells were restricted to POMC cells of the AL and IL (Fig. 1D) and were not present in other POMC-expressing tissues, such as skin or hypothalamic POMC neurons (data not shown), in agreement with the highly pituitary-specific expression of Tpit (Lamolet et al. 2001).
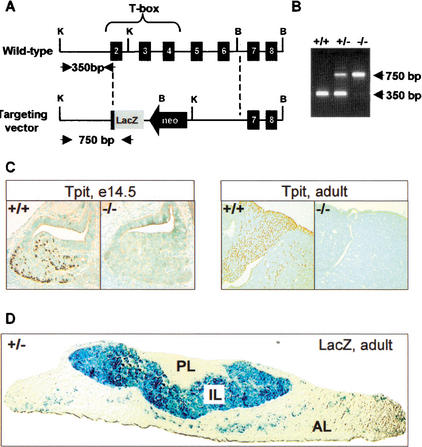
Figure 1.
Targeted disruption of the mouse Tpit gene. (A) Homologous recombination between the mouse Tpit gene (top) and the targeting vector (bottom) will result in replacement of almost all of the coding region by a lacZ coding gene. K, KpnI; B, BamHI. (B) PCR assay for genotyping using oligonucleotides identified by arrows in A. Wild-type and Tpit−/− PCR products are 350 and 750 bp, respectively. (C) Tpit immunohistochemical analysis of pituitaries from wild-type and Tpit−/− mice showing absence of Tpit protein in E14.5 mutant embryo and adult. (D) LacZ staining of pituitary from Tpit+/− mouse showing expression of β-galactosidase throughout intermediate lobe (IL) melanotroph cells and in a subset of anterior lobe (AL) cells (corticotrophs), whereas no expression is detected in posterior lobe (PL).
Tpit is required for late POMC lineage differentiation but not for lineage commitment
The developing pituitary of Tpit-null mice (E14.5) has apparently normal histology (Fig. 2A). However, the number of POMC-positive cells is greatly reduced, with only a few cells remaining. The great reduction in POMC-expressing cells does not appear to be sensitive to gene dosage, because +/+ and +/− mice had indistinguishable numbers of POMC-positive cells and POMC expression (data not shown). The loss of pituitary POMC expression in these mice results in very low plasma ACTH, with pathophysiological consequences that are extremely similar to human early-onset isolated ACTH deficiency (IAD), a condition that was poorly delineated until we showed a high frequency of TPIT gene mutation in these patients (Pulichino et al. 2003). The normal morphology of the pituitary gland and the appearance of lacZ-expressing cells in Tpit−/− mice (Fig. 2C) suggest that the corticotroph differentiation process is initiated. To further test this, we assessed the expression of another corticotroph-specific marker, NeuroD1, which was expressed at similar levels in the AL of Tpit−/− and +/− mice (Fig. 2B), showing that the presumptive corticotroph cells are present (at about normal abundance) but are not able to reach terminal differentiation (POMC expression). Thus, Tpit is required for late POMC lineage differentiation but not for lineage commitment. We next analyzed the adult gland to see how this incomplete differentiation process was reflected later in development. The AL of Tpit−/− pituitaries still had lacZ-positive cells, although much fewer than in heterozygous animals (Fig. 2D) or by comparison to corticotrophs in normal pituitaries. The IL of Tpit−/− mice is hypoplastic, with all cells expressing lacZ (Fig. 2D). We next assessed POMC expression in the adult pituitary of Tpit−/− mice. A small number of AL cells express POMC, and in the IL, it is clear that only a small fraction of lacZ-positive cells also expressed POMC (Fig. 2E). Thus, most lacZ-positive cells are POMC-negative.
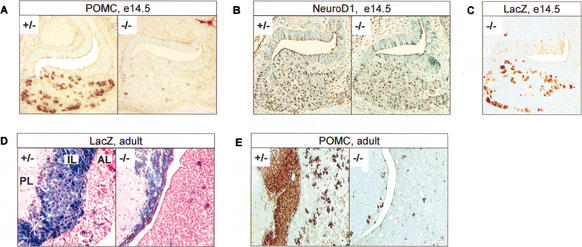
Figure 2.
Tpit is required for late POMC lineage differentiation but not for lineage commitment. Immunohistochemistry showing almost complete disappearance of pituitary POMC-expressing cells in Tpit−/− mice compared to heterozygotes (+/−) or wild-type (+/+, not shown), in both E14.5 embryo (A) and adult (E). No difference was observed between +/+ and +/− mice throughout these analyses. (B) Immunohistochemistry showing normal distribution of NeuroD1 expression in E14.5 Tpit−/− pituitary. (C) Immunohistochemistry showing lacZ expression in Tpit−/− E14.5 pituitary. Distribution of lacZ-positive cells is similar to normal POMC and NeuroD1 expression at this stage of development. (D) X-gal staining showing lacZ expression in both Tpit+/− and Tpit−/− adult pituitaries. In Tpit−/− pituitary, a few positive cells are present in anterior lobe (AL), and intermediate lobe (IL) is hypoplastic, with all lacZ-positive cells. (E) POMC staining reveals a few POMC-positive cells in AL and IL.
Alternate pituitary cell fates in absence of Tpit
The lacZ-positive POMC-negative pituitary cells of Tpit−/− mice may be blocked in their differentiation process (as suggested by the pattern of NeuroD1 expression in the developing AL), but they may also adopt another cell fate. To test this hypothesis, we investigated the expression of other pituitary hormones in Tpit−/− pituitaries (Fig. 3). PRL and GH were normally expressed in these pituitaries; that is, only in the AL (Fig. 3A). αGSU, a marker of both gonadotrophs and thyrotrophs, seemed normally expressed in the AL but was, surprisingly, ectopically expressed in the IL. These IL αGSU-positive cells may reflect inappropriate differentiation or may reflect bona fide differentiation into thyrotrophs and/or gonadotrophs. To investigate these possibilities, we assessed the expression of the β subunits of the glycoprotein hormones as well as relevant transcription factors. The presence of βTSH-positive cells (very few) and that of βLH- and βFSH-positive cells in the IL of Tpit−/− pituitaries (Fig. 3A) clearly suggests that these cells have adopted a new cell fate.
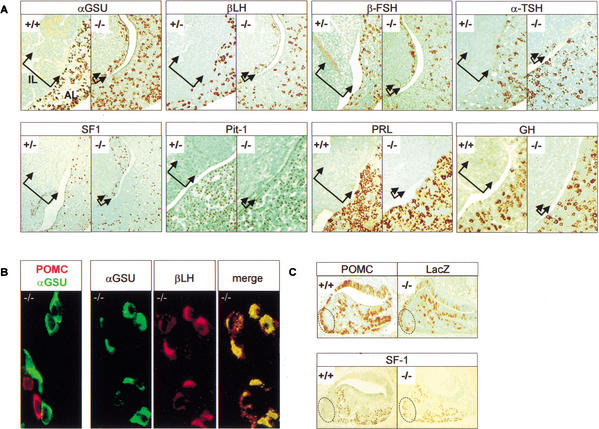
Figure 3.
Alternate pituitary cell fates in the absence of Tpit. The hypoplastic intermediate lobe (IL, bracketed by arrows) of Tpit−/− pituitaries contains gonadotroph and Pit-1-independent thyrotroph cells in addition to the few POMC-positive cells (Fig. 2E). (A) Gonadotroph cells are revealed using immunohistochemical analysis for αGSU, βLH, βFSH, and SF1. Thyrotrophs are revealed using anti-TSH antibody. Very few TSH-positive clusters were present in all mutant pituitaries examined. Pit-1 immunoreactivity was never detected in hypoplastic IL, including in sections adjacent to TSH-positive cell clusters. PRL and GH were never detected in hypoplastic IL. (B) Colocalization immunohistochemistry indicates that hypoplastic IL cells are either POMC lineage or gonadotroph. Colabeling with POMC and αGSU never showed any cells positive for both. Colocalization between αGSU and βLH showed all βLH cells to be αGSU-positive. (C) Ectopic expression of SF1 in AL of E16.5 Tpit−/− pituitaries. The dotted area represents a cluster of POMC-positive cells usually observed on the ventrocaudal side of normal (+/+) E16.5 pituitaries. A similar pattern of lacZ-positive cells was observed in Tpit−/− pituitary. This area is usually devoid of SF1-positive cells in normal (+/+) pituitaries, but Tpit−/− pituitaries exhibit SF1-positive cells in this area.
During development, two populations of thyrotrophs are generated. A transient Pit-1-independent lineage appears first in the rostral part of the gland and disappears by birth (Lin et al. 1994). The role and mechanism of differentiation of this cell population are poorly defined. The definitive Pit-1-dependent thyrotrophs appear later in the dorsal region of the gland and persist in the adult. Pit-1 is also expressed in AL somatotrophs and lactotrophs (Ingraham et al. 1988). In the present study, Pit-1 was normally expressed in Tpit−/− mice: the large number of AL somatotroph and lactotroph cells have nuclear Pit-1, and no Pit-1 was detected in the hypoplastic IL of Tpit−/− mice, including in sections consecutive to those where βTSH expression was shown. The absence of Pit-1 in these later cells indicates that the thyrotrophs in the hypoplastic Tpit−/− IL are similar to the Pit-1-independent lineage.
To investigate whether the βLH and βFSH-positive cells in the hypoplastic IL are gonadotrophs, we assessed the expression of a marker of normal gonadotroph differentiation, SF1, an orphan nuclear receptor that plays essential roles at multiple levels of the reproductive axis (Parker and Schimmer 1997). The large number of SF1-positive cells in the Tpit−/− IL supports the idea that these cells are bona fide gonadotrophs (Fig. 3A). Colocalization experiments showed that Tpit−/− IL cells express POMC or αGSU, never both, and that αGSU and βLH expression colocalize (Fig. 3B). These colocalization experiments are in agreement with the conclusion that, in the absence of Tpit, cells of the IL predominantly differentiate into gonadotrophs together with a few melanotrophs and Pit-1-independent thyrotrophs, and that they do not appear to have a mixed or abnormal cell identity. Because all of the cells of the Tpit−/− IL express the β-gal gene inserted in the Tpit locus, these data clearly support the interpretation that cells originally destined to become melanotrophs have instead differentiated into gonadotrophs or Pit-1-independent thyrotrophs.
We analyzed E16.5 embryos to determine whether cell fate changes also occur in the AL. Indeed, pituitary cells normally expressing POMC in the caudal part of wild-type mice now express both lacZ and SF1 in the Tpit−/− mice (Fig. 3C). At this early developmental timepoint, this caudal part of the pituitary does not normally have SF1- or glycoprotein hormone-expressing cells. These correlative observations suggest that cell fate changes between corticotrophs and gonadotrophs may also occur in the AL of Tpit−/− mice.
Tpit is a repressor of the gonadotroph lineage
The appearance of gonadotroph and Pit-1-independent thyrotroph cells in the IL of Tpit−/− mice might reflect a default differentiation pathway, and/or it may be suggestive of a Tpit activity as a repressor of the gonadotroph lineage. To better assess these possibilities, we designed a gain-of-function experiment in transgenic mice using the αGSU promoter to drive Tpit expression in the gonadotroph lineage (Fig. 4). The pituitaries of these mice have slightly more Tpit-positive (Fig. 4A) and POMC-positive cells (Fig. 4B) in the AL. The number of αGSU-positive cells is reduced (Fig. 4C), whereas βTSH-positive cells are present in normal number (Fig. 4D). Most strikingly, βLH is no longer detectable in transgenic pituitaries (Fig. 4E), whereas the level of βFSH is greatly reduced (Fig. 4F). αGSU-Tpit pituitaries also have less SF1-positive cells, and the remaining SF1-positive cells (mostly on the ventral side of the gland) express low SF1 levels (Fig. 4G). The use of the αGSU promoter in this experiment is a limiting factor, because it appears that its expression is itself subject to Tpit repression. Taken together, these results indicate that Tpit represses at least late events of gonadotroph differentiation as assessed by hormone and SF1 expression.
Figure 4.
Repression of gonadotroph differentiation in transgenic mice expressing Tpit under control of αGSU promoter. Expression of marker genes was assessed by immunohistochemistry on sections of pituitaries from wild-type or transgenic mice (four transgenics showed a similar phenotype). A slight increase in the number of anterior lobe (AL) Tpit-positive (A) and POMC-positive (B) cells is observed in transgenic pituitary, whereas a decrease of αGSU (C) and βFSH (F) expression is observed. LHβ is no longer detectable (E), whereas βTSH (D) expression appears to be relatively normal. The number of SF1-positive cells (G) is decreased in the transgenic pituitaries.
Trans-repression of Tpit and SF1 activity
Tpit may repress the gonadotroph phenotype by different mechanisms. In view of the decreased expression of SF1 and of SF1-dependent genes such as βLH (Halvorson et al. 1996, 1998; Tremblay and Drouin 1999; Tremblay et al. 1999), we investigated the possibility of a transcriptional interaction between Tpit and SF1. Using an SF1-dependent reporter in αT3 cells that express endogenous SF1, we found that increasing amounts of Tpit antagonized SF1-dependent transcriptional activity (Fig. 5A). Conversely, Tpit-dependent activity of a reporter containing the Tpit/Pitx target sequence (Lamolet et al. 2001) was reversed in the presence of increasing amounts of SF1 (Fig. 5B). Mutual trans-repression by these two transcription factors is thus one mechanism by which they may influence differentiation of pituitary precursors and expression of cell-specific target genes. Trans-repression is the reciprocal antagonism of transcription produced through protein–protein interactions between two activators of transcription. On a given target gene, DNA binding activity is only required for the activating factor but not for the repressing one. This mechanism of repression was best characterized for GR and AP-1 (Yang-Yen et al. 1990), GR and NFκ-B (Ray and Prefontaine 1994; Scheinman et al. 1995), and for GR and NFGI-B (Philips et al. 1997). In support of this mode of action, we used the I171T Tpit mutant that has lost DNA binding activity (Pulichino et al. 2003) to show Tpit repression of SF1 activity even in absence of DNA binding by Tpit (Fig. 5C). We also observed in pull-down assays that the two proteins interact directly in vitro (Fig. 5D). In addition, Tpit may directly repress the expression of gonadotroph-specific genes, and this could be shown for the αGSU promoter (Fig. 5C). In similar transfection experiments, the available βLH, βFSH, βTSH, GH, and PRL promoter constructs (Tremblay et al. 1998) were not affected by Tpit (data not shown). Also, the available mouse SF1 promoter was not found to be affected by Tpit; it is however noteworthy that a 50-kb SF1 promoter fragment was recently shown to be insufficient for gonadotroph expression (Stallings et al. 2002). In view of the undetectable βLH expression in the αGSU-Tpit transgenic mice (Fig. 4E), these negative transfection results may reflect the absence of relevant regulatory sequences in the available promoter constructs. Another cell-specific regulator of gonadotroph differentiation is GATA-2 (Steger et al. 1994; Dasen et al. 1999). In similar transfection experiments using either the mouse GATA-2 promoter or a reporter dependent on tandemly repeated GATA sites, we could not detect any effect of Tpit on GATA-dependent transcription (data not shown). These results suggest that Tpit-dependent trans-repression is restricted to SF1 and is not exerted on the other gonadotroph-specific factor GATA-2.
Figure 5.
Trans-repression between Tpit and SF1 as a mechanism for antagonism between the two factors. (A) Increasing amounts (0–250 ng expression plasmid) of Tpit repress SF1-dependent activity in gonadotroph-derived αT3 cells. SF1-RE-luc reporter (containing three copies of SF1-RE) activity was stimulated with SF1 (100 ng). (B) Increasing amounts of SF1 (0–250 ng expression plasmid) repress Tpit-dependent activity in αT3 cells. Tpit/Pitx reporter (Tpit/Pitx-RE-luc containing three tandem copies of Tpit/Pitx-RE) activity was enhanced with Tpit expression plasmid (50 ng). (C) Repression of SF1-RE-luc activity does not require Tpit DNA binding activity. Increasing amounts (0–250 ng) of Tpit or its DNA-binding-deficient I171T mutant repress reporter activity in SF1-expressing αT3 cells. (D) In vitro interaction between Tpit and SF1. MBP-Tpit and MBP-βGal columns were used in pull-down assays to show Tpit interaction with in vitro translated SF1. (E) αGSU promoter (−5 kb) is repressed by Tpit (0–100 ng expression plasmid) in αT3 cells. Data are means ± S.E.M. of three to five experiments, each performed in duplicate.
Discussion
The role of Tpit as a positive regulator of differentiation for both pituitary POMC lineages is very consistent with the highly cell-restricted expression of this factor. Conversely, the absence of Tpit in other adult pituitary lineages did not suggest a role of this factor in these lineages: the discovery of its role as negative regulator of gonadotroph differentiation is therefore surprising. The present work thus defines previously unknown relationships among four pituitary lineages, namely melanotrophs, corticotrophs, gonadotrophs, and the transient population of Pit-1-independent thyrotrophs. These lineages are thus clearly demarcated relative to the other three pituitary lineages which are Pit-1-dependent, namely the somatotrophs, lactotrophs, and Pit-1-dependent thyrotrophs. In this context, we propose a scheme for pituitary cell differentiation that is divided into two alternate pathways (Fig. 6).
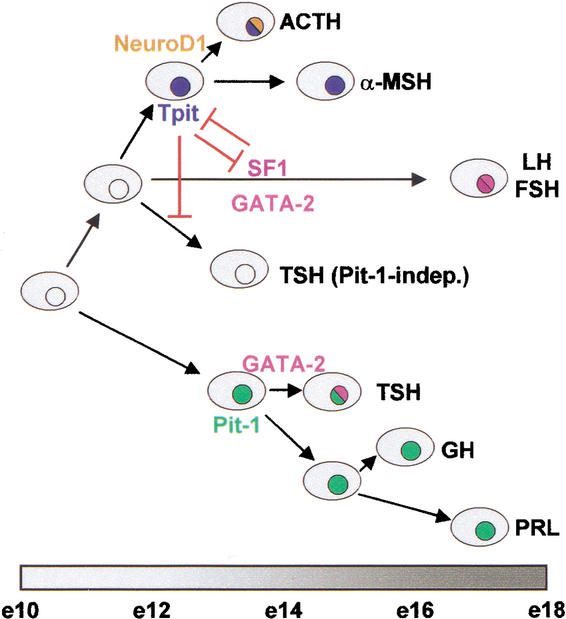
Figure 6.
A binary model of pituitary cell differentiation. The present work provides support for a model of pituitary cell differentiation that relies on sequential choices between alternate fates. In this model, the cortico/melanotroph (ACTH, αMSH) and gonadotroph (LH, FSH) lineages arise from a common precursor (present work) that is different from precursors of Pit-1-dependent lineages (GH, PRL, TSH). In the cortico/melano/gonadotroph lineage, expression of (and antagonism between) Tpit and SF1 establishes the POMC or gonadotroph lineage, respectively. In this branch of the pathway, GATA-2 contributes to the gonadotroph phenotype, whereas in the Pit-1-dependent branch of the pathway, it acts together with Pit-1 for differentiation of thyrotrophs (Dasen et al. 1999). The model is roughly aligned with a timeline of mouse pituitary development.
Tpit is a positive regulator in POMC-expressing cells
Tpit was identified as a cell-specific transcription factor of the POMC gene. Its role in this context is entirely dependent on Pitx1 (Lamolet et al. 2001), and the bHLH factor NeuroD1/Beta2 also plays a crucial role for promoter activity (Poulin et al. 2000). We have now shown that Tpit is very important for the last step of corticotroph differentiation, namely POMC gene expression (Fig. 2A,E). However, the AL of Tpit−/− pituitaries contains an about normal number of lacZ-positive (Fig. 2C) and NeuroD1-positive (Fig. 2B) cells at E14.5, and the IL is normally formed in these pituitaries (Fig. 2A–C). These data indicate that corticotroph precursors, pre-corticotrophs, form in apparently normal number in the absence of Tpit. Thus, Tpit is not essential for commitment of POMC lineages.
In addition to its role in late differentiation of corticotrophs and melanotrophs revealed through failure of POMC expression, the role of Tpit in the maintenance of those cells is highlighted by the present findings. Indeed, adult pituitaries of Tpit−/− mice have very few POMC-positive and lacZ-positive cells remaining in the AL (Fig. 2D,E). Also, the IL is hypoplastic, with only a few POMC-expressing melanotrophs (Fig. 2D,E). Because the pituitary histology and abundance of pre-corticotrophs and pre-melanotrophs appear relatively normal at E14.5, it is likely that these cells do not proliferate between fetus and adult, or that they are lost during that period of growth. Irrespective of which of these two possibilities accounts for the deficit of melanotrophs and corticotrophs in adults, these observations indicate that Tpit has a role in maintenance of these cells.
Tpit is a negative regulator of gonadotroph differentiation
In the absence of Tpit, IL cells destined to differentiate into melanotrophs (lacZ-positive in Tpit−/− pituitaries) differentiate instead into gonadotrophs or Pit-1-independent thyrotrophs (Fig. 3A). These cells do not have mixed identity (Fig. 3B) and have the hallmarks of bona fide gonadotrophs or Pit-1-independent thyrotrophs. These data indicate that Tpit normally represses these differentiation pathways (Fig. 6). Transgenic gain-of-function experiments confirm this interpretation, because Tpit overexpression in gonadotrophs leads to extinction of βLH expression and to decreased expression of αGSU, βFSH, and SF1 (Fig. 4).
The change of IL cell fate suggests that similar pathways may be implicated in cortico/melanotroph and in gonadotroph differentiation in both the IL and AL. A specific signal for cortico/melanotroph differentiation may trigger this program through activation of Tpit expression, whereas a competing signal may initiate gonadotroph differentiation by induction of SF1 expression. Antagonism between Tpit and SF1 ensures that once a cell has responded to one signal by expression of either Tpit or SF1, the expressed factor prevents action of the other. This antagonism establishes a unique program of gene expression and determines cell identity. This model implies that signals for cortico/melanotroph and gonadotroph differentiation operate on a common pool of precursor cells. These precursors have not yet been identified, and there are no markers to differentiate these from other precursors, such as those of the Pit-1-dependent somato-lactotroph and definitive thyrotroph lineages (Fig. 6).
The presence of a few Pit-1-independent thyrotrophs in the IL of Tpit−/− pituitaries suggests that this transient lineage is also related to the cortico/melanotroph and gonadotroph lineages. Appearance of these cells is not dependent on SF1 in the normal pituitary (Lin et al. 1994), and they also appear to be SF1-negative in Tpit−/− pituitaries (data not shown). In both knockout and transgenic gain-of-functions, Tpit did not appear to affect the other thyrotroph lineage, that is, the definitive Pit-1-dependent thyrotrophs (Figs. 3A, 4B). These data clearly support previous models in which these two thyrotroph lineages have different origins (Lin et al. 1994).
Tpit action may repress the gonadotroph phenotype by different mechanisms. First, the nonoverlapping patterns of SF1 and Tpit expression suggest that the expression of both factors is mutually exclusive in vivo. Second, Tpit was shown to directly repress transcription of the αGSU promoter (Fig. 5E), indicating that part of Tpit's repressor activity may be through direct action on gonadotroph-specific coding genes. Thirdly, we showed that Tpit and SF1 antagonize each other's activity on cognate reporters (Fig. 5A,B). This antagonism appears to result from a mechanism of trans-repression in which DNA binding activity is not required for the repressing factor (Fig. 5C) and which involves protein–protein interactions (Fig. 5D), as shown for other factors that antagonize each other's activity by trans-repression (Yang-Yen et al. 1990; Ray and Prefontaine 1994; Scheinman et al. 1995; Philips et al. 1997). In these examples of trans-repression that involve GR, the mechanism of trans-repression remains elusive, although recent work has revealed a unique pattern of CTD phosphorylation of RNA Polymerase II complexes that are paused as a result of trans-repression between GR and NFκB (Nissen and Yamamoto 2000). Trans-repression may not rest on recruitment of corepressors but may involve coactivators (Rogatsky et al. 2001). Although repressor domains have been identified in other T-box factors, such as Tbx2 and Tbx3 (Carreira et al. 1998; He et al. 1999), Tpit does not have sequences that are homologous to these domains.
A binary model of pituitary cell differentiation
All pituitary cells differentiate from a common pool that originates in the epithelial folds of Rathke's pouch. The precise relationships among pituitary lineages are not yet clear, but a model of signal gradients has been proposed to account for differentiation of these lineages (Ericson et al. 1998; Treier et al. 1998). By providing evidence for a common precursor for both cortico/melanotroph and gonadotroph lineages and by demarcating these lineages in comparison to Pit-1-dependent lineages, the present work can be taken to support a binary model of pituitary cell differentiation (Fig. 6). Indeed, early pituitary precursors may initially choose, possibly under the influence of signaling gradients, either the cortico/melano/gonadotroph or Pit-1-dependent pathways. Next, cortico/melano/gonadotroph precursors will take either the cortico/melanotroph or gonadotroph path, depending on expression of Tpit or SF1, respectively. GATA-2 was shown to influence differentiation of one lineage in each branch of the differentiation pathway (Dasen et al. 1999). In the cortico/melano/gonadotroph pathway, it promotes gonadotroph differentiation (in combination with SF1), whereas in the Pit-1-dependent pathway, it acts together with Pit-1 for differentiation of definitive thyrotrophs. The absence of Pit-1-dependent cells in the IL of Tpit−/− mice taken together with the antagonistic actions of Tpit and SF1 clearly supports a model (Fig. 6) in which the initial binary choice is between Tpit and Pit-1-dependent lineages, with Tpit being expressed earlier than Pit-1 in the AL (Dolle et al. 1990; Lamolet et al. 2001). Secondary cell fate choices would then involve SF1 and/or GATA-2. For POMC lineages, NeuroD1 is important for corticotroph, but not melanotroph, differentiation (B. Lamolet, K. Chu, G. Poulin, F. Guillemot, M.J. Tsai, and J. Drouin, in prep.). NeuroD1 expression starts at E12 in corticotrophs (Poulin et al. 2000); that is, at the same time as Tpit (Lamolet et al. 2001), and NeuroD1 deficiency prevents POMC, but not Tpit, expression (B. Lamolet, K. Chu, G. Poulin, F. Guillemot, M.J. Tsai, and J. Drouin, in prep.). Thus, Tpit and NeuroD1 appear to be regulated in parallel and independently of each other, both being similarly required for terminal corticotroph differentiation and POMC expression.
The present work has provided the first evidence to demarcate the cortico/melanotroph and gonadotroph lineages in opposition to the Pit-1-dependent somatolactotrophs and definitive thyrotrophs. Taken together, our data support a model in which differentiation of pituitary cells is established through a series of binary choices that oppose each other and lead to establishment of lineage identity.
Materials and methods
Gene targeting, transgenics, and genotyping
The murine Tpit gene was cloned from a 129sv genomic library (gift from J.P. Julien, McGill University, Montréal, Quebec, Canada). To construct the targeting vector, a 4.3-kb NcoI/KpnI fragment containing part of intron 1 and exon 2 and a 2.7-kb BamhI/Mscl fragment containing exons 7 and 8 were subcloned in pUC19 and used as 5′ and 3′ recombination targets. Tpit exons 3–6 were replaced by a pGKneo-pA cassette (gift from D. Lohnes, Clinical Research Institute of Montréal, Montréal, Quebec, Canada), and a lacZ coding gene was inserted in frame with exon 2, leaving seven amino acids of this exon. Mutant ES cell lines were obtained as described (Lanctôt et al. 1999). Homologous recombination occurred at the Tpit locus in 15 out of 480 transfectants that were picked. Two different ES cell clones were injected into blastocysts, and mouse lines were established for both. Tpit mutant animals were crossed with 129sv and Balb/c mice. All exhibited the same pituitary phenotype. ES cells lines and the first 50 mice were genotyped by genomic Southern blotting with 5′ and 3′ probes. Other mice were genotyped by PCR using DNA isolated from tails or umbilical cords. Transgenic mice were generated as described (Lamolet et al. 2001), and embryos were taken by caesarean section at E18.5.
Sections and lacZ staining
Paraffin sections were performed as described (Lanctôt et al. 1997). For lacZ staining, tissues were fixed in 4% paraformaldehyde (PFA) for 15 min, rinsed with PBS, and stained overnight at 30°C in X-gal solution (5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6*3H2O, 1m M MgCl2, 0.01% sodium desoxycholate, 0.02% NP-40, 0.1% X-gal), rinsed with PBS, and postfixed in 4% PFA.
Cells and transfections
αT3 cells were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum and antibiotics; then 250,000 cells were transfected in 12-well dishes with Lipofectamine (Invitrogen) using 500 ng reporter plasmid, up to a total of 1.5 μg DNA per assay. Cells were harvested 48 h later.
Pull-down assays
All MBP fusion proteins were produced, and [35S]-labeled SF1 was synthesised in vitro as described (Batsche et al. 1998). Labeled proteins were incubated with 400 ng immobilized MBP-lacZ or MBP-Tpit constructs in 150 μL of TNEN50 (50 mM TRIS at pH 7.5, 5 mM EDTA, 50 mM NaCl, 0.1% NP-40) with 1 mM PMSF and 2% BSA for 2 h at 4°C. Beads were washed at 4°C twice in TNEN250 and twice in TNEN125. Bound proteins were resolved on SDS-PAGE, stained with Coomassie blue to ensure that similar amounts of fusion proteins were recovered, and then autoradiographed.
Immunohistochemistry and immunofluorescence
Immunohistochemistry was performed as described (Lanctôt et al. 1997). Antibodies were used as follows: rabbit anti-Tpit 1:200 (Lamolet et al. 2001), mouse anti-POMC 1:500 (Cortex Biochem), mouse anti-lacZ 1:500 (ICN Pharmaceuticals), rabbit anti-SF1 1:1500 (kind gift from K. Morohashi, National Institute for Basic Biology, Okazaki, Japan), rabbit anti-Pit-1 1:50 (Santa Cruz Biotechnology), rabbit anti-αGSU 1:500, rabbit anti-βFSH 1:200, rabbit anti-prolactin 1:1000, rabbit anti-GH 1: 1670, guinea pig anti-βLH 1:200 (all pituitary hormones antibodies were kindly provided by A.F. Parlow, Pituitary Hormones and Antisera Center, Torrance, CA). All secondary antibodies were used 1:150 (Vector Laboratories). NeuroD1 was detected with rabbit anti-NeuroD1 1:10 (Poulin et al. 2000) with the TSA biotin system (PerkinElmer Life Sciences). For immunofluorescence, sections were treated as above. For αGSU/POMC colocalization, anti-αGSU was incubated overnight, mouse anti-POMC (1:200) and anti-rabbit-biotinylated (1:200, Vector) were added, and finally, anti-mouse-rhodamine (1:200, ImmunoPure Antibody) and avidin-fluorescein (1:200, Vector) were added. For αGSU/βLH colocalization, anti-βLH (1:200) was incubated overnight, rabbit anti-αGSU (1:200) and anti-guinea pig-biotinylated (1:200, Vector) were added next, and then anti rabbit-fluorescein (1:200, Vector) and avidin-rhodamine (1:200, Vector) were added. Sections were placed in blocking solution (5% dried skim milk in PBS, 0.2% Tween20) between each step.
Acknowledgments
We thank Drs. Marc Therrien and Guy Sauvageau for critical comments on this manuscript. We are very grateful to Drs. K. Morohashi and A.F. Parlow of the NIH Pituitary Hormone Program for antibodies against SF1 and pituitary hormones, respectively. We thank Dr. Keith Parker for SF1 and SF1-RE plasmids; Dr. David Lohnes for targeting vectors; and Dr. David Gordon for the αGSU reporter plasmid. The 129sv genomic library was kindly provided by Drs. Jean-Pierre Julien and Janet Rossant, and ES R1 cells were a generous gift of Dr. Andras Nagy. Dr. Pamella Mellon kindly provided the αT3 cells. We are most thankful to Dr. Qianzhang Zhu and Michel Robillard of the IRCM Transgenesis Service for production of Tpit knockout mouse lines and transgenic mice, to Ms. Annie Vallée of the IRCM Histology Laboratory for her expert assistance, and to Ms. Julie D'Amours for her help with animal husbandry. The unrelenting secretarial support of Lise Laroche is greatly appreciated. A.M.P. was supported by a studentship from Canadian Institutes of Health Research, and S.V.K. by a Bourse d'études internationales de l'Institut Lilly and A.DE.RE.M. This work was supported by the National Cancer Institute of Canada with funds provided by the Canadian Cancer Society.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL drouinj@ircm.qc.ca; FAX (514)987-5575.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1065703.
References
- Batsche E, Muchardt C, Behrens J, Hurst HC, Cremisi C. RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol Cell Biol. 1998;18:3647–3658. doi: 10.1128/mcb.18.7.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner M, Castrillo JL, Theill LE, Deerinck T, Ellisman M, Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988;55:505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Carreira S, Dexter TJ, Yavuzer U, Easty DJ, Goding CR. Brachyury-related transcription factor Tbx2 and repression of the melanocyte-specific TRP-1 promoter. Mol Cell Biol. 1998;18:5099–5108. doi: 10.1128/mcb.18.9.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku S, Chikamori M, Adachi T, Maki Y. Effect of the basal diencephalon on the development of Rathke's pouch in rats: A study in combined organ cultures. Dev Biol. 1982;90:198–202. doi: 10.1016/0012-1606(82)90225-1. [DOI] [PubMed] [Google Scholar]
- Dasen JS, O'Connell SM, Flynn SE, Treier M, Gleiberman AS, Szeto DP, Hooshmand F, Aggarwal AK, Rosenfeld MG. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell. 1999;97:587–598. doi: 10.1016/s0092-8674(00)80770-9. [DOI] [PubMed] [Google Scholar]
- Dolle P, Castrillo JL, Theill LE, Deerinck T, Ellisman M, Karin M. Expression of GHF-1 protein in mouse pituitaries correlates both temporally and spatially with the onset of growth hormone gene activity. Cell. 1990;60:809–820. doi: 10.1016/0092-8674(90)90095-v. [DOI] [PubMed] [Google Scholar]
- Drouin J, Sun YL, Nemer M. Regulatory elements of the pro-opiomelanocortin gene. Pituitary specificity and glucocorticoid repression. Trends Endocrinol Metab. 1990;1:219–225. doi: 10.1016/1043-2760(90)90056-9. [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh HY, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Halvorson LM, Kaiser UB, Chin WW. Stimulation of luteinizing hormone β gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1. J Biol Chem. 1996;271:6645–6650. doi: 10.1074/jbc.271.12.6645. [DOI] [PubMed] [Google Scholar]
- Halvorson LM, Ito M, Jameson JL, Chin WW. Steroidogenic factor-1 and early growth response protein 1 act through two composite DNA binding sites to regulate luteinizing hormone β-subunit gene expression. J Biol Chem. 1998;273:14712–14720. doi: 10.1074/jbc.273.24.14712. [DOI] [PubMed] [Google Scholar]
- He M, Wen L, Campbell CE, Wu JY, Rao Y. Transcription repression by Xenopus ET and its human ortholog TBX3, a gene involved in ulnar-mammary syndrome. Proc Natl Acad Sci. 1999;96:10212–10217. doi: 10.1073/pnas.96.18.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham HA, Chen R, Mangalam HJ, Elsholtz HP, Flynn SE, Lin CR, Simmons DM, Swanson L, Rosenfeld MG. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988;55:519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes & Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J. A pituitary cell-restricted T-box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859. doi: 10.1016/s0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Lanctôt C, Lamolet B, Drouin J. The bicoid-related homeoprotein Ptx1 defines the most anterior domain of the embryo and differentiates posterior from anterior lateral mesoderm. Development. 1997;124:2807–2817. doi: 10.1242/dev.124.14.2807. [DOI] [PubMed] [Google Scholar]
- Lanctôt C, Moreau A, Chamberland M, Tremblay ML, Drouin J. Hindlimb patterning and mandible development require the Ptx1 gene. Development. 1999;126:1805–1810. doi: 10.1242/dev.126.9.1805. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu R, Izpisua Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphegenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Lin SC, Li S, Drolet DW, Rosenfeld MG. Pituitary ontogeny of the Snell dwarf mouse reveals Pit-1-independent and Pit-1-dependent origins of the thyrotrope. Development. 1994;120:515–522. doi: 10.1242/dev.120.3.515. [DOI] [PubMed] [Google Scholar]
- Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes & Dev. 2000;14:2314–2329. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Schimmer BP. Steroidogenic factor 1: A key determinant of endocrine development and function. Endocr Rev. 1997;18:361–377. doi: 10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- Philips A, Maira MH, Mullick A, Chamberland M, Lesage S, Hugo P, Drouin J. Antagonism between Nur77 and glucocorticoid receptor for control of transcription. Mol Cell Biol. 1997;17:5952–5959. doi: 10.1128/mcb.17.10.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin G, Turgeon B, Drouin J. NeuroD1/BETA2 contributes to cell-specific transcription of the POMC gene. Mol Cell Biol. 1997;17:6673–6682. doi: 10.1128/mcb.17.11.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin G, Lebel M, Chamberland M, Paradis FW, Drouin J. Specific protein–protein interaction between basic Helix-Loop-Helix transcription factors and homeoproteins of the Pitx family. Mol Cell Biol. 2000;20:4826–4837. doi: 10.1128/mcb.20.13.4826-4837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulichino, A.-M., Vallette-Kasic, S., Couture, C., Gauthier, Y., Brue, T., David, M., Malpuech, G., Deal, C., Van Vliet, G., De Vroede, M., et al. 2003. Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency. Genes & Dev. (this issue). [DOI] [PMC free article] [PubMed]
- Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-κ B and the glucocorticoid receptor. Proc Natl Acad Sci. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Zarember KA, Yamamoto KR. Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J. 2001;20:6071–6083. doi: 10.1093/emboj/20.21.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS., Jr Characterization of mechanisms involved in transrepression of NF-κ B by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng HZ, Westphal H. Early steps in pituitary organogenesis. Trends Genet. 1999;15:236–240. doi: 10.1016/s0168-9525(99)01742-4. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Zhadanov AB, Mosinger B, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science. 1996;272:1004–1007. doi: 10.1126/science.272.5264.1004. [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Stallings NR, Hanley NA, Majdic G, Zhao L, Bakke M, Parker KL. Development of a transgenic green fluorescent protein lineage marker for steroidogenic factorMol. Endocrinol. 2002;16:2360–2370. doi: 10.1210/me.2002-0003. [DOI] [PubMed] [Google Scholar]
- Steger DJ, Hecht JH, Mellon PL. GATA-binding proteins regulate the human gonadotropin α-subunit gene in the placenta and pituitary gland. Mol Cell Biol. 1994;14:5592–5602. doi: 10.1128/mcb.14.8.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke's pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes & Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, O'Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang PT, McMahon AP, Rosenfeld MG. Hedgehog signaling is required for pituitary gland development. Development. 2001;128:377–386. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone β gene transcription. Mol Cell Biol. 1999;19:2567–2576. doi: 10.1128/mcb.19.4.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay JJ, Lanctôt C, Drouin J. The pan-pituitary activator of transcription, Ptx-1 (pituitary homeobox1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol. 1998;12:428–441. doi: 10.1210/mend.12.3.0073. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Marcil A, Gauthier Y, Drouin J. Ptx1 regulates SF-1 activity by an interaction that mimics the role of the ligand-binding domain. EMBO J. 1999;18:3431–3441. doi: 10.1093/emboj/18.12.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, Karin M. Transcriptional interference between c-jun and the glucocorticoid receptor: Mutual inhibition of DNA binding due to direct protein–protein interaction. Cell. 1990;62:1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development. 2001;128:147–154. doi: 10.1242/dev.128.2.147. [DOI] [PubMed] [Google Scholar]