Abstract
In Caenorhabditis elegans, the decision to enter a developmentally arrested dauer larval stage is triggered by a combination of signals from sensory neurons in response to environmental cues, which include a dauer pheromone. These sensory inputs are coupled to the parallel DAF-2/insulin receptor-like and DAF-7/TGFβ-like signaling pathways. Although sensory inputs have been shown to physiologically regulate DAF-7/TGFβ expression, no such regulation of insulin-like ligands in the DAF-2 pathway has been reported. We show here that daf-28 encodes an insulin-like protein, which when mutated causes dauer arrest and down-regulation of DAF-2/IR signaling. A daf-28∷GFP fusion gene is expressed in ASI and ASJ, two sensory neurons that regulate dauer arrest. daf-28∷GFP expression in ASI and ASJ is down-regulated under dauer-inducing conditions and in mutants of DAF-11/guanylyl cyclase, a predicted component of the dauer-pheromone-sensing pathway. Thus, daf-28 expression in sensory neurons is regulated by the environmental cues that normally trigger dauer arrest. Among the 38 C. elegans insulin genes, daf-28 is so far the only insulin mutant to affect dauer arrest. daf-28 was revealed from this functional redundancy by a dominant-negative allele that disrupts a probable proteolytic processing site required for insulin maturation. This DAF-28 mutant is likely to be poisonous to wild-type DAF-28 and other insulins.
Keywords: Insulin signaling, TGFβ signaling, Caenorhabditis elegans, dauer, pheromone, furin
Environmental conditions determine whether Caenorhabditis elegans grows directly into adulthood or arrests at an alternative L3 larval stage to form a dauer larva (Cassada and Russell 1975). Dauer larvae are induced by harsh environmental conditions such as starvation, high population density, or high temperature. Dauers can survive in harsh environments because of their distinctive adaptive features in morphology, behavior, metabolism, and life span. When environmental conditions improve, a dauer undergoes a series of developmental changes and reenters the reproductive cycle by molting into an L4 larva and subsequently into an adult.
Molecular genetic analysis of dauer arrest has revealed that the dauer versus reproductive growth decision is controlled by at least two signaling cascades: the DAF-2 (insulin/IGF-1 receptor-like; Kimura et al. 1997) and the DAF-7 (TGFβ-like) pathways (Ren et al. 1996; Schackwitz et al. 1996). A decrease in either of the signals causes dauer arrest, indicating that both pathways are required for reproductive growth. Downstream components of the DAF-2 pathway include the AGE-1/PI3 kinase (Morris et al. 1996), PDK-1/PDK1 (Paradis et al. 1999), AKT-1 and AKT-2/Akt/PKB (Paradis and Ruvkun 1998), DAF-18/PTEN (Ogg and Ruvkun 1998), and the DAF-16/forkhead transcription factor (Lin et al. 1997; Ogg et al. 1997). Down-regulation of DAF-2 results in nuclear localization and thus activation of the DAF-16 transcription factor (Henderson and Johnson 2001; Lee et al. 2001; Lin et al. 2001). The daf-2 signaling pathway also regulates metabolism and aging, analogously and probably homologously to the regulation of metabolism by insulin signaling in mammals. When daf-2 signaling is decreased, life span is greatly extended (Kenyon et al. 1993; Larsen et al. 1995). Insulin-like signaling has recently been shown to regulate longevity in mice as well (Bluher et al. 2003; Holzenberger et al. 2003). Because the dauer arrest, longevity, and metabolic-shift phenotypes caused by the daf-2(lf) (lf: loss-of-function) or age-1(lf) mutation are fully suppressed by a daf-16(lf) mutation (Gottlieb and Ruvkun 1994), daf-16 is the major output of DAF-2/IR signaling.
daf-7 encodes a divergent member of the TGFβ superfamily that is a ligand for the parallel neuroendocrine pathway (Ren et al. 1996; Schackwitz et al. 1996). Downstream components of the DAF-7 pathway include the DAF-1/TGFβ type I receptor (Georgi et al. 1990; Gunther et al. 2000), DAF-4/TGFβ type II receptor (Estevez et al. 1993), DAF-8, DAF-14 and DAF-3/Smads (Patterson et al. 1997; Inoue and Thomas 2000), and DAF-5 (Riddle et al. 1981; Thomas et al. 1993). A nearly congruent mammalian TGFβ signaling pathway has also been detected in mammals, and although its regulation of metabolism and convergence with insulin signaling have not yet been noted, there are indications that these pathways do intersect (McPherron et al. 1997; McPherron and Lee 2002). Similarly to daf-16 in the insulin-like pathway, daf-3 and daf-5 are the major outputs of the DAF-7/TGFβ-like pathway.
Sensory neurons mediate initiation of dauer arrest in response to environmental input of population density (Albert et al. 1981; Perkins et al. 1986; Vowels and Thomas 1994). A pheromone reflecting this population density is the normal physiological trigger of dauer arrest. Although its biochemical identity remains unknown, dauer pheromone has been surmised to be a small fatty acid, based on its partitioning pattern. Addition of a crude pheromone preparation to wild-type C. elegans causes dauer arrest (Golden and Riddle 1982, 1984). Of all the sensory inputs to dauer arrest, although food and temperature are modulatory, dauer pheromone is the most potent regulator and can overcome, for example, low temperature and high food concentrations, which favor reproductive development.
Proper morphology and function of sensory neurons are necessary for pheromone-induced dauer arrest (Albert et al. 1981; Perkins et al. 1986; Vowels and Thomas 1994). Mutant animals defective in ciliated sensory neurons or the glial-like sheath cells that facilitate exposure of sensory neurons to the environment do not arrest as dauers when exposed to dauer pheromone, but do form dauers if the animals also carry a mutation in the insulin-like or TGFβ-like neuroendocrine signaling pathways. This indicates that sensory neurons are involved in responding to dauer pheromone to, in turn, regulate the production of neuroendocrine insulin-like and TGFβ-like signals. A set of 12 sensory neurons in the head amphid sensilla has ciliated endings, and 8 of them are directly exposed to the environment (Perkins et al. 1986). Laser ablation of three of these sensory neurons, ADF, ASG, and ASI, causes dauer arrest, indicating that these neurons signal reproductive growth and lack of such signals causes dauer arrest (Bargmann and Horvitz 1991). Similarly, ablation of the sensory neuron ASJ inhibits dauer recovery (Bargmann and Horvitz 1991). The DAF-7 TGFβ-like protein is clearly one of those neuroendocrine signals: a daf-7∷GFP transcriptional fusion gene is expressed in the ASI neuron, and its expression is down-regulated by dauer pheromone (Ren et al. 1996; Schackwitz et al. 1996).
The dauer pheromone is likely to be detected by an as yet unidentified pheromone receptor that couples to a cyclic GMP signaling pathway that includes daf-11. Mutations in DAF-11, a transmembrane guanylyl cyclase that is expressed in ciliated sensory neurons, including ASI and ASJ, cause dauer arrest (Birnby et al. 2000). This observation suggests that the signaling pathway downstream of the pheromone receptor uses a cGMP second messenger, as do G-protein-coupled receptors involved in olfaction and vision (Birnby et al. 2000). Moreover, cGMP-gated channels have also been implicated in regulation of dauer arrest (Coburn and Bargmann 1996; Komatsu et al. 1996; Ailion and Thomas 2000). Because dauer arrest caused by daf-11(lf) can be partially suppressed either by daf-3(lf) or daf-16(lf), DAF-11 is likely to regulate both the DAF-7/TGFβ and the DAF-2/IR pathways (Thomas et al. 1993; Gottlieb and Ruvkun 1994; Riddle 1997).
Many of the 37 C. elegans ins (insulin-like) genes identified by genome analysis are expressed in sensory neurons (Pierce et al. 2001), consistent with the expected sensory input to the daf-2 pathway. Sensory neurons have been implicated in control of life span (Apfeld and Kenyon 1998), perhaps by regulating the production and/or secretion of insulin-like ligands, which, in turn, engage the daf-2 pathway. However, there is not yet direct evidence linking sensory neuron function to ins gene activities.
Although multiple components of the DAF-2/IR pathway have been isolated from various genetic screens, no loss-of-function (lf) mutation has been found in an ins gene that might encode a ligand for DAF-2/IR. Failure to isolate such an lf mutation may be attributed to the possible redundant functions of the many ins genes in dauer arrest. Among the ins genes, ins-1 and ins-18 have been shown to regulate dauer arrest and life span, respectively. Increased ins-1 gene dosage promotes dauer arrest, most likely by antagonizing DAF-2 activity (Pierce et al. 2001). INS-18 has been suggested to regulate life span by activating DAF-2, based on RNAi (RNA interference) experiments (Kawano et al. 2000).
daf-28(sa191) has been isolated from a screen for mutants that arrest at the dauer stage (Malone and Thomas 1994). daf-28(sa191) causes transient dauer arrest and a 10% extension in life span. daf-28(sa191) dauer arrest is partially suppressed by a daf-16(lf) mutation . Because daf-28(sa191) shares the extended life span and suppression by daf-16 features with mutations in the daf-2 insulin-like pathway, daf-28 was predicted to function in the daf-2 pathway (Malone et al. 1996). Genetic analysis also showed that daf-28(sa191) is a semidominant allele that poisons daf-28 wild-type function (Malone and Thomas 1994).
We show here that daf-28 encodes an insulin-like protein that regulates DAF-16 nuclear localization. Therefore, we propose that DAF-28 functions as a ligand of DAF-2. A daf-28∷GFP transcriptional fusion is expressed in two pairs of sensory neurons, ASI and ASJ, and this expression is down-regulated in response to dauer-inducing environmental and sensory signals. We propose that daf-28 is expressed in sensory neurons to inhibit dauer arrest as a DAF-2 agonist. The daf-28(sa191) mutation R37C disrupts a probable proteolytic cleavage site that is expected to be necessary for processing the DAF-28 propeptide into a mature insulin. We propose that the DAF-28R37C mutant protein acts as a competitive inhibitor to down-regulate the activities of DAF-28+ and other INS proteins. Consistent with this hypothesis, increased gene dosage of DAF-28+ or its closest relatives INS-4 or INS-6 suppresses the dauer arrest caused by DAF-28R37C. daf-28 has a broadened expression pattern in aged animals and increased expression levels in animals defective in sensory functions, suggesting that the production of insulin-like neuroendocrine signals is influenced by aging-related factors and sensory neuron functions.
Results
daf-28 encodes an insulin/IGF-1-like protein
daf-28(sa191) has been genetically mapped to the right arm of Chromosome V, between exp-3 and rol-9 (Malone and Thomas 1994). To further determine the molecular interval in which daf-28 resides, we used an SNP (single nucleotide polymorphism) mapping strategy (Wicks et al. 2001). By SNP mapping in 30 recombinants between the exp-3 and rol-9 visible markers, we found a pair of SNP markers that define the left and right molecular boundaries of the region containing the daf-28 mutation (for details, see Materials and Methods). These two SNP markers reside in Y116F11B.2 and Y116F11B.5, respectively. Of the two predicted genes between Y116F11B.2 and Y116F11B.5, Y116F11B.1 encodes a β-type insulin/IGF-1-like molecule that is most similar to INS-4 and INS-6. Y116F11B.1 was not identified in the previous searches for ins genes (Pierce et al. 2001), because the sequences in this particular region were only recently included in the database. Given that daf-28 is likely to couple to the DAF-2/IR signaling pathway, Y116F11B.1 was a good candidate for daf-28.
Sequencing the predicted coding region of Y116F11B.1 from the daf-28(sa191) strain revealed a C-to-T transition in the first exon, typical of EMS (ethylmethanesulfonate) mutagenesis. This mutation causes an arginine-to-cysteine substitution at position 37 in the protein sequence. No additional mutation was found in the 4-kb interval that should include the coding and the regulatory sequences of Y116F11B.1. This R37C substitution in DAF-28 disrupts a probable proteolytic cleavage site that is predicted to be required to excise the F-peptide (Fig. 1A,B). The 37 known ins genes in C. elegans have been grouped into three classes based on the arrangement of intramolecular disulfide bonds (Pierce et al. 2001). The γ-type is predicted to form the three canonical disulfide bonds found in mammalian insulin and insulin-related proteins and therefore is structurally most similar to human insulin. The β-type is predicted to form an extra disulfide bond in addition to the canonical three. The α-type is predicted to form three disulfide bonds but with different positioning compared with the γ-type. Based on the predicted proteolytic cleavage sites, these INS proteins fall into three different groups (Fig. 1B, a–c). The β-type INS-1 and the γ-type INS-18 are the only two insulins in C. elegans that are predicted to have a C-peptide with flanking dibasic residue cleavage sites; upon processing, the A and the B chains are joined by disulfide bonds to form a mature insulin, like human insulin. In the remaining INS proteins, the A and B domains probably stay attached to form a continuous mature peptide, like human insulin-like growth factors. Most members of the β-type, including INS-4 and INS-6, contain an F-peptide at the N terminus of the proinsulin that is predicted to be removed by cleavage at an RXXR site. The remaining proinsulins in C. elegans do not have obvious proteolytic processing sites (Pierce et al. 2001).
Figure 1.
(A) Alignment of the propeptide sequences of the β-type insulin-like genes in C. elegans, except for that of INS-1 and INS-10. The partial protein sequences only exclude the predicted signal peptides (predomain) from the predicted full-length prepropeptide sequences. The blue box outlines the furin-like cleavage site RXXR (also see B legend); the first arginine residue is mutated to cysteine in daf-28(sa191). The red boxes indicate the aligned four pairs of cysteine residues. Hydrophobic residues important for helix formation are in bold. The numbers in green are the percentages of identical residues of each propeptide shared with DAF-28. (B) Three groups of C. elegans insulins classified on the basis of predicted proteolytic processing sites. All preproinsulins in C. elegans at least contain a signal peptide (predomain) and A and B domains. The signal peptide is removed by signal peptidase to form a proinsulin, and the A and B domains become part of a mature insulin. (a) DAF-28 and eight other β-type insulins are predicted to have a cleaved F-peptide between the signal peptide and the B domain; the predicted proteolytic processing site between the F-peptide and the B domain is RXXR, which is recognized by a proprotein convertase similar to mammalian protease furin. The consensus and the minimal sequences for furin cleavage are RXRR and RXXR, respectively. (b) β-Type INS-1 and γ-type INS-18 are the only two insulins in C. elegans that are predicted to bear a C-peptide. The C-peptide is flanked by a dibasic residue cleavage site on either side, which are predicted to be recognized by a proprotein convertase similar to the mammalian protease PC2. (c) The remaining proinsulins in C. elegans do not have obvious endoproteolytic sites. (d) Human insulin has a cleaved C-peptide.
The dauer arrest phenotype of daf-28(sa191) is semidominant. Therefore, we expected transgenes containing the wild-type daf-28 gene to suppress the dauer arrest phenotype of daf-28(sa191) homozygotes. To test whether Y116F11B.1 contains the wild-type activity of daf-28, we transformed daf-28(sa191) animals with a 4-kb PCR fragment of Y116F11B.1, amplified from wild-type N2 genomic DNA. This fragment contains 3.3 kb 5′ to the coding region, the entire 700-bp coding sequence, and 45 bp 3′ to the coding region of Y116F11B.1 (also see Table 1). Transgenic lines carrying this PCR fragment suppress daf-28(sa191) dauer arrest, indicating that Y116F11B.1 carries daf-28+ activity (Fig. 2). The equivalent PCR fragment amplified from daf-28(sa191) genomic DNA did not suppress daf-28(sa191) dauer arrest (Fig. 2). A transgene generated from daf-28(sa191) genomic DNA with C37 reverted to the wild-type R completely restored the daf-28+ activity (Fig. 2), indicating that the original R37C substitution found in daf-28(sa191) is causal for the dauer arrest phenotype. A daf-28 transgene containing a R37A substitution only partially suppressed the dauer arrest caused by daf-28(sa191) homozygotes, suggesting that R37 contributes to the DAF-28 wild-type activity. This is consistent with R37 being part of the predicted proteolytic cleavage site. We conclude that DAF-28 is encoded by Y116F11B.1 and the dauer arrest caused by daf-28(sa191) is due to the R37C substitution.
Table 1.
Transgenes containing daf-28 and ins genes
| Gene
|
Size of 5′-flanking region (kb)
|
Size of 3′-flanking region (kb)
|
Sequences of the forward primer
|
Sequences of the reverse primer
|
|---|---|---|---|---|
| daf-28 | 3.3 | 0.45 | TTCCACCATTGGTAGGATAGTTGGTTGCCG | TAGAGGAAGAGCGGGGGGGGGAGATC |
| ins-2 | 4.0 | 0.16 | TTGTAACCTATCGATTAATGTTCTCAATC | CGCATTGATATCATATGATACATTACGTGC |
| ins-4 | 5.5 | 0.11 | GCTGATCGGTCGCTTAGAATTG | AGGAATTGGTTGCACATGG |
| ins-6a | 1.7 | 0.12 | CATTTGACTAATTGGATCCCAG | TTCGCATGGAAATAGACTTCCG |
| ins-7a | 0.66 | 0.18 | CTATTCATCCATCCTTGCTTCC | GAAGGTTGAGATTGTTTCAAGC |
| ins-9a | 1.2 | 0.15 | GACGAGCAGGCACAAGAATAAACG | ATATCAGTATAAATTATTTAACCTGAACCG |
| ins-17 | 3.9 | 0.57 | GGTCGACTTCCAGAATTATGATAAGTG | CAAGCATGATTGGTGTGGACATAC |
| ins-21 | 3.0 | 2.2 | CCGAAACATTCATCATTCCGATAC | TGATCAACAAGGTTTATTGAACGG |
| ins-22a | 2.2 | 1.6 | as above | as above |
| ins-23a | 1.6 | 0.14 | as above | as above |
aMaximum lengths of promoter sequences were included without including the upstream genes.
Figure 2.
(A) Percentages of animals that bypassed dauer arrest in the daf-28(sa191) mutant that either carry the indicated transgene (first 12 columns from left) or the ins-1(nr2091) deletion (right-most column). Above each column is the total number of animals scored. The numbers represent the summary of at least two, and in most cases three, independent transgenic lines for each construct. The error bars are standard deviations among the transgenic lines.
The model of daf-28(sa191) encoding a poison product also predicts that a transgene containing DAF-28R37C will cause dauer arrest in a wild-type background. We observed a low level of dauer arrest in wild-type animals carrying the DAF-28R37C transgene, although the effect is weak and variable (data not shown). However, in a daf-28+/Df (Df: deficiency) strain, in which the dose of daf-28+ is reduced to half, the transgene carrying DAF-28R37C caused more penetrant dauer arrest. Two independent lines of daf-28+/Df animals carrying such transgenes displayed 29% (n = 231) and 17.6% (n = 136) dauer arrest, respectively. In contrast, the daf-28+/Df animals carrying the DAF-28+ transgene displayed 0% dauer arrest (n = 200). This result suggests that DAF-28R37C is weakly dominant, consistent with the model that DAF-28R37C poisons DAF-28+ activity in vivo.
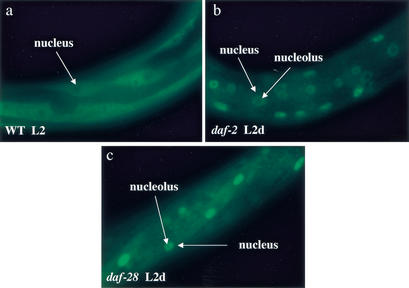
DAF-16 protein localizes to the nucleus upon down-regulation of DAF-2 activity (Henderson and Johnson 2001; Lee et al. 2001; Lin et al. 2001). Detection of DAF-16∷GFP subcellular localization in vivo provides a sensitive measure of DAF-2 activity. To test whether daf-28(sa191) causes dauer arrest because it down-regulates daf-2 signaling, we examined the subcellular localization of DAF-16∷GFP in daf-28(sa191) animals. At the L2d stage, a preparatory stage for dauer arrest, we observed nuclear localization of DAF-16∷GFP in daf-28(sa191) animals, in contrast to cytoplasmic localization of DAF-16∷GFP in wild-type animals at an equivalent developmental stage, L2 (Fig. 3). This nuclear localization occurred in multiple cell types, including hypodermal, muscle, and neuronal cells, similar to what has been observed in daf-2(lf) animals (Henderson and Johnson 2001; Lee et al. 2001; Lin et al. 2001). These data suggest that daf-28(sa191)causes dauer arrest by antagonizing DAF-2 activity.
Figure 3.
DAF-16∷GFP subcellular localization in mostly hypodermal cells located in the middle section of the animal (40× optics). (a) Cytoplasmic localization of DAF-16∷GFP in wild-type N2 at the L2 larval stage. (b) Nuclear localization of DAF-16∷GFP in the daf-2(e1370) animals (Lee et al. 2001) at the L2d stage, a stage prior to dauer arrest. (c) Nuclear localization of DAF-16∷GFP in daf-28(sa191) animals at the L2d stage. White arrows indicate the nucleus and nucleolus.
daf-28 is expressed in sensory neurons ASI and ASJ and is down-regulated by pheromone signaling
To study the expression pattern and regulation of daf-28, we fused the probable daf-28 promoter to GFP (Chalfie et al. 1994), and generated three integrated arrays of the Pdaf-28∷GFP transgene (see Materials and Methods). From late L1 to L2, Pdaf-28∷GFP is strongly expressed in two pairs of ciliated sensory neurons: ASI and ASJ (Fig. 4A). This time period of expression matches the temperature-sensitive period for a daf-2(ts) mutation (Swanson and Riddle 1981) and therefore is when daf-2 signaling is expected to be regulated to control the dauer arrest versus reproductive growth decision. This expression pattern continues through L3, L4, and early adult stages. Pdaf-28∷GFP is also expressed in the hindgut as well as a tail neuron identified as PQR, a neuron with rudimentary cilia (Fig. 4A). From late embryogenesis to early L1, Pdaf-28∷GFP was seen in many cell types including pharyngeal muscle, hypodermal, and many head cells (data not shown).
Figure 4.
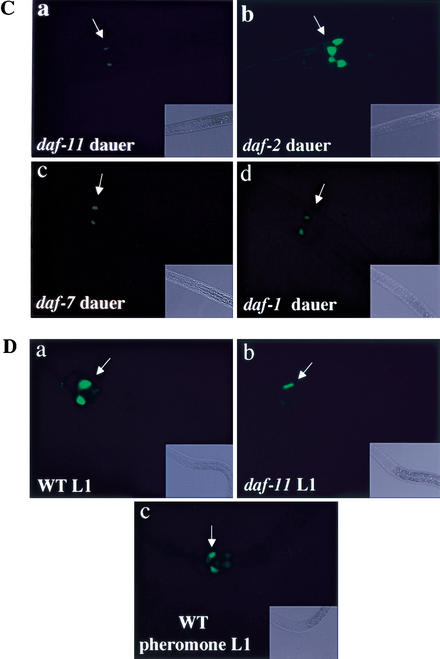
(A) Pdaf-28∷GFP expression in wild-type animals at the L3 larval stage (side view, anterior left and ventral down). Indicated are two sensory neurons, ASI and ASJ (both neurons exist as bilaterally symmetric pairs; indicated are one of each pair) in the head containing projections extended to the tip of the nose and projections joining the nerve ring, the hindgut cells, and the tail neuron PQR. (B–D) Comparison of Pdaf-28∷GFP intensity in the animals that are reproductively growing versus animals reared under dauer-inducing conditions or in dauer arrest mutant backgrounds. White arrows indicate ASI and ASJ neurons. WT, wild type. (B, panel a) Wild-type N2 at the L3 larval stage (side view). (Panel b) A wild-type N2 dauer induced by starvation and high population density (pheromone). (Panel c) A wild-type N2 dauer induced by dauer pheromone. (Panel d) A daf-22(m130) dauer induced by starvation. g.a., gut auto-fluorescence. (C, panel a) A daf-11(mg295) dauer. (Panel b) A daf-2(e1368) dauer, showing strong GFP expression in two ASI and two ASJ cells (dorsal view). (Panel c) A daf-7(e1372) dauer. (Panel d) A daf-1(m40) dauer, showing weak GFP expression in two ASI cells (dorsal view). (D, panel a) Wild-type N2 at the L1 larval stage. (Panel b) daf-11(mg295) animal at the L1 stage. (Panel c) Wild-type L1 reared with extracted dauer pheromone. The corresponding Nomarski pictures are shown at the lower-right corner of each fluorescence images (16× optics).
To determine whether daf-28 expression responds to environmental cues, we tested Pdaf-28∷GFP expression levels in dauers induced by different conditions. The concentration of the dauer pheromone produced by a population varies with the population density and controls the reproductive versus dauer arrest life cycle of that population. A crowded population produces high concentrations of pheromone to induce dauer arrest. In natural dauers, which are induced by a combination of starvation and dauer pheromone, Pdaf-28∷GFP is down-regulated (Fig. 4B, panel b). In dauers induced by treatment with crude pheromone extract, Pdaf-28∷GFP is dramatically down-regulated as well (Fig. 4B, panel c). In a daf-22 mutant that is defective in pheromone release (Golden and Riddle 1985), the Pdaf-28∷GFP expression is dramatically reduced in dauers induced by starvation (Fig. 4B, panel d), suggesting that daf-28 also responds to the food signal. As shown in Figure 5A, the GFP level is ∼16- to 19-fold lower in pheromone or starvation-induced dauers than in reproductively growing larvae. Because the Pdaf-28∷GFP fusion gene only contains daf-28 promoter sequences, these results indicate that daf-28 expression is negatively regulated at the level of transcription by dauer-inducing conditions. Moreover, this down-regulation is initiated at the L1 larval stage, when DAF-2 activity is required for inhibition of dauer arrest. However, Pdaf-28∷GFP down-regulation in L1 larvae is variable. On dauer pheromone plates that induce ∼33% dauer arrest, 16% (n = 92) of L1 larvae display low levels of GFP expression as shown in Figure 4D (panel c). These results are consistent with the view that the wild-type function of daf-28 is to activate daf-2 to inhibit dauer arrest because daf-28 is expressed under conditions of high daf-2 signaling and is down-regulated under dauer-inducing conditions.
Figure 5.
(A) Quantification of Pdaf-28∷GFP intensity in animals that are reproductively growing and affected by dauer-inducing conditions or mutations. (B) Quantification of Pdaf-28∷ GFP intensity in wild-type animals and animals defective in various sensory neuronal functions. The error bars in A and B reflect the variation among individual animals. Above each column is the total number of animals scored. a.u., arbitrary unit.
To determine whether the daf-28-mediated insulin-like pathway is regulated by the daf-11/guanylyl cyclase signal, a possible component of the dauer pheromone signal transduction pathway in sensory neurons (Birnby et al. 2000), we analyzed Pdaf-28∷GFP expression in a daf-11(lf) background. Pdaf-28∷GFP is down-regulated in daf-11 dauers (Figs. 4C, panel a, 5A), indicating that depletion of cyclic GMP signal down-regulates daf-28 transcription. This down-regulation is initiated during the L1 stage (Fig. 4D, panel b), although it is not as dramatic as at the dauer stage. Of daf-11(lf) L1 larvae, 26% (n = 87) displayed a low level of GFP expression as shown in Figure 4D (panel b). The daf-11(mg295) mutant analyzed (Fig. 4C, panel a) was originally identified in a screen for positive regulators of Pdaf-7∷GFP expression in ASI. daf-11(mg295) is a G-to-A transition at the first position of intron 7 and therefore is predicted to disrupt the 5′ splicing site. Pdaf-28∷GFP is also down-regulated in a daf-11(m47) background (data not shown). This result supports the hypothesis that the daf-11 signal functions in sensory neurons to regulate insulin production.
In mammals, insulin expression and release in pancreatic β-cells is coupled to insulin receptor signaling in an autocrine fashion (Leibiger et al. 1998). To investigate whether daf-28 expression is regulated by the DAF-2/IR pathway, we introduced the Pdaf-28∷GFP integrated transgene into daf-2(lf) mutants e1368 and e1370, respectively. Pdaf-28∷GFP is not down-regulated in dauers caused by either daf-2(lf) allele (Figs. 4C, panel b, 5A), indicating that there is not a feedback signal from the insulin-like receptor DAF-2 to daf-28 transcription.
To investigate whether daf-28 expression is regulated by DAF-7/TGFβ signaling, we introduced the Pdaf-28∷GFP integrated transgene into the daf-7(lf) mutant e1372. We found that Pdaf-28∷GFP expression is dramatically reduced in daf-7(lf) dauers (Figs. 4C, panel c, 5A). Similarly, in the DAF-1/TGFβ receptor mutant m40, Pdaf-28∷GFP is also down-regulated, but less dramatically, possibly owing to the difference in the severity of the mutations in these two genes (Figs. 4C, panel d, 5A). These results indicate that there is cross-talk between the TGFβ-like and insulin-like pathways in regulation of insulin-like ligand production.
In daf-2(lf) dauers, Pdaf-28∷GFP remained strongly expressed in two pairs of neurons. In contrast, in all the dauers described above in which Pdaf-28∷GFP is down-regulated, GFP is often faintly visible in only one pair of neurons, as though one of the ASI and ASJ pairs is preferentially down-regulated. This pattern is more consistent in daf-7(lf) and daf-1(lf) backgrounds. In daf-1(lf) dauers in which Pdaf-28∷GFP is less down-regulated than in daf-7(lf) dauers, we determined that 100% (n = 45) of the animals have weak GFP expression in ASI but virtually none in ASJ (see also Fig. 4C, panel d). These observations suggest that DAF-28 expression in ASJ is more subject to regulation by the ASI-expressed DAF-7/TGFβ signal and possibly also by pheromone and DAF-11 signals.
Dauer arrest is also induced in wild-type and a large variety of sensitized genetic backgrounds by growth at high temperature, 27°C (Ailion and Thomas 2000). In contrast to the pheromone and low food signals, high temperature does not affect daf-28 expression.Pdaf-28∷GFP remained bright in 100% (n = 63) of dauers induced by high temperature (27°C).
The daf-28 expression pattern is affected by age and sensory neuron functions
Microarray analysis has shown that the expression levels of several ins genes change as animals age (Lund et al. 2002). daf-28 was not included in this analysis. To determine whether daf-28 expression is altered when animals age, we examined the Pdaf-28∷GFP expression pattern in 1-day-old, 7-day-old, and 17-day-old animals. We found that the number of Pdaf-28∷GFP-expressing cells increases as animals age (Fig. 6A,B). In contrast, as a control, the number of TTX-3∷GFP-expressing cells remained constant, indicating that not all neuronally expressed genes are dysregulated as animals age. More interestingly, in the 17-day-old animals, the somatic gonad showed strong Pdaf-28∷GFP expression (Fig. 6A, panel d). Of these old animals, 35/36 gonads displayed this expression. The gonadal GFP expression was not observed either in the younger animals (day 1 and day 7) carrying Pdaf-28∷GFP or in animals carrying the TTX-3∷GFP at any stages (data not shown). These results suggest that age affects the regulation of DAF-28 in head neurons and in the somatic gonad; and both of these tissues have been implicated in life-span regulation previously (Apfeld and Kenyon 1999; Arantes-Oliveira et al. 2002).
Figure 6.
Images (A) and quantification (B) showing the number of cells expressing Pdaf-28∷GFP increases with age. (A) Head neurons expressing Pdaf-28∷GFP in day 1 (panel a), day 7 (panel b), and day 17 (panel c) adults. (Panel d) Somatic gonad of a 17-day-old adult. s.g., somatic gonad; g.a., gut auto-fluorescence. The corresponding Nomarski pictures are shown at the lower-right corner of the fluorescence images (16× optics). (B) The numbers of Pdaf-28∷GFP-expressing cells in the head in adults of day 1, day 7, and day 17. The numbers of total animals scored at each time point are indicated in parentheses. Data on TTX-3∷GFP-expressing cells are presented as a control. The error bars reflect the variation among individual animals.
The overall level of daf-28 expression is also elevated in several mutants defective in sensory neuron functions. These mutants include daf-6(lf), osm-1(lf), and tax-4(lf). In the daf-6(lf) mutant, the ciliated neurons are not exposed to the environment because of the defective morphology of the amphid sheath cells (Albert et al. 1981). The osm-1(lf) animals are defective in response to osmolarity, and tax-4(lf) animals are defective in chemotaxis (Dusenbery et al. 1975). tax-4 encodes an α subunit of the cyclic nucleotide-gated channel expressed in sensory neurons (Komatsu et al. 1996). In ASI and ASJ, the Pdaf-28∷GFP levels in the sensory neuron mutants are 9- to 11-fold higher than that in the wild type at the L3 larval stage (Fig. 5B). These results suggest that daf-28 expression is modulated during the aging process and according to the perception by sensory neurons of conditions in the environment.
daf-28 functions in concert with other ins gene(s) in dauer arrest
DAF-28 is most related to the other β-type INS proteins in C. elegans; most of the β insulins, including DAF-28, have a predicted F-peptide in proinsulin (Fig. 1A,B). To test the possible redundant function of daf-28 in dauer arrest with other ins genes, we tested several ins genes for their ability to suppress daf-28(sa191) dauer arrest when introduced as high-copy transgenes. We tested ins-4, ins-6, ins-7, ins-9, ins-17, and a combination of ins-21, ins-22, and ins-23 because they are arranged in tandem in a 4.7-kb region of the genome. ins-4, ins-6, ins-7, and ins-9 are β-type, whereas ins-17 is γ-type, and ins-21, ins-22, and ins-23 are α-type (Pierce et al. 2001). Because both the γ- and the α-types of insulins are predicted to form three intramolecular disulfide bonds, they are more divergent from DAF-28 than the β-type insulins. We generated daf-28(sa191) transgenic lines carrying each of the ins genes (Table 1) and scored the proportion of animals that bypassed dauer arrest. Among these ins genes, only ins-4 and ins-6 at high gene dosage suppress daf-28(sa191) (Fig. 2). Consistent with this result, INS-4 and INS-6 are most similar to DAF-28 among the INS proteins in C. elegans. We conclude that ins-4 or ins-6 at high gene dosage can functionally replace daf-28+, possibly because of similarity in the protein sequences. These data strongly support the view that daf-28(sa191) encodes an insulin-like antagonist, which may down-regulate DAF-2 activity by interfering with activities of daf-28+ and several other insulin-like genes.
ins-1 is the only β-type ins gene that bears a C-peptide (Pierce et al. 2001). ins-1 antagonizes daf-2 activity; high gene dosage of ins-1 in wild-type animals causes a low percentage of dauer arrest and enhances dauer arrest in animals carrying weak daf-2(lf) mutations. Therefore, it is a formal possibility that daf-28(sa191) dauer arrest is caused by up-regulation of ins-1. To address whether daf-28(sa191) dauer arrest is dependent on ins-1 activity, we tested an ins-1(nr2091); daf-28(sa191) double mutant for suppression of dauer arrest. The nr2091 mutation is a deletion and therefore is likely to deplete the ins-1 activity (Pierce et al. 2001). This double mutant displayed 100% dauer arrest, similar to the daf-28(sa191) single mutant (Fig. 2). Thus daf-28(sa191) dauer arrest is independent of ins-1 activity.
Discussion
daf-28 expression is regulated in response to environmental cues for dauer arrest
We have found that daf-28 encodes a β-type insulin, which is predicted to form four intramolecular disulfide bridges (Pierce et al. 2001). In the C. elegans genome, there are 11 genes encoding such insulins. Although this type of insulin is found in the mollusc Lymnaea, where it is expressed in a neuron that regulates metabolism (Smit et al. 1996), it has not yet been found in higher organisms. We have shown that several members of this insulin family function as DAF-2/IR agonists to inhibit dauer arrest.
Although insulin signaling has been implicated in dauer arrest, and many insulin-like genes have been identified by genome analysis, until this study, no insulin-like genes had been shown to be regulated by the known physiological regulators of dauer arrest. We have shown that daf-28 is expressed in two pairs of sensory neurons, ASI and ASJ. These two ciliated sensory neurons have been shown by laser ablation to be critical for dauer arrest (Bargmann and Horvitz 1991). daf-28 expression at the L2/L3 stage is more restricted compared with other ins genes, which tend to be expressed in more head neurons, including the ciliated sensory neurons (Pierce et al. 2001). This difference may indicate that DAF-28 has evolved to carry out specific roles in the dauer-regulating neurons. Furthermore, we have shown that transcription of daf-28 in these two neurons is responsive to environmental cues that normally regulate dauer arrest: the dauer pheromone and starvation.
daf-28 transcription responds to the DAF-11/guanylyl cyclase signal, which functions in the sensory neurons to transduce dauer pheromone signals to cyclic GMP gated ion channels to, in turn, control dauer arrest. The dauer arrest phenotype of daf-11(lf) can be partially suppressed by either daf-3(lf) or daf-16(lf), indicating that the daf-11 signal feeds into both the DAF-2 and the DAF-7 pathways (Thomas et al. 1993; Gottlieb and Ruvkun 1994; Riddle 1997). daf-11+ regulates the DAF-7 pathway by activating daf-7 transcription in ASI, and DAF-11 functions at least in ASI and ASJ to regulate dauer arrest based on cell-specific daf-11 rescue experiments (Murakami et al. 2001). We have found that daf-11+ may regulate the DAF-2 pathway in part by activating daf-28 transcription in ASI and ASJ.
Interestingly, whereas down-regulation of daf-28 expression under dauer-inducing conditions is most obviously observed at the dauer arrest stage, in daf-28(sa191), DAF-16∷GFP is nuclear-localized at the L2d stage, a preparatory stage for dauer arrest, indicating that daf-28 functions at the L2d stage or earlier. Consistent with this hypothesis, we observe that Pdaf-28∷GFP expression begins to be down-regulated at the L1 stage in the daf-11(lf) background or in wild-type animals exposed to dauer pheromone. However, if the function of daf-28 were controlled solely at the level of transcription, we would have observed a more robust down-regulation of Pdaf-28∷GFP in L1 larvae. This may be because of the intrinsic stability of GFP in ASI and ASJ, except that daf-7∷GFP shows more dramatic L1 down-regulation (Ren et al. 1996). It is also possible that DAF-28 function is initially regulated at posttranscriptional levels, such as insulin maturation and secretion. In mammals, insulin production is known to be regulated by glucose at least at the levels of transcription, translation, and secretion (German 2000; Rhodes 2000). Insulin maturation may also be glucose-regulated, and it is coupled to the regulated secretory process (Orci et al. 1987; Rhodes 2000).
Under pheromone-inducing conditions or in daf-11(lf), daf-28 transcriptional down-regulation could also be a secondary response to a reduced DAF-7/TGFβ signal. Consistent with this possibility, the daf-7 pathway positively regulates daf-28 transcription. Interestingly, the temperature-sensitive period for daf-7 is mid L1, whereas for daf-2 it is late L1 (Swanson and Riddle 1981), suggesting a cascade of signaling. It has also been reported that DAF-16∷GFP is nuclearly localized in a daf-7(lf) background at the L2d stage, indicating that daf-7 signaling somehow intersects with daf-2 signaling (Lee et al. 2001). Our results provide an explanation for this observation: daf-7 signaling may have an impact on daf-2 signaling by regulating production of the insulin ligands including DAF-28. However, it is unclear whether the transcriptional down-regulation of daf-28 under dauer-inducing conditions or in daf-11(lf) is completely mediated by the DAF-7/TGFβ signal or there is another transcriptional cascade of direct regulation. Nevertheless, this interaction between the insulin-like and the TGFβ-like pathways explains mechanistically how these two major signaling pathways are coordinated to regulate dauer arrest.
Unlike in a daf-11 mutant, Pdaf-28∷GFP is up-regulated in a set of sensory neuron mutants. This result is consistent with these mutants being suppressors of daf-11(lf) dauer arrest, as well as defective in dauer arrest induced by dauer pheromone (Ailion and Thomas 2000). Pdaf-28∷GFP expression is increased in tax-4(lf) but decreased in daf-11(lf), suggesting that daf-28 transcriptional control is not along the cGMP signaling pathway from the DAF-11 guanylyl cyclase to the TAX-4 channel. The elevated levels of daf-28 expression in the sensory mutants may reflect their defects in transducing environmental cues; for examples, these neurons may constitutively signal replete conditions and thus continually express DAF-28 and other insulins. An interesting aspect of these mutants is that they undergo dauer arrest at a much higher rate at 27°C. We found that in tax-4(lf) dauers induced at 27°C, Pdaf-28∷GFP is not down-regulated (data not shown), just as in the wild-type dauers induced at 27°C. Our results suggest that the 27°C dauer arrest is regulated independently of daf-28 function. It is probable that this temperature regulation couples to other insulin genes or other neuroendocrine signals.
daf-28 encodes an insulin-like molecule that is likely to promote reproductive growth by activating the DAF-2/IR pathway
Our results suggest that daf-28 is likely to function at least partially through the daf-2 pathway. We have shown that in the daf-28(sa191) background, DAF-16∷GFP is nuclearly localized, which is a hallmark for down-regulation of daf-2 activity. This result supports the view that DAF-28 is a ligand for DAF-2. Also consistent with this model are the findings by Hua et al. in this issue that a C. elegans β insulin INS-6, which we have shown here genetically interacts with DAF-28, folds into an insulin structure and can compete with human insulin for binding to the human insulin receptor (Hua et al. 2003). However, genetic epistasis analysis indicates that daf-28(sa191) is only partially suppressed by daf-16(lf), at the rate of 44% (Malone et al. 1996). daf-28 may also function through another parallel signaling cascade besides the DAF-2/DAF-16 pathway. There are other RTKs (receptor tyrosine kinases) in C. elegans (Popovici et al. 1999; Dlakic 2002), which could also serve as receptors for DAF-28 and other insulins. Furthermore, orphan G-protein-coupled receptors are also implicated in transducing the insulin-like signal (Hsu et al. 2002). A genetic screen for mutations that suppress daf-28(sa191) dauer arrest may reveal the components in this parallel pathway.
Results from high gene dosage experiments with daf-28 are consistent with the genetic evidence suggesting that daf-28(sa191) is a dominant-negative or antimorphic type of gf (gain-of-function) allele. Because 10% of daf-28(sa191)/+ animals arrest at the dauer stage, whereas 0% of daf-28+/Df animals arrest at the dauer stage, daf-28(sa191) has been proposed to encode a product that poisons daf-28+ function in promoting reproductive growth (Malone and Thomas 1994). For this type of mutation, increasing the dose of wild-type DAF-28 should reduce the toxicity of the mutant product. Indeed, we found that high-copy transgenes of daf-28+ effectively suppress daf-28(sa191) dauer arrest. The potent suppression of daf-28(sa191) dauer arrest by high gene dosage of daf-28+ rules out the possibility that DAF-28(sa191) is a hyperactive protein.
We attempted various RNAi approaches to determine the lf phenotype of daf-28 in longevity and dauer arrest. These approaches include feeding animals with bacteria bearing double-stranded RNA (dsRNA), injection of dsRNA, and generating snap-back transgenes that transcribe dsRNA in vivo (Fire et al. 1998; Timmons and Fire 1998; Tavernarakis et al. 2000). None of these approaches could, for example, revert the daf-28(sa191) phenotype, nor did they induce dauer phenotypes in wild type. In addition, RNAi experiments in which a mixture of ins-4, ins-6, and daf-28 dsRNA was injected into the RNAi-sensitive mutant rrf-3(lf) (Sijen et al. 2001; Simmer et al. 2002) did not display a dauer arrest phenotype, and their life span is normal (data not shown). These results are inconclusive, however, because the current RNAi techniques are less effective in targeting genes that are expressed in neurons, where daf-28 is expressed (Fraser et al. 2000; Tavernarakis et al. 2000; Simmer et al. 2002).
The daf-28 expression pattern and regulation further support the view that the daf-28+ function is to promote reproductive growth. Expression of daf-28 in ASI and ASJ is down-regulated in natural dauers and pheromone-induced dauers, indicating that daf-28 is inhibited during normal dauer arrest. This regulation of daf-28 is opposite to that of ins-1, which has been shown to promote dauer arrest (Pierce et al. 2001); ins-1 is up-regulated in dauers based on SAGE analysis (Jones et al. 2001). Moreover, daf-28 is regulated in a similar manner to DAF-7/TGFβ, which promotes reproductive growth.
The view of redundant functions among the ins genes in animals is supported by our results showing that the daf-28(sa191) dauer arrest is suppressed by high gene dosage of daf-28+, ins-4, or ins-6 (Fig. 2). Given that INS-4 and INS-6 are the closest relatives of DAF-28 in C. elegans and are both expressed in sensory neurons (Pierce et al. 2001), INS-4 and INS-6 may functionally replace the DAF-28 protein because of similarity in protein sequences. An alternative model is that the endogenous activity of INS-4 or INS-6 is down-regulated in daf-28(sa191) and the suppression of dauer arrest results from restoration of the INS-4 or the INS-6 activity. Both models suggest that INS-4 and INS-6 function in concert with DAF-28 to promote reproductive growth, possibly as ligands for DAF-2.
The R37C substitution in DAF-28 disrupts a predicted proteolytic cleavage site, suggesting that DAF-28 is processed to release the F-peptide (Fig. 1A,B). The prediction of proteolytic processing sites in worm proinsulins was based on the known functions of proprotein convertases. Proprotein convertases are a family of proteases that process proproteins into their mature forms. Seven proprotein convertases are found in mammals. Among these, furin has been shown to cleave after an RXXR motif and is capable of processing a wide range of proproteins, including hormones, receptors, and growth factors, such as pro-TGFβ1 and pro-IGF-I (Nakayama 1997). Another mammalian convertase, PC2, has been shown to cleave at dibasic residue sites and function in neuroendocrine tissues to process prohormones including human insulin (Nakayama 1997; Rhodes 2000). The C. elegans genome sequence reveals four proprotein convertases with at least 14 isoforms (Thacker and Rose 2000). Among them, BLI-4 and KPC-1 are most similar to furin (Thacker et al. 1995; Thacker and Rose 2000), and EGL-3 is most similar to mammalian PC2 (Kass et al. 2001). Thus, worm proinsulins may be processed in the same manner as prohormones in mammals. However, given that no dauer arrest phenotype has been observed in animals bearing any single mutant of these four convertases (Thacker and Rose 2000), it is unclear which convertases are responsible for insulin processing or whether there is functional redundancy among these convertases.
Because the daf-28(sa191) mutation affects the probable proprotein convertase cleavage site, and because daf-28(sa191) displays dominant-negative features and yet the dauer arrest phenotype can be completely suppressed by extra copies of daf-28+ or ins-4+, we speculate that DAF-28R37C acts as a competitive inhibitor to down-regulate activities of DAF-28, INS-4, and INS-6, possibly by interfering with insulin processing. We hypothesize that dauer arrest in 10% of daf-28(sa191)/+ animals results from interference of the R37C propeptide form with the convertase machinery that is necessary for DAF-28+ processing, which, in turn, leads to inadequate levels of mature DAF-28. Because the same convertase cleavage site is conserved in other β-type insulins, including INS-4 and INS-6, DAF-28R37C may interfere with the maturation of other β insulins as well. Additionally, DAF-28R37C may also interfere with other types of insulins in C. elegans because of possible overlapping functions among proprotein convertases. A DAF-28R37A substitution can only partially suppress daf-28(sa191) dauer arrest, in contrast to the strong suppression by high gene dosage of INS-4, which is only 51% identical to DAF-28. This result suggests that DAF-28R37A has impaired DAF-28 activity and, therefore, the R37 residue is likely to be important for DAF-28 processing. However, given that R37A displayed partial suppression of daf-28(sa191) dauer arrest, R37A retains more DAF-28 activity compared with R37C, or alternatively, R37A is less capable of interfering with processing or activities of other insulins. For example, it could be less stable than R37C. In this model, DAF-28R37C would be expected to interfere with processing of other β insulins that are expressed in the same cells as DAF-28, that is, the ASI and ASJ neurons. The β insulins expressed in other cell types would not be affected. This model predicts that it is β-insulin signaling from these two neurons that is key to dauer arrest. Given the apparent redundant function among the ins genes, it makes sense that a dominant-negative mutation such as R37C that may affect activities of multiple insulins would be necessary to reveal functional involvement of the ins genes in dauer arrest.
Like DAF-28, DAF-7 is also expressed in ASI as a dauer inhibitory ligand that contains a furin-like cleavage site (Ren et al. 1996). Thus, it is possible that DAF-7 processing is also affected in daf-28(sa191). However, because dauer arrest in daf-28(sa191) is not significantly suppressed by lf mutations in downstream target genes daf-3 (0% suppression) and daf-5 (9% suppression), dauer arrest in daf-28(sa191) is not primarily attributable to the down-regulation of DAF-7. Presently, it is unknown if production of the mature DAF-7 or DAF-28 is regulated at the level of proprotein processing. Regulated processing steps in addition to transcriptional control of daf-28 and daf-7 by dauer-inducing sensory signals would help to explain why a cleavage-site mutation in a single insulin could have such a dramatic effect.
It is also possible that the apparent stronger toxicity of R37C in comparison to R37A is derived from creation of an extra cysteine residue in an unprocessed propeptide. Unpaired cysteine residues have been reported in mice to cause insulin misfolding and, in turn, cause β pancreatic cell dysfunction by triggering stress response and global misregulation of gene expression (Wang et al. 1999). However, it is unlikely that DAF-28R37C causes overall dysfunction in ASI and ASJ because dauers caused by daf-28(sa191) recover very well even at the nonpermissive temperature, and ASJ has been shown to be critical for dauer recovery by laser ablation (Bargmann and Horvitz 1991). Therefore, if the extra cysteine in DAF-28R37C induces misfolding of itself or other insulins, the resulting dauer arrest is the outcome of misregulation of the DAF-2/IR signaling pathway instead of general cellular toxicity in the insulin secretary cells. Finally, it is possible that the uncleaved DAF-28R37C binds to DAF-2 receptor in a manner distinct from DAF-28+ to antagonize rather than agonize signaling.
ins-1 and ins-18 have previously been reported to function in the daf-2 pathway (Kawano et al. 2000; Pierce et al. 2001). ins-1 is likely to promote dauer arrest because overexpression of ins-1 causes dauer arrest (Pierce et al. 2001). We considered the possibility that daf-28(sa191) dauer arrest is caused by up-regulation of ins-1 activity: if DAF-28 and INS-1 have opposite roles in dauer arrest and they share the DAF-2 receptor, down-regulation of DAF-28 may free the DAF-2 receptor for INS-1 signaling. However, because ins-1(nr2091);daf-28(sa191) animals still arrest at the dauer stage, daf-28(sa191) acts independently of ins-1.
ins-18 has not been implicated in dauer arrest. However, ins-18(RNAi) causes increased longevity, suggesting that ins-18 is also a DAF-2 agonist (Kawano et al. 2000). We have not found daf-28 to play a major role in life-span regulation. We measured the life span of wild-type animals carrying the high gene dosage of daf-28+, and their life span is not different from that of the wild type (data not shown). The reported long-life phenotype in daf-28(sa191) is quite mild (Malone et al. 1996), and this phenotype could be mediated by perturbation of the activity of another insulin-like gene, such as ins-18.
daf-28 expression is age-sensitive
Although we have not found direct evidence linking daf-28 function to longevity, daf-28 expression is age-sensitive. Pdaf-28∷GFP is expressed throughout adulthood, unlike daf-7 expression, which is limited to larval stages. This is consistent with the view that the TGFβ-like pathway is not primarily involved in regulation of longevity. The observations that Pdaf-28∷GFP expression in head neurons increases from an average of 4 neurons to 8 neurons as animals age, and that gonadal GFP expression is only observed in old animals, suggest that daf-28 expression is subject to regulation by aging-related factors. In addition, the overall level of Pdaf-28∷GFP expression is elevated in several mutants with sensory neuron defects, which also live longer than wild-type animals (Apfeld and Kenyon 1999). This observation suggests that daf-28 may be regulated by some sensory factors that also regulate longevity. DAF-28 alone may not be a determinant in longevity, but alteration of its expression pattern may reflect the regulation of ins genes as animals age. In fact, many ins genes have been shown to change expression levels during the aging process (Lund et al. 2002). It will not be surprising if the higher levels of expression observed with microarray and SAGE analyses are the result of broadened expression patterns such as those we have observed for daf-28. As in dauer arrest, regulation of longevity may involve combinatorial activities of multiple ins genes to ensure a fine-tuned outcome.
The dauer pheromone is produced at all stages of development; its concentration is a surrogate measure of C. elegans population density. The response to dauer pheromone suggests that individuals in a C. elegans community signal each other to compute as a population the future demand for resources by a reproductively growing population and, if the population density is too high, the entire community switches to the dauer arrest life cycle. Because dauer larvae consume much less food than reproductively growing C. elegans and are a dispersal stage, the switch to dauer arrest matches the population to resources and sends the nonfeeding arrested animals off to forage elsewhere. daf-28 and daf-7 constitute neuroendocrine outputs of this sensory computation by a C. elegans community. Part of this computation regulates social behavior: daf-7 mutants, like the NPR-1/neuropeptide Y receptor mutants, feed in groups rather than in a solitary manner (de Bono and Bargmann 1998). daf-28 does not affect this behavioral axis but is a major factor in another community decision, that is, whether to develop reproductively or disperse from a crowded ecosystem.
Materials and methods
Strains
The following strains were used for experiments described in this manuscript; they are either obtained from CGC, our collection, or constructed using strains from these sources (the sur-5∷gfp integrated array was kindly provided by Min Han at the University of Colorado at Boulder): N2 (wild type), CB4856; JT191, daf-28(sa191) V; GR1352: daf-16[mgDf47]; mg Is41[daf-16∷gfp]; GR1353: daf-2(e1370) III; mgIs41[daf-16∷gfp]; GR1317: mgIs18[ttx-3∷gfp]; GR1354: daf-28(sa191) rol-9(sa148) V; GR1355: exp-3(n2372) daf-28(sa191) rol-9(sa148) V; GR1356: ins-1(nr2091) IV; daf-28(sa191) V; GR1357: daf-11(mg295) V; mgIs40[Pdaf-28∷gfp]; GR1358: daf-2(e1368) III; mgIs40; GR1359: daf-7(e1372) III; mgIs40; GR1360: daf-22(m130) II; mgIs40; GR1361: daf-6(e1377) X; GR1362: tax-4(pr678) III; mgIs40; GR1363: osm-1(p808) X; mgIs40; GR1364: daf-1(m40) IV; mgIs40; GR1356: daf-28(sa191) V; CU217: kuIs[sur-5∷gfp] I.
The above strains were maintained or constructed using standard methods (Sulston and Hodgkin 1988). ins-1(nr2091) IV; daf-28(sa191) V was constructed by assaying for the presence of molecular lesions of both nr2091 and sa191. daf-28(sa191) is a C-to-T transition that creates an AluI site.
SNP mapping
exp-3(n2372) daf-28(sa191) rol-9(sc148) V was constructed to serve as a parental strain for SNP analysis to define the molecular boundaries of daf-28(sa191). This triple mutant was mated with males of the SNP-containing strain CB4856. Among the second-generation progeny (F2) of this cross, we selected segregants in which recombination occurred between the exp-3 and the rol-9 genes. Such recombinants contain recombined chromosomal regions between the Exp and the Rol markers, which are ∼3.4 cM apart. The recombinants were then assayed for presence of SNP markers located between exp-3 and rol-9. The information on the SNPs was either obtained from http://genome.wustl.edu or by sequencing certain genomic regions of CB4856.
Constructs and transgenic procedures
All PCR fragments used to generate transgenic lines are pooled from at least four individual PCR reactions to alleviate the influence of PCR errors. The forward and reverse primers for PCR amplification of daf-28 or ins genes are listed in Table 1. The daf-28+ transgene refers to a PCR fragment amplified from N2 wild-type genomic DNA using the primer pair listed in Table 1; this PCR fragment include a 3.3-kb promoter region, the entire daf-28 coding sequence, and a 45-bp 3′-flanking region. The daf-28(sa191) or DAF-28R37C transgene refers to an equivalent PCR fragment amplified from the daf-28(sa191) genomic DNA. The DAF-28 transgenes containing either C37R revertant or R37A substitutions were PCR fragments obtained using the SOE (splicing by overlap extension) by PCR method (Horton et al. 1989). The DAF-28R37A PCR fragment was amplified from genomic DNA of daf-28(sa191) instead of N2 wild type to avoid possible contamination of the daf-28+ product in the PCR mix. Transgenes of various ins genes are PCR fragments containing the coding and regulatory sequences of the corresponding ins genes. The transgene ins-21; ins-22; ins-23 refers to a 7-kb PCR fragment containing all three genes. All the PCR fragments were injected at the concentration of 0.017 mM, except that the DAF-28R37C fragment was also injected at 1/5 of this concentration into the daf-28+/Df strain to generate the second transgenic line that displayed a lower percentage of dauer arrest (see Results). The transgenic marker for all PCR fragments used for this study is pTG96/SUR-5∷GFP (Gu et al. 1998; Yochem et al. 1998).
The Pdaf-28∷GFP transgene was constructed by cloning a PCR fragment containing 3.3 kb of the 5′-flanking region of daf-28. The vector ppD95.67 was kindly provided by Andy Fire at Carnegie Institution of Washington, and it carries a nuclear-localization signal for GFP. The Pdaf-28∷GFP plasmid was either transformed alone (mgIs40) or with pRF4/rol-6 (Mello et al. 1991). mgIs40(5X) was used for imaging and quantification to generate the data presented in this manuscript. The TTX-3∷GFP integrated array and the DAF-16∷GFP integrated array were previously reported (Hobert et al. 1997; Lee et al. 1999).
Functional assays for transgenes
Suppression of dauer arrest caused by daf-28(sa191) transgenic animals, normally reared at the permissive temperature 15°C, were allowed to lay eggs at the nonpermissive temperature 25°C for 5 h. The dauer arrest phenotype of the transgenic progeny was scored after 45–48 h. Because daf-28(sa191) dauer arrest is transient and the window of time for scoring the phenotype is only several hours, the nontransgenic population is always scored as an internal control. The data on transgenic lines presented in Figure 2 were obtained at the time point when virtually all the nontransgenic animals were arrested as dauers (data not shown).
Microscopy and related assays
Identification of ciliated sensory neurons expressing GFP was facilitated by treatment of live animals with DiI (Sigma), a dye that stains ciliated neurons including ASI and ASJ. The intensity of Pdaf-28∷GFP was quantified using the Openlab software (http://www.improvision.com). Images prepared for quantification were obtained at low-power optics 16×, and the brightest focal plane was focused on for each animal. For Figures 4B and C and 5A, staged animals reared at 25°C with a similar population density (<100/plate) were scored, except for the natural and daf-22(lf) dauers; these dauers were collected from starved and crowded populations reared at 25°C by 1% SDS (0.5 h) treatment, which allows only dauers to survive. For Figure 5B, L3 larvae from a population reared at 20°C were scored. For Figure 6A and B, to ensure the age of adult animals, the first-day wild-type adults carrying Pdaf-28∷GFP were transferred onto plates containing FUdR (fluorodeoxyuridine), a drug that causes animals to be sterile. Smaller data sets obtained from animals reared on non-drug plates showed similar results (data not shown), indicating that our results were not influenced by utilization of FUdR. For Figure 6A, panels b and c, images of two focal planes were combined using Photoshop to generate each picture. For Figure 6B, GFP-expressing cells were scored under 16× optics. Crude pheromone extract was prepared as previously described (Golden and Riddle 1984). The concentration of pheromone used to induce dauer arrest is 1 arbitrary unit, equivalent to the concentration that causes 33% dauer arrest in N2 at 25°C. L4 larvae or young adults were transferred onto pheromone plates and allowed to lay ∼50 eggs; the progeny were kept well-fed and the dauers were picked for imaging.
Computational analysis
SignalP V1.1 (http://www.cbs.dtu.dk/services/SignalP) was used to predict the signal peptides of the insulin-like propeptides. ClustalW (http://www.ebi.ac.uk/clustalw) was used to align the INS sequences to generate Figure 1A.
Acknowledgments
We thank S.B. Pierce, A. Pasquinelli, H. Mak, Q. Ch'ng, L. Xue, J. Xu, S. Joksimovic, S. Milstein, S. Satterlee, H.A. Tissenbaum, G. Patterson, J.H. Thomas, J.M. Kaplan, A. Hart, D. Altshuler, P. Sengupta, J. Avruch, B. Seed, W.B. Wood, members of the Kaplan group, and past and present members of the Ruvkun group for assistance and discussions. We also thank M.A. Weiss for advice and communication of results before publication, M. Han for CU217, A. Fire for GFP vectors, A. Coulson for cosmids, and T. Stiernagle (CGC) for worm strains. This work was supported by grants RO1AG16636 and RO1AG14161 from the NIH to G.R. and NRSA fellowships from the NIH to W.L. and S.G.K.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL ruvkun@molbio.mgh.harvard.edu; FAX (617) 726-5937.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1066503.
References
- Ailion M, Thomas JH. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics. 2000;156:1047–1067. doi: 10.1093/genetics/156.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PS, Brown SJ, Riddle DL. Sensory control of dauer larva formation in Caenorhabditis elegans. J Compar Neur. 1981;198:435–451. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell. 1998;95:199–210. doi: 10.1016/s0092-8674(00)81751-1. [DOI] [PubMed] [Google Scholar]
- ————— Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Cassada RC, Russell R. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- Dlakic M. A new family of putative insulin receptor-like proteins in C. elegans. Curr Biol. 2002;12:R155–R157. doi: 10.1016/s0960-9822(02)00729-7. [DOI] [PubMed] [Google Scholar]
- Dusenbery DB, Sheridan RE, Russell RL. Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics. 1975;80:297–309. doi: 10.1093/genetics/80.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez M, Attisano L, Wrana JL, Albert PS, Massague J, Riddle DL. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993;365:644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Georgi LL, Albert PS, Riddle DL. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990;61:635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- German M. Insulin gene regulation. In: LeRoith D, et al., editors. Diabetes mellitus: a fundamental and clinical text. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 11–19. [Google Scholar]
- Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- ————— The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- ————— A gene affecting production of the Caenorhabditis elegans dauer-inducing pheromone. Mol General Genet. 1985;198:534–536. doi: 10.1007/BF00332953. [DOI] [PubMed] [Google Scholar]
- Gottlieb S, Ruvkun G. daf-2, daf-16 and daf-23: Genetically interacting genes controlling dauer formation in Caenorhabditis elegans. Genetics. 1994;137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T, Orita S, Han M. Caenorhabditis elegans SUR-5, a novel but conserved protein, negatively regulates LET-60 Ras activity during vulval induction. Mol Cell Biol. 1998;18:4556–4564. doi: 10.1128/mcb.18.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther CV, Georgi LL, Riddle DL. A Caenorhabditis elegans type I TGF β receptor can function in the absence of type II kinase to promote larval development. Development. 2000;127:3337–3347. doi: 10.1242/dev.127.15.3337. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Hobert O, Mori I, Yamashita Y, Honda H, Ohshima Y, Liu Y, Ruvkun G. Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron. 1997;19:345–357. doi: 10.1016/s0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: Gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- Hua, Q., Nakagawa, S.H., Wilken, J., Ramos, R.R., Jia, W., Bass, J., and Weiss, M.A. 2003. A divergent INS protein in Caenorhabditis elegans structurally resembles human insulin and activates the human insulin receptor. Genes & Dev. (this issue). [DOI] [PMC free article] [PubMed]
- Inoue T, Thomas JH. Targets of TGF-β signaling in Caenorhabditis elegans dauer formation. Dev Biol. 2000;217:192–204. doi: 10.1006/dbio.1999.9545. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Riddle DL, Pouzyrev AT, Velculescu VE, Hillier L, Eddy SR, Stricklin SL, Baillie DL, Waterston R, Marra MA. Changes in gene expression associated with developmental arrest and longevity in Caenorhabditis elegans. Genome Res. 2001;11:1346–1352. doi: 10.1101/gr.184401. [DOI] [PubMed] [Google Scholar]
- Kass J, Jacob TC, Kim P, Kaplan JM. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci. 2001;21:9265–9272. doi: 10.1523/JNEUROSCI.21-23-09265.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Ito Y, Ishiguro M, Takuwa K, Nakajima T, Kimura Y. Molecular cloning and characterization of a new insulin/IGF-like peptide of the nematode Caenorhabditis elegans. Biochem Biophys Res Commun. 2000;273:431–436. doi: 10.1006/bbrc.2000.2971. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Leibiger IB, Leibiger B, Moede T, Berggren PO. Exocytosis of insulin promotes insulin gene transcription via the insulin receptor/PI-3 kinase/p70 s6 kinase and CaM kinase pathways. Mol Cell. 1998;1:933–938. doi: 10.1016/s1097-2765(00)80093-3. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Lund J, Tedesco P, Duke K, Wang J, Kim SK, Johnson TE. Transcriptional profile of aging in C. elegans. Curr Biol. 2002;12:1566–1573. doi: 10.1016/s0960-9822(02)01146-6. [DOI] [PubMed] [Google Scholar]
- Malone EA, Thomas JH. A screen for nonconditional dauer-constitutive mutations in Caenorhabditis elegans. Genetics. 1994;136:879–886. doi: 10.1093/genetics/136.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone EA, Inoue T, Thomas JH. Genetic analysis of the roles of daf-28 and age-1 in regulating Caenorhabditis elegans dauer formation. Genetics. 1996;143:1193–1205. doi: 10.1093/genetics/143.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: Extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Murakami M, Koga M, Ohshima Y. DAF-7/TGF-β expression required for the normal larval development in C. elegans is controlled by a presumed guanylyl cyclase DAF-11. Mech Dev. 2001;109:27–35. doi: 10.1016/s0925-4773(01)00507-x. [DOI] [PubMed] [Google Scholar]
- Nakayama K. Furin: A mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327:625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell. 1998;2:887–893. doi: 10.1016/s1097-2765(00)80303-2. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Storch MJ, Anderson RG, Vassalli JD, Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987;49:865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes & Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes & Dev. 1999;13:1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GI, Koweek A, Wong A, Liu Y, Ruvkun G. The DAF-3 Smad protein antagonizes TGF-β-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes & Dev. 1997;11:2679–2690. doi: 10.1101/gad.11.20.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, et al. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes & Dev. 2001;15:672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovici C, Roubin R, Coulier F, Pontarotti P, Birnbaum D. The family of Caenorhabditis elegans tyrosine kinase receptors: Similarities and differences with mammalian receptors. Genome Res. 1999;9:1026–1039. doi: 10.1101/gr.9.11.1026. [DOI] [PubMed] [Google Scholar]
- Ren P, Lim C, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-β homologue. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ. Processing of the insulin molecule. In: LeRoith D, et al., editors. Diabetes mellitus: a fundamental and clinical text. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 20–38. [Google Scholar]
- Riddle DL. Genetic and environmental regulation of dauer larva development. In: Riddle DL, et al., editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 739–768. [PubMed] [Google Scholar]
- Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RH, Fire A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- Simmer F, Tijsterman M, Parrish S, Koushika SP, Nonet ML, Fire A, Ahringer J, Plasterk RH. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr Biol. 2002;12:1317–1319. doi: 10.1016/s0960-9822(02)01041-2. [DOI] [PubMed] [Google Scholar]
- Smit AB, Spijker S, Van Minnen J, Burke JF, De Winter F, Van Elk R, Geraerts WP. Expression and characterization of molluscan insulin-related peptide VII from the mollusc Lymnaea stagnalis. Neuroscience. 1996;70:589–596. doi: 10.1016/0306-4522(95)00378-9. [DOI] [PubMed] [Google Scholar]
- Sulston J, Hodgkin J. Methods. In: Wood WB, editor. The nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. pp. 587–606. [Google Scholar]
- Swanson MM, Riddle DL. Critical periods in the development of the Caenorhabditis elegans dauer larva. Dev Biol. 1981;84:27–40. doi: 10.1016/0012-1606(81)90367-5. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Wang SL, Dorovkov M, Ryazanov A, Driscoll M. Heritable and inducible genetic interference by double-stranded RNA encoded by transgenes. Nat Genet. 2000;24:180–183. doi: 10.1038/72850. [DOI] [PubMed] [Google Scholar]
- Thacker C, Rose AM. A look at the Caenorhabditis elegans Kex2/subtilisin-like proprotein convertase family. Bioessays. 2000;22:545–553. doi: 10.1002/(SICI)1521-1878(200006)22:6<545::AID-BIES7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Thacker C, Peters K, Srayko M, Rose AM. The bli-4 locus of Caenorhabditis elegans encodes structurally distinct kex2/subtilisin-like endoproteases essential for early development and adult morphology. Genes & Dev. 1995;9:956–971. doi: 10.1101/gad.9.8.956. [DOI] [PubMed] [Google Scholar]
- Thomas JH, Birnby DA, Vowels JJ. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics. 1993;134:1105–1117. doi: 10.1093/genetics/134.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Vowels JJ, Thomas JH. Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics. 1994;138:303–316. doi: 10.1093/genetics/138.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks SR, Yeh RT, Gish WR, Waterston RH, Plasterk RH. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet. 2001;28:160–164. doi: 10.1038/88878. [DOI] [PubMed] [Google Scholar]
- Yochem J, Gu T, Han M. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics. 1998;149:1323–1334. doi: 10.1093/genetics/149.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]