Abstract
During testis development, the rapid morphological changes initiated by Sry require the coordinate integration of many signaling pathways. Based on the established role of the platelet-derived growth factor (PDGF) family of ligands and receptors in migration, proliferation, and differentiation of cells in various organ systems, we have investigated the role of PDGF in testis organogenesis. Analysis of expression patterns and characterization of the gonad phenotype in Pdgfr-α−/− embryos identified PDGFR-α as a critical mediator of signaling in the early testis at multiple steps of testis development. Pdgfr-α−/− XY gonads displayed disruptions in the organization of the vasculature and in the partitioning of interstitial and testis cord compartments. Closer examination revealed severe reductions in characteristic XY proliferation, mesonephric cell migration, and fetal Leydig cell differentiation. This work identifies PDGF signaling through the α receptor as an important event downstream of Sry in testis organogenesis and Leydig cell differentiation.
Keywords: Sex determination, platelet-derived growth factor, Leydig cells, endothelial cells
Organogenesis of the gonad is unique in that both the testis and ovary are derived from an initially bipotential tissue, the genital ridge. Development diverges when Sry is expressed during a narrow window of time between 10.5 and 12.5 days postcoitum (dpc) in the XY gonad, resulting in rapid morphological changes that produce a characteristic testis morphology by 12.5 dpc. At this stage, the organization of testis cords defines two compartments of the testis. Inside testis cords are the Sertoli cells, which support the development of primordial germ cells, are the cell type that expresses Sry, and are believed to orchestrate testis organogenesis. Peritubular myoid cells surround the cords and participate with Sertoli cells in the production of basal lamina. The interstitial compartment lies between testis cords and contains steroid-secreting Leydig cells, fibroblasts, and the characteristic vasculature of the testis.
Although no direct targets of Sry have been identified, several developmental processes in the gonad depend on Sry and occur soon after Sry is expressed. One early event is an increase in proliferation of cells at the coelomic surface of the XY gonad (Schmahl et al. 2000). Proliferation occurs in two stages. The first stage of proliferation occurs between 11.2 and 11.5 dpc and gives rise to Sertoli cell precursors. The second stage of proliferation occurs between 11.5 and 12.0 dpc and gives rise to other, uncharacterized somatic cells of the testis (Karl and Capel 1998; Schmahl et al. 2000). The adjacent mesonephros is an additional source of cells for the XY gonad. Migration of cells from the mesonephros is dependent on Sry (Capel et al. 1999) and is required for organization of testis cords (Buehr et al. 1993; Merchant-Larios et al. 1993; Martineau et al. 1997; Tilmann and Capel 1999). The migrating cell population includes peritubular myoid cells and many endothelial and perivascular cells that contribute to divergent vascular development in the XY gonad (Brennan et al. 2002). The origin of fetal Leydig cells has not been clarified, but several sources have been suggested, which include the coelomic epithelium (Karl and Capel 1998), a migrating mesonephric population (Merchant-Larios and Moreno-Mendoza 1998; Nishino et al. 2001) that may include neural crest cells (Middendorff et al. 1993; Mayerhofer et al. 1996), or a common early precursor of the adrenal steroid cells (Hatano et al. 1996).
Very little is known about the genetic signaling pathways operating downstream of Sry that control the cellular mechanisms of testis organogenesis. Using a candidate approach, we investigated the platelet-derived growth factor (PDGF) family of ligands and receptors, which have well-characterized roles in the proliferation and migration of mesenchymal cell types, particularly smooth muscle and endothelial cells (Betsholtz et al. 2001). There are two identified PDGF receptors, α and β, and four ligands, A, B, C, and D. Different combinations of ligands and receptors can homo- and heterodimerize and activate several distinct and overlapping intracellular pathways (for review, see Heldin and Westermark 1999; Klinghoffer et al. 2001). PDGFR-α has a broader specificity for ligand binding and can bind PDGF-A, PDGF-B, and PDGF-C homodimers as well as PDGF-AB heterodimers, whereas PDGFR-β can bind only PDGF-B and PDGF-D homodimers. Generally, Pdgf ligands are expressed by epithelial or endothelial cells, whereas mesenchymal cells express Pdgf receptors. Proliferation and migration of mesenchymal cells in response to PDGF signaling contribute to the morphogenesis and integrity of epithelial and endothelial structures as shown by genetic disruptions that affect tissues such as the lung, the kidney, and the intestine (Soriano 1994; Bostrom et al. 1996; Lindahl et al. 1998; Karlsson et al. 2000).
Previous expression data (Gnessi et al. 1995) and recent genetic data have provided some insight into the involvement of PDGFs in the postnatal development of the testis. In the Pdgf-A−/− testis, very few adult Leydig cells develop, leading to a reduction in testis size and spermatogenic arrest by 32 days postnatal (dpn). Adult Leydig cells are not derived from fetal Leydig cells, but instead arise as a separate, distinct cell population after birth (Ariyaratne et al. 2000). Although fetal stages were not examined, two pieces of evidence implied that fetal Leydig cell development was normal. First, normal numbers of fetal Leydig cells were reported in Pdgf-A−/− gonads at 10–25 dpn, just before they are replaced by adult Leydig cells. Second, testicular descent and masculinization occurred normally, indicating that fetal Leydig cells were present during fetal development and produced testosterone. (Gnessi et al. 2000). The role of PDGF signaling in the embryonic testis has not been investigated in detail. A recent study using the PDGFR tyrosine kinase inhibitor, AG1296, implicated PDGF signaling in rat fetal testis cord development (Uzumcu et al. 2002). Large, dilated cords were noted in the treated samples, although the cellular and molecular basis for the defect was not characterized, and the role of specific PDGF receptors and ligands was not addressed.
Our examination of gonads from Pdgfr-β−/− mice (Soriano 1994) revealed no overt defects in early XY or XX development at 11.5–13.5 dpc, ruling out a critical role for this receptor (K. Tilmann, unpubl.). These data led us to focus our study on PDGFR-α and its ligands PDGF-A, PDGF-B, and PDGF-C. We found that Pdgfr-α and Pdgf-A display a sexually dimorphic expression pattern in the gonad. To examine the potential roles of these factors in gonad development, we analyzed gonad development in Pdgfr-α−/− mice. These studies uncovered a sex-specific role for PDGFR-α signaling in promoting cord formation, proliferation, endothelial cell migration, and Leydig cell differentiation. We also provide data placing PDGF signaling in a parallel pathway with Dhh, which is expressed in Sertoli cells and is the only other fetal Leydig cell factor identified thus far (Yao et al. 2002).
Results
Pdgfr-α and its ligands are expressed in unique patterns during early testis development
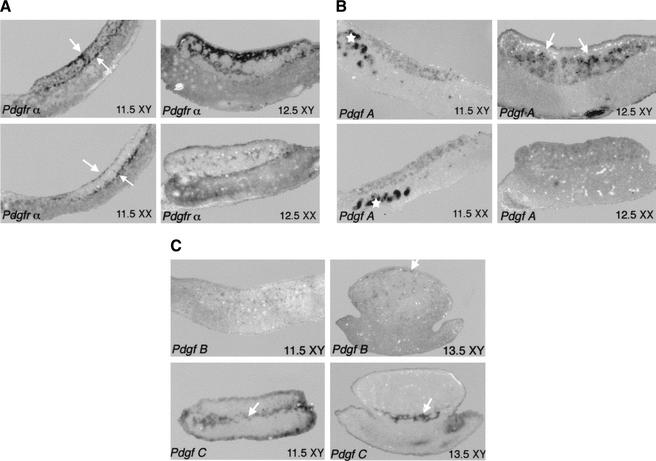
We examined the RNA expression patterns of Pdgfr-α and its ligands in XX and XY gonads between 11.5 and 13.5 dpc. Pdgfr-α was expressed at low levels in the mesenchyme of the mesonephros at 11.5 dpc in both sexes (Fig. 1A). High expression was detected in the coelomic epithelium, and at the gonad–mesonephros border at 11.5 dpc in both sexes (Fig. 1A, white arrows). However, expression was seen on cells in the interior of the XY, but not the XX gonad. By 12.5 dpc, expression was very strong in the interstitial cells of the XY gonad, particularly in the region near the coelomic epithelium, whereas, in the XX gonad, there was expression in only a few scattered cells (Fig. 1A).
Figure 1.
Expression of Pdgfr-α and Pdgf ligands at early stages of gonad development. In all figures anterior is to the left and the gonad is located on top of the mesonephros, positioned between the two white arrows as in A. (A) Pdgfr-α was expressed at the coelomic epithelium and mesonephros/gonad boundary (arrows) as well as on scattered cells in the interior of XY but not XX gonads. Expression was also detected at lower levels on mesenchymal cells of the mesonephros. Expression of the receptor was very strong in interstitial cells of the XY gonad at 12.5 dpc, but at much lower levels in the XX gonad. (B) Pdgf-A was expressed in both XY and XX gonads at 11.5 dpc as well as in the mesonephric tubules (*). High levels of Pdgf-A were detected inside the testis cords of XY gonads at 12.5 dpc (arrows), whereas expression was uniformly detected at low levels in 12.5-dpc XX gonads. (C) Pdgf-B is expressed in the gonad at low levels at 11.5 dpc. By 13.5 dpc, expression is exclusively endothelial, concentrated in the coelomic vessel of XY gonads (arrow). Pdgf-C expression is mainly concentrated at the mesonephros/gonad boundary of both XY gonads from 11.5 to 13.5 dpc (arrow).
Three ligands bind the PDGFR-α receptor: PDGF-A, PDGF-B, and PDGF-C. Expression of Pdgf-A was detected in both XX and XY gonads and in the mesonephric tubules (Fig. 1B, asterisk) at 11.5 dpc (Fig. 1B). By 12.5 dpc, Pdgf-A was strongly expressed in Sertoli cells within testis cords of the XY gonad in contrast to a much lower level of expression detected in the XX gonad (Fig. 1B). Pdgf-B expression was also present in XX and XY gonads at early stages, but at low levels in a small number of cells, which could easily be identified as exclusively endothelial by 13.5 dpc (Fig. 1C, arrow). Pdgf-C, a newly characterized ligand that binds and activates the Pdgfr-α receptor, was expressed in a similar, unique pattern in both sexes. At 11.5 dpc, we detected expression of Pdgf-C in the coelomic epithelium as previously reported (Ding et al. 2000), as well as strong expression at the gonad/mesonephros boundary and at lower levels in a scattered population of cells within the gonad. By 12.5 dpc, expression was restricted to the mesonephros/gonad boundary with little or no expression in the gonad itself (Fig. 1C, arrow).
Because of the robust, sexually dimorphic expression of Pdgfr-α and Pdgf-A, they represent the strongest candidates for PDGF signaling in the testis. However, the nonoverlapping expression patterns of the three PDGF ligands suggest they may perform unique, restricted functions during testis development.
Pdgfr-α−/− XY gonads have defects in testis cord formation and vascular development
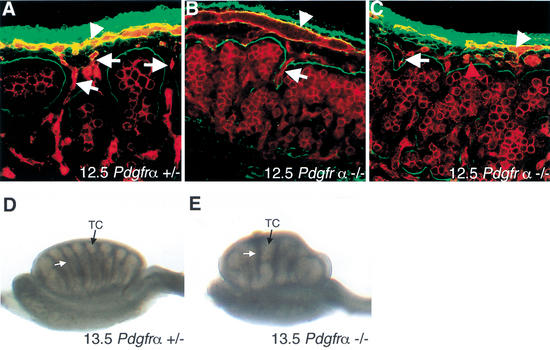
To determine the role of PDGF signaling in testis development, we first examined testis structure in Pdgfr-α−/− gonads. We labeled 12.5-dpc gonads with antibodies against laminin (Fig. 2, green), which outlined testis cords, and PECAM (Fig. 2, red), which labeled endothelial and germ cells. By 12.5 dpc, wild-type and heterozygous XY gonads began to separate into testis cord and interstitial compartments (Fig. 2A). The coelomic vessel formed at the dorsal/ventral boundary under the coelomic epithelium (arrowhead), and vessels branched from the coelomic vessel into the interstitium between testis cords of the XY gonad (arrows). No differences were seen between wild-type and heterozygous littermates. In contrast, Pdgfr-α−/− XY gonads failed to subdivide into proper testis cord and interstitial compartments. Although the coelomic vessel was present (Fig. 2B,C, arrowhead), fewer branches descended between forming testis cords in all samples analyzed (n = 8). In 2 out of 8 gonads, there was a defect in the organization of the coelomic vessel, and an increased number of endothelial cells were observed under the coelomic surface that was not organized as part of the coelomic vessel (Fig. 2C, red arrowhead). By 13.5 dpc, XY gonads from Pdgfr-α−/− mice had formed some testis cords, but they were reduced in number and abnormally shaped (Fig. 2E) as compared with control littermates (Fig. 2D). No defects in ovarian development were detected in Pdgfr-α−/− XX gonads (data not shown).
Figure 2.
Analysis of Pdgfr-α−/− testis structure at 12.5–13.5 dpc. (A) At 12.5 dpc, Pdgfr-α+/+ and Pdgfr-α+/− XY gonads have begun to form testis cords as outlined by laminin deposition (green). Staining with the endothelial and germ-cell marker PECAM (red) shows the formation of the coelomic vessel (arrowhead) and branches from the vessel that extend between testis cords (arrows). (B,C) In 12.5-dpc Pdgfr-α−/− XY samples, subdivision of testis cord and interstitial compartments had not occurred and the coelomic vessel (arrowhead) had formed few distinct branches between cords (arrows). (C) In a small proportion of samples, the coelomic vessel was not organized normally, and an increased number of endothelial cells was observed under the coelomic surface that were not organized as part of the coelomic vessel (red arrowhead). (D) By 13.5 dpc, testis cords (TC, black arrow) separated by regular interstitial spaces (white arrow) are well formed in Pdgfr-α+/− gonads. (E) At 13.5 dpc, Pdgfr-α−/− XY gonads had formed large, abnormally shaped testis cords (TC, black arrow) separated by irregular interstitial spaces (white arrow).
Fetal Leydig cell differentiation requires PDGFR-α
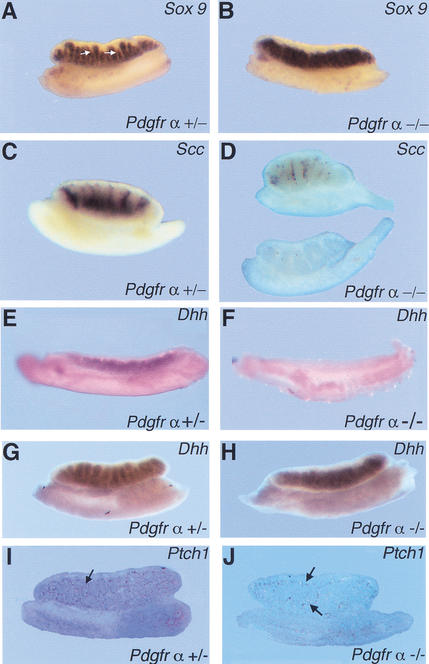
To determine if PDGFR-α is required for the specification of XY cell types, we examined Pdgfr-α−/− gonads for the expression of markers specific to the Sertoli and Leydig cell lineages between 12.5 and 13.5 dpc by in situ hybridization. Expression levels of the Sertoli cell marker, Sox9, were similar in 12.5-dpc wild-type, heterozygous (Fig. 3A), and mutant (Fig. 3B) gonads. Sox9 negative areas corresponding to the interstitium between testis cords (Fig. 3A, arrows) were absent or severely reduced in 12.5-dpc Pdgfr-α−/− gonads (Fig. 3B). The Leydig cell marker, P450 side chain cleavage (Scc), was expressed by many interstitial cells in wild-type XY gonads (Fig. 3C). However, in Pdgfr-α−/− XY gonads, Scc was expressed at much lower levels or not at all (Fig. 3D), indicating that fetal Leydig cell differentiation in these gonads was severely impaired. It has been proposed that the steroid-secreting cells of the gonad and adrenal gland share a common progenitor (Hatano et al. 1996). Normally, Scc-positive cells are readily detected by 12.5 dpc in adrenal samples, as in XY gonads. This expression is unaltered in adrenals from Pdgfr-α−/− embryos (data not shown), suggesting that the defect in Leydig cell differentiation observed in XY gonads does not occur at the level of a common precursor for these two steroid cell populations.
Figure 3.
Expression of male-specific markers in Pdgfr-α−/− XY gonads. (A) Sox9 was expressed in the testis cords of Pdgfr-α+/− XY gonads at 12.5 dpc. Arrows point to interstitial space between testis cords in the Pdgfr-α+/− sample. (B) Sox9 was expressed in 12.5-dpc Pdgfr-α−/− XY gonads, but interstitial spaces between testis cords were absent. (C) Scc, a Leydig cell marker, is expressed extensively in the interstitium of Pdgfr-α+/− XY gonads at 13.5 dpc. (D) Scc was expressed at low levels or was absent in 13.5-dpc Pdgfr-α−/− XY gonads (two representative samples are shown). In 11.5-dpc Pdgfr-α−/− XY gonads, expression of Dhh is absent (F) compared with wild-type or heterozygous littermates (E). By 12.5 dpc, Dhh expression is expressed at normal levels in Pdgfr-α−/− XY gonads (H) compared with control XY gonads (G). (I) At 13.5 dpc, Ptch1 is normally expressed in interstitial cells (arrows). (J) In Pdgfr-α−/− XY gonads, Ptch1 expression is significantly reduced (arrows).
Desert hedgehog (Dhh) signaling was recently shown to be necessary for fetal Leydig cell development (Yao et al. 2002). We examined whether these two pathways interact to direct Leydig cell differentiation. We found that expression of Pdgfr-α and Pdgf-A (data not shown) was unaffected in Dhh−/− XY gonads. Dhh expression was absent in Pdgfr-α−/− XY gonads at 11.5 dpc, when expression has normally initiated in wild-type XY gonads (Fig. 3E,F), but by 12.5 dpc expression was similar to normal levels (Fig. 3G,H). In contrast, expression of the Dhh receptor, Ptch1, is still significantly reduced at 13.5 dpc (Fig. 3J) compared with control samples (Fig. 3I).
Pdgfr-α−/− XY gonads have defects in sex-specific proliferation
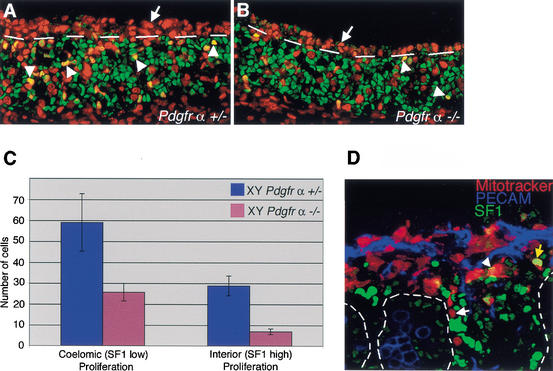
PDGFs have been shown to act as potent mitogens for many different cell types (for review, see Heldin and Westermark 1999). The severe reduction in interstitial and Leydig cells in Pdgfr-α−/− XY gonads could result from a defect in proliferation. To examine proliferation in Pdgfr-α−/− XY gonads, we injected pregnant females with BrdU at stages when characteristic XY proliferation at the coelomic epithelium has been shown to occur (Schmahl et al. 2000). The first wave of XY proliferation (11.2–11.5 dpc) occurs in cells at the coelomic surface that express high levels of Steroidogenic factor 1 (SF1, green) and divide to produce Sertoli cells as well as interstitial cells (Karl and Capel 1998; Schmahl et al. 2000). At 11.5–12.0 dpc, Pdgfr-α+/− and Pdgfr-α−/− XY gonads contained equivalent numbers of SF1-positive cells (green), indicating that initial proliferation was not defective in mutant samples. However, the proportion of proliferating SF1-positive cells within the gonad (below dashed line) was reduced by ∼70% [Fig. 4A,B; arrowheads indicate yellow cells positive for BrdU (red) and SF1 (green)]. In addition, we found that the second wave of proliferation, which occurs in cells at the coelomic surface that express low levels of SF1, was reduced by ∼60% compared with wild-type levels (Fig. 4A,B, red cells above dashed line).
Figure 4.
PDGFR-α is required for the proliferation of interstitial precursors at early stages of testis development. (A,B) At stages between 11.5 and 12.0 dpc, samples were stained with BrdU (red) to detect proliferating cells and SF1 (green) to stain Sertoli and Leydig cell precursors. (A) The coelomic epithelium of Pdgfr-α+/− XY gonads was highly proliferative in a population of cells that expressed low levels of SF1 (red cells above broken line, arrows). There were also many proliferating cells expressing SF1 at high levels within the gonad (arrowheads, yellow nuclei below broken line). (B) Pdgfr-α−/− XY gonads had 60% fewer proliferating cells at the coelomic epithelium (above broken line). SF1-positive proliferation within the gonad (arrowheads, below broken line) was also reduced by 70% compared with controls, as shown graphically in C. (C) Proliferation rates of SF1-low cells at the coelomic epithelium and SF1-high population in the gonad in Pdgfr-α+/− XY gonads (blue) and Pdgfr-α−/− XY gonads (pink). Error bars represent the standard deviation of the sample mean. (D) Mitotracker vital dye (red) was used to label cells at the coelomic epithelium at 22–23 ts in wild-type XY gonads. Samples were cultured for 40 h then stained with PECAM (blue) and SF1 (green). During culture, cells moved into the gonad, but only rarely expressed high levels of SF1, characteristic of Leydig cells (yellow arrow). Most cells derived from the coelomic epithelium were SF1 negative (white arrow) or SF1 low (arrowhead). Cord boundaries are outlined by dashed lines.
Cells proliferating at the coelomic surface at this stage (11.5–12.0 dpc) give rise to unidentified interstitial cells, which could represent progenitors of the Leydig cell population. To test the hypothesis that proliferating cells at the coelomic epithelium represent a significant source for the fetal Leydig cell population during the second stage of proliferation, we labeled the coelomic epithelium of gonads with Mitotracker Orange (Molecular Probes) at the 22–23-tail-somite stage (between 11.5 and 12.0 dpc). Following culture for 40 h, samples were fixed and stained with SF1 (Fig. 4D, green) and PECAM (Fig. 4D, blue). By 12.5 dpc (or after culture of normal gonads for 40 h), cells expressing high levels of SF1 also express Scc, designating these cells as fetal Leydig cells (Yao et al. 2002). After Mitotracker cell labeling, cells migrated into the interstitium during the culture period, as previously described (Karl and Capel 1998). Interestingly, in the cultured samples, labeled cells were mainly SF1 negative (82%, white arrow) and SF1 low (15%, arrowhead), and only rarely expressed high levels of SF1 (3%, yellow arrow). This suggests that most of the cells derived from the coelomic epithelium at this stage do not become Leydig cells and that this cell population does not represent a major source for the fetal Leydig cell lineage.
Pdgfr-α is required in the gonad for normal mesonephric cell migration and Leydig cell differentiation
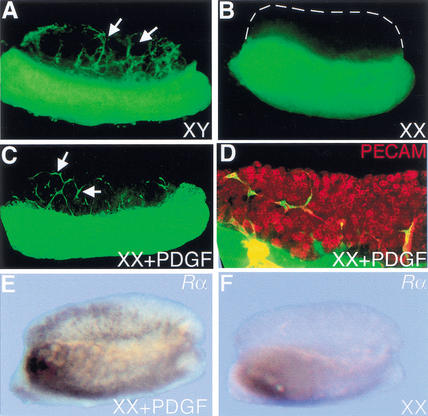
Migration of cells from the mesonephros is an early XY-specific event downstream of Sry (Buehr et al. 1993; Merchant-Larios et al. 1993; Martineau et al. 1997; Capel et al. 1999). The expression pattern of Pdgf-A in Sertoli cells of the XY gonad and the expression of the α receptor on cells of the mesonephros and interstitial cells of the gonad suggested that PDGF-A could be a candidate for the migration factor that induces mesonephric cell migration in XY gonads. To test whether PDGF factors could induce migration in the gonad, we cultured XX gonads in combination with GFP-expressing mesonephrons in the presence of purified PDGF-AA, PDGF-BB, or PDGF-AB factors. In control cultures with no exogenous PDGF, XY gonads recruited cells from the mesonephros (Fig. 5A, arrows), whereas XX gonads did not (Fig. 5B). The addition of 50 ng of PDGF-AA, PDGF-BB, or PDGF-AB to the culture media induced recruitment of cells into the XX gonad after 48 h in culture (Fig. 5C, arrows). Induced samples were labeled with the endothelial marker PECAM (Fig. 5D, red), which showed that all migrating GFP cells were PECAM-positive endothelial cells. Expression of Pdgfr-α was also up-regulated in induced XX gonads compared with cultured XX controls (Fig. 5E,F), although other XY markers such as Sox9 and Scc were not expressed in induced XX samples (data not shown).
Figure 5.
PDGFs induce endothelial cell migration into XX gonads. (A) XY gonads recruited cells (arrows) from GFP mesonephrons when recombinant cultures were assembled at 11.5 dpc and cultured for 48 h, whereas XX gonads did not (B, XX gonad outlined with broken lines). (C) When XX gonads were incubated in culture with 50 ng of PDGF-AA, PDGF-BB, or PDGF-AB, GFP-positive mesonephric cells were recruited into the gonad. (D) Following PDGF induction, samples were labeled with the endothelial and germ-cell marker, PECAM (red), which double-labeled all migrating cells and identified this population as endothelial. (E) Pdgfr-α was up-regulated in induced XX cultures compared with XX control cultures (F).
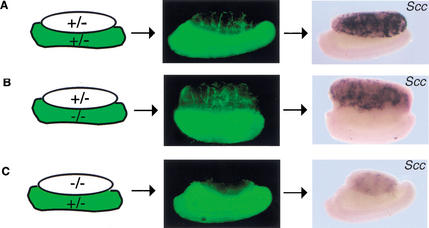
The migration-inducing effect of PDGFs led us to examine whether mesonephric cell migration was abnormal in Pdgfr-α−/− XY gonads. We transferred the GFP transgene onto the Pdgfr-α+/− background to assay for migration in recombinant organ cultures. We assembled gonad/mesonephros cultures at 11.5 dpc (data not shown) and 12.5 dpc in the combinations outlined in Figure 6. By examining combinations with either Pdgfr-α−/− mesonephroi or Pdgfr-α−/− gonads, we could determine whether the requirement for the Pdgfr-α was in the gonad or in the migrating mesonephric cells. After 48 h in culture, samples were examined for the presence of GFP-positive mesonephric cells in the gonad. As shown in Figure 6A, in control cultures, XY gonads recruited mesonephric cells into the interstitium of the gonad. When the gonadal portion of the culture was Pdgfr-α+/− and the mesonephroi was Pdgfr-α−/− (Fig. 6B), cell migration into the gonad was similar to normal control cultures, demonstrating that the Pdgfr-α is not required on migrating mesonephric cells for migration to occur. However, when the gonadal portion was Pdgfr-α−/− and the mesonephric portion was Pdgfr-α+/−, migration was severely impaired (Fig. 6C). This result indicates that PDGFR-α signaling acts indirectly in the cells of the gonad to promote migration by activating a secondary signal.
Figure 6.
Analysis of migration and Leydig cell differentiation in Pdgfr-α−/− recombinant organ cultures. (A) At 12.5 dpc, Pdgfr-α+/+ or Pdgfr-α+/− XY gonads cultured with GFP-positive mesonephrons for 48 h recruited cells from the mesonephros (green, arrows). Following culture, Leydig cells differentiated as assayed by SCC expression. (B) When the gonadal portion of the culture was Pdgfr-α+/− and the mesonephros component was Pdgfr-α−/−, migration of mesonephric cells occurred normally (arrows), and Leydig cell development was normal compared with Pdgfr-α+/+ or Pdgfr-α+/− XY samples (A). (C) When the gonadal portion of the culture was Pdgfr-α−/−, cell migration (arrow) and Leydig cell development were both disrupted.
Samples that were analyzed for GFP migration were subsequently fixed and examined for expression of Scc to assay for Leydig cell development. In Pdgfr-α+/− mesonephros/gonad combinations or when the mesonephric portion of the organ culture was mutant for the receptor, Leydig cells differentiated normally (Fig. 6A,B). However, when the gonadal portion of the culture was Pdgfr-α−/−, Leydig cells failed to differentiate (Fig. 6C), indicating that differentiation of Leydig cells also requires Pdgfr-α in the cells of the gonad and cannot be rescued by a wild-type mesonephros.
Discussion
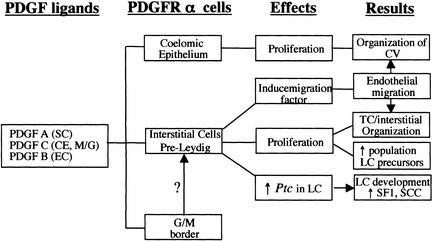
Our studies provide genetic evidence that signaling through PDGFR-α is required for several distinct aspects of early testis development. In Pdgfr-α−/− XY gonads, there are defects in cell proliferation, cell migration, vascularization, and cell fate determination (Fig. 7). These data provide insight into the pathways active downstream of Sry that control early divergent development of the testis.
Figure 7.
Effects of PDGFR-α signaling in the XY gonad. (A) Diagram of the expression of Pdgfr-α and its ligands and the cellular effects promoted by PDGF signaling in the XY gonad. CV, coelomic vessel; CE, coelomic epithelium; EC, endothelial cell; M/G, mesonephros/gonad border; SC, Sertoli cell; LC, Leydig cell.
Mesonephric cell migration, vascular patterning, and development of testis cords are coordinately impaired in Pdgfr-α null mutants
Examination of Pdgfr-α null gonads revealed a delay in cord formation at 12.5 dpc, which resolved by 13.5 dpc into a smaller number of large, dilated cord structures. Coincident with this disruption in testis cord partitioning, we found defects in the formation and branching of the characteristic coelomic vessel of the testis in Pdgfr-α mutants. In wild-type XY gonads, the relatively unpatterned vasculature at 11.5 dpc is significantly remodeled through the induction of endothelial cell migration from the mesonephros into the testis. Most of the vascular growth at this stage occurs through angiogenesis rather than vasculogenesis. Migrating cells contribute heavily to development of the coelomic artery, which forms by 12.5 dpc at the dorso–ventral margin of the XY gonad, and branches in positions where testis cords partition (Brennan et al. 2002). Its descending branches come to lie in close apposition to the boundaries of Sertoli cell cords. It is not yet clear how the formation and branching of this vessel is integrated with the structural development and organization of testis cords. Endothelial or perivascular cells may provide signals that contribute to the morphological development of the testis cord lamina. Evidence for the importance of endothelial cells in organ development and patterning was recently presented in both the liver and pancreas (Lammert et al. 2001; Matsumoto et al. 2001). In Pdgfr-α−/− XY gonads, the disruption of endothelial migration and organization may contribute to the abnormal cord organization that was observed. Further functional studies will be required to address this question.
Normally, the population of Pdgfr-α-expressing cells near the coelomic epithelium expands dramatically in XY gonads between 11.5 and 12.5 dpc as testis cords partition. This proliferation is reduced by 60% in the absence of Pdgfr-α. It is known that this proliferating cell population gives rise to interstitial cells, but their specific cell fate has not been determined. Proliferation in the region where the coelomic vessel normally forms may be required to establish a cellular guidance tract, in which secondary signals direct the migration and assembly of cells to form this artery. Another possibility is that the expansion of this population and their movement to a position between aggregates of Sertoli and germ cells may regulate the efficient partitioning of testis cords through a more general mesenchymal-to-epithelial signaling mechanism. Alternatively, there could be a differentiation or signaling defect in a specific cell type, such as the peritubular myoid cell, leading to disruptions in testis cord patterning.
Our induction experiments showed that PDGFs induce the ectopic recruitment of endothelial cells into XX gonads. Interestingly, our recombinant organ culture experiments indicated that PDGFR-α was required on the cells of the gonad rather than on mesonephric cells to promote endothelial cell migration. These data indicate that PDGF acts as an indirect migratory factor, rather than a primary factor as originally hypothesized.
PDGFR-α is required for early stages of Leydig cell differentiation
The origin of fetal Leydig cells remains controversial (Hatano et al. 1996; Morohashi 1997; Merchant-Larios and Moreno-Mendoza 1998; Nishino et al. 2001). Our data are consistent with the idea that fetal Leydig cells do not have a single lineage origin but are induced among a competent gonadal somatic cell population by a network of signaling interactions.
The near complete absence of Leydig cells in our recombinant assays when the gonadal compartment is Pdgfr-α−/− and the inability of a wild-type mesonephros to rescue development of Leydig cells suggest that PDGFR-α is required in gonadal cells for the specification or expansion of fetal Leydig cells. These results support the idea that Leydig cell precursors are already present in the gonad by 11.5 dpc, and are not derived from the mesonephros after this stage. We cannot rule out the possibility that PDGF signaling is required at earlier stages before a progenitor reaches the gonad; however, two pieces of evidence from this work support an argument against this explanation. (1) The principle evidence for such a progenitor comes from data linking the origin of steroid precursors in the adrenal and the gonad (Hatano et al. 1996). Yet, in Pdgfr-α−/− mice, steroid cell development in the adrenal is normal (data not shown), suggesting that if such a precursor exists, it is not under the control of PDGFR-α signaling. (2) The neural crest is another migratory population that is a source for neuro-endocrine cells that could include Leydig cell precursors (Middendorff et al. 1993; Mayerhofer et al. 1996). Severe defects in some derivatives of the neural crest have been reported in Pdgfr-α−/− mice (Soriano 1997). To investigate whether neural crest contributes to the fetal Leydig cell population, we examined two neural crest reporter lines, P0∷Cre;R26R and Wnt1∷Cre;R26R (Yamauchi et al. 1999; Chai et al. 2000), but found no evidence for a significant contribution of neural crest to the Leydig cell lineage (data not shown).
We initially speculated that the reduced proliferation observed at the coelomic epithelium of Pdgfr-α−/− XY gonads was responsible for the reduction in fetal Leydig cells. However, experiments labeling the coelomic epithelium during this proliferative stage rarely labeled a cell that later differentiated as a Leydig cell as assayed by high levels of SF1 expression (Yao et al. 2002). These experiments suggest that although Leydig cells may differentiate from this population, it is not the major source for this lineage.
PDGFR-α could be important for the early allocation of the Leydig cell lineage or for the subsequent expansion of cells once their fate has been determined. In Pdgfr-α−/− gonads, there are normal numbers of SF1-positive cells at 11.5 dpc, indicating that the early proliferation of somatic precursors occurred normally. Based on the reduction in the second stage of proliferation of SF1-positive cells within the gonad, we propose that PDGF signaling normally promotes the expansion of the Leydig precursor population.
Thus far, Dhh is the only other molecule that has a designated role in fetal Leydig cell specification. As in Pdgfr-α null mutants, fetal Leydig cells are reduced or absent in Dhh−/− gonads (Yao et al. 2002). We found that expression levels of Pdgf-A and Pdgfr-α are unaffected in Dhh−/− gonads, but Dhh expression is delayed in Pdgfr-α−/− XY gonads. The interstitial defects observed in Pdgfr-α−/− XY gonads could contribute to this delay in Dhh expression. Normally, DHH up-regulates expression of its receptor, Ptch1, in cells exposed to DHH. In the XY gonad, this occurs soon after the onset of Dhh expression, but in Pdgfr-α−/− samples, Ptch1 remained at low levels at 13.5 dpc, more than 24 h after the onset of Dhh expression. This suggests that Pdgfr-α may function in parallel with Dhh to promote the expansion and/or differentiation of Ptch1-positive cells. Based on our data, there are two likely ways in which this could occur. (1) Defects in proliferation could alter the number of Ptch1 precursor cells. (2) The structural and endothelial defects in Pdgfr-α−/− gonads could interfere with the organizational and signaling cues normally required to specify Leydig cell fate. Ongoing experiments are aimed at distinguishing between these two possibilities.
Thus far, the only known critical factors for Leydig cell differentiation are Dhh and a newly identified factor Arx (Kitamura 2002). Our data identify Pdgfr-α as another critical factor and provide additional insight into the origin and differentiation of the fetal Leydig cell lineage.
PDGFR-α ligands induce distinct aspects of testis development
There are three potential PDGF ligands that activate PDGFR-α signaling. By in situ hybridization, we could detect expression of Pdgf-B in the testis at low levels in scattered endothelial cells between 11.5 and 13.5 dpc. Because of its restricted expression pattern and low levels of expression, we suspect that PDGF-B is not the major ligand for PDGFR-α, but may contribute to the vascular defects in XY gonads. Pdgf-A is expressed at high levels throughout the XY gonad and coelomic epithelium 11.5–12.0 dpc, before expression is sequestered inside testis cords. This expression pattern puts PDGF-A in the correct position for promoting the proliferation of cells at the coelomic epithelium during these stages as well as promoting mesonephric cell migration and/or establishing the vascular domain. Loss of signaling in this pathway may contribute to vascular defects, and to the delay and defects in cord formation. Defects in the development of adult Leydig cells have been reported in Pdgf-A−/− mice (Gnessi et al. 2000). Direct examination of Scc expression in Pdgf-A−/− testes at 12.5–14.5 dpc was not reported; however, indirect evidence suggested that Pdgf-A−/− mice had normal numbers of fetal Leydig cells (Gnessi et al. 2000). This implied that another ligand is important for signaling through the α receptor to support fetal Leydig cell development early in testis development.
Pdgf-C, which is expressed at high levels at the gonad/mesonephros border and in scattered cells in both the XX and XY gonad at early stages, could be involved in fetal Leydig cell development. Pdgf-C is expressed in an identical pattern in both XX and XY gonads; thus, PDGF signaling could promote development of steroid cell precursors in both XX and XY gonads. In the XX gonad, these cells could remain dormant until signals are present to direct differentiation into theca cells, the analogous steroid-secreting cell in the XX gonad. Although we could induce migration and the up-regulation of Pdgfr-α in XX gonads with exogenous PDGF, we could not detect Leydig cell differentiation. This suggests that an additional step is required. The absence of Leydig cell differentiation in XX gonads could be attributed to the presence of female-specific factors that repress Leydig cell development. One female-specific factor, WNT4, has a demonstrated role in repressing steroid cell development in the XX gonad (Vanio et al. 1999). Recently, this activity has been shown to be a function of the inhibition of ectopic steroid cell migration from the adrenal gland rather than endogenous steroid cell development within the gonad (Heikkila et al. 2002), and thus is probably not relevant to the signaling pathways that normally control Leydig cell development in the XY gonad. Alternatively, there could be a requirement for other XY-specific factors, such as DHH, to trigger Leydig cell differentiation.
Our experiments have identified PDGFR-α as a key player in the pathway downstream of Sry that orchestrates the morphological and cellular development of the early testis. Signaling through this receptor is critical for several important events including organization of endothelial cells, partitioning of testis cords, proliferation, cell migration, and Leydig cell development. Future experiments will address the interaction of PDGF with other signaling pathways that promote morphological and cellular development in the testis.
Materials and methods
Mouse strains, matings, and organ culture
129SV/J females were mated to C57BL/6 Pdgfr-α+/− males (kindly provided by Philippe Soriano) to yield male and female F1 offspring heterozygous for the Pdgfr-α null allele. Siblings were then intercrossed to yield Pdgfr-α−/− embryos for experimental analysis. There was no detectable difference in phenotype of heterozygous and wild-type littermates; therefore, control samples were designated Pdgfr-α+/− and reflect either a wild-type or heterozygous sample. For assays involving the analysis of mesonephric cell migration, random-bred females possessing a ubiquitously expressed GFP transgene [TgN(GFPU)5Nagy; Hadjantonakis et al. 1998] were mated to C57BL/6 Pdgfr-α+/− males to generate hybrid offspring that were hemizygous for the GFP transgene and heterozygous for the Pdgfr-α null allele. Siblings were then intercrossed to yield embryos for organ culture experiments. Gonads and mesonephrons from these embryos were dissected in PBS, assembled in agar blocks, and cultured for 48 h as previously described (Martineau et al. 1997). Dhh−/− mice (mixed genetic background) were kindly provided by Wendy Whoriskey (Curis, Inc.). Pdgfr-α and Pdgfr-β mice were kindly provided by Phillipe Soriano.
To sex embryos before sexual dimorphism was apparent, amnions from individual embryos were saved and stained for the presence of nuclei with the condensed chromatin body (Barr body) found in XX cells (Palmer and Burgoyne 1991). Tail somite (ts) stages were counted as previously described (Hacker et al. 1995), where 18 ts corresponds to 11.5 dpc and 30 ts corresponds to 12.5 dpc.
Genotyping
Genotypes of Pdgfr-α mice and embryos were determined by isolating genomic DNA from tissue samples incubated in buffer (0.05 M Tris at pH 7.5, 0.1 M EDTA, 0.5% SDS in water, and 50 μg/mL Proteinase K) at 55°C overnight. DNA was prepared for PCR by phenol:chloroform extraction and ethanol precipitation. The PCR primer sets were PDGFR-α1, CCCTTGTGGT CATGCCAAAC; PDGFR-α2, GCTTTTGCCTCCATTACAC TGG; Neo, ACGAAGTTATTAGGTCCCTCGAC. PDGFR-α1 and PDGFR-α2 amplified a wild-type fragment of ∼500 bp. PDGFR-α1 and Neo amplified a mutant fragment of ∼250 bp. The annealing temperature was 62°C for both primer sets.
Immunohistochemistry, cell labeling, and in situ hybridization
Samples that were used for immunohistochemistry were fixed in 4% paraformaldehyde/PBS at 4°C overnight. On the following day, samples were rinsed twice in PBS and blocked at room temperature for 1 h in PBS/1% HIGS (heat-inactivated goat serum)/5% BSA (bovine serum albumin)/0.1% Triton-X 100. Primary antibody incubations were carried out at 4°C overnight in blocking solution (1/500 dilution of rabbit anti-laminin1 antibody, provided by Harold Erikson; 1/500 dilution of rat anti-PECAM antibody, Pharmingen; 1/200 dilution of rabbit anti-SF1, provided by Ken Morohashi). Secondary antibody incubations were performed at 4°C overnight with 1/500 dilution of fluorescently conjugated secondary antibodies (Jackson Immunochemicals). Washes were three times for 1 h in PBS plus 0.1% Triton X-100.
For BrdU experiments, pregnant females were injected with 50 mg/kg BrdU 2 h before dissection of embryonic gonads. Staining was carried out as previously described (Schmahl et al. 2000). Samples were mounted on glass slides in DABCO and subjected to confocal microscopy to detect immunofluoresence. Images were collected on a Zeiss LSM confocal microscope and processed using Adobe Photoshop. To obtain comparable sections, the three most central sections from each gonad were counted and averaged. An XY gonad from three different Pdgfr-α−/− embryos was collected and compared with four control gonads.
For labeling of coelomic epithelial cells, a mixture of Mitotracker Orange (Molecular Probes, Inc.) and CRSE (Molecular Probes, Inc.) was pipetted onto the surface of the gonad as previously described (Karl and Capel 1998; Darnell et al. 2000). Samples were cultured on agar plates for 40 h. Fixed samples were embedded in 3:1 Oct:20% sucrose and cryosectioned at 10 μm thickness and stained with anti-SF1 and anti-PECAM antibodies. Representative sections (where labeled cells had migrated beneath the coelomic vessel into regions of SF1-positive cells) from four different gonads were counted and averaged separately for each gonad and then averaged together for a final average percentage of SF1-negative, SF1-low, and SF1-high cells.
In situ hybridization was performed according to Henrique et al. (1995) with probes against Pdgf-A, Pdgf-B, Pdgfr-α, Pdgfr-β (provided by Christer Betsholtz), Pdgf-C (purchased from Incyte), Dhh (provided by Andrew McMahon), Ptch1 and Sox9 (provided by Peter Koopman), and Scc (provided by Keith Parker).
Acknowledgments
We are grateful to Philippe Soriano for providing Pdgfr-α and Pdgfr-β mice. We also thank Wendy Whoriskey (Curis, Inc.) for sending Dhh mice for analysis and Erik Myers for providing P0∷Cre;R26R and Wnt1∷Cre;R26R mice. In situ probes were kindly provided by Christer Betsholz (Pdgr-α, Pdgr-β, Pdgf-A, Pdgf-B), Andrew McMahon (Dhh), Peter Koopman (Ptch1, Sox9), and Keith Parker (Scc). Valuable antibodies were generously provided by Ken-ichirou Morohashi (SF1) and Harold Erikson (Laminin-1). We appreciate thoughtful comments on the manuscript provided by Humphrey Yao and Andrea Ross. This work was funded by a grant from the NIH (HL63054-01).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL b.capel@cellbio.duke.edu; FAX (919) 684-5481.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1052503.
References
- Ariyaratne S, Mendis-Handagama C, Hales B, Mason I. Studies on the onset of Leydig precursor cell differentiation in the prepubertal rat testis. Biol Reprod. 2000;63:165–171. doi: 10.1095/biolreprod63.1.165. [DOI] [PubMed] [Google Scholar]
- Betsholtz C, Karlsson L, Lindahlm P. Developmental roles of platelet-derived growth factors. Bioessays. 2001;23:494–507. doi: 10.1002/bies.1069. [DOI] [PubMed] [Google Scholar]
- Bostrom H, Willetts K, Pekny M, Leveen P, Lindahl P, Hedstrand H, Pekna M, Hellstrom M, Gebre-Medhin S, Schalling M, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- Brennan J, Karl J, Capel B. Divergent vascular mechanisms downstream of Sry establish the arterial system in the XY gonad. Dev Biol. 2002;244:418–428. doi: 10.1006/dbio.2002.0578. [DOI] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- Capel B, Albrecht KH, Washburn LL, Eicher EM. Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech Dev. 1999;84:127–131. doi: 10.1016/s0925-4773(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Darnell DK, Garcia-Martinez V, Lopez-Sanchez C, Yuan S, Schoenwolf GC. Dynamic labeling techniques for fate mapping, testing cell commitment, and following living cells in avian embryos. Methods Mol Biol. 2000;135:305–321. doi: 10.1385/1-59259-685-1:305. [DOI] [PubMed] [Google Scholar]
- Ding H, Wu X, Kim I, Tam PP, Koh GY, Nagy A. The mouse Pdgfc gene: Dynamic expression in embryonic tissues during organogenesis. Mech Dev. 2000;96:209–213. doi: 10.1016/s0925-4773(00)00425-1. [DOI] [PubMed] [Google Scholar]
- Gnessi L, Emidi A, Jannini EA, Carosa E, Maroder M, Arizzi M, Ulisse S, Spera G. Testicular development involves the spatiotemporal control of PDGFs and PDGF receptors gene expression and action. J Cell Biol. 1995;131:1105–1121. doi: 10.1083/jcb.131.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnessi L, Basciani S, Mariani S, Arizzi M, Spera G, Wang C, Bondjers C, Karlsson L, Betsholtz C. Leydig cell loss and spermatogenic arrest in platelet-derived growth factor (PDGF)-A deficient mice. J Cell Biol. 2000;149:1019–1025. doi: 10.1083/jcb.149.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Non-invasive sexing of preimplantation stage mammalian embryos. Nat Genet. 1998;19:220–222. doi: 10.1038/893. [DOI] [PubMed] [Google Scholar]
- Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF1. Genes Cells. 1996;1:663–671. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vaino S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Lindahl P, Heath JK, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-α deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–3466. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–369. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- Klinghoffer RA, Mueting-Nelsen PF, Faerman A, Shani M, Soriano P. The two PDGF receptors maintain conserved signaling in vivo despite divergent embryological functions. Mol Cell. 2001;7:343–354. doi: 10.1016/s1097-2765(01)00182-4. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rβ signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Lahr G, Seidl K, Eusterschulte B, Christoph A, Gratzl M. The neural cell adhesion molecule (NCAM) provides clues to the development of testicular Leydig cells. J Andrology. 1996;17:223–230. [PubMed] [Google Scholar]
- Merchant-Larios H, Moreno-Mendoza N. Mesonephric stromal cells differentiate into Leydig cells in the mouse fetal testis. Exp Cell Res. 1998;244:230–238. doi: 10.1006/excr.1998.4215. [DOI] [PubMed] [Google Scholar]
- Merchant-Larios H, Moreno-Mendoza N, Buehr M. The role of the mesonephros in cell differentiation and morphogenesis of the mouse fetal testis. Intl J Dev Biol. 1993;37:407–415. [PubMed] [Google Scholar]
- Middendorff R, Davidoff M, Holstein AF. Neuroendocrine marker substances in human Leydig cells—Changes by disturbances of testicular function. Andrologia. 1993;25:257–262. doi: 10.1111/j.1439-0272.1993.tb02722.x. [DOI] [PubMed] [Google Scholar]
- Morohashi K. The ontogenesis of the steroidogenic tissues. Genes to Cells. 1997;2:95–106. doi: 10.1046/j.1365-2443.1997.1060304.x. [DOI] [PubMed] [Google Scholar]
- Nishino K, Yamanouchi K, Naito K, Tojo H. Characterization of mesonephric cells that migrate into the XY gonad during testis differentiation. Exp Cell Res. 2001;267:225–232. doi: 10.1006/excr.2001.5238. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne PS. In situ analysis of fetal, prepubertal and adult XX–XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development. 1991;112:265–268. doi: 10.1242/dev.112.1.265. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127:65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF β-receptor mutant mice. Genes & Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- ————— The PDGF α receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- Tilmann K, Capel B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999;126:2883–2890. doi: 10.1242/dev.126.13.2883. [DOI] [PubMed] [Google Scholar]
- Uzumcu M, Dirks KA, Skinner MK. Inhibition of platelet-derived growth factor actions in the embryonic testis influences normal cord development and morphology. Biol Reprod. 2002;66:745–753. doi: 10.1095/biolreprod66.3.745. [DOI] [PubMed] [Google Scholar]
- Vanio S, Heikkila M, Kispert A, Chin N, McMahon A. Female development in mammals is regulated by Wnt-4 signaling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Abe K, Mantani A, Hitoshi Y, Suzuki M, Osuzu F, Kuratani S, Yamamura K. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol. 1999;212:191–203. doi: 10.1006/dbio.1999.9323. [DOI] [PubMed] [Google Scholar]
- Yao HH, Whoriskey W, Capel B. Desert hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes & Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]