Abstract
The nef gene of human and simian immunodeficiency viruses encodes a 27–32-kDa myristoylated protein that is expressed at high levels early after infection. Many functions have been ascribed to the Nef protein, including the down-regulation of cell surface CD4 and a role in viral infectivity. This report describes a novel effect of the Nef protein on human T cells. Electron microscopy was used to examine human T cell lines stably expressing functionally active simian or human immunodeficiency virus type 1 Nef proteins. These studies revealed that the subcellular morphology of Nef-expressing cells was dramatically altered as compared with control cells. The Nef-expressing cells contained numerous membrane-bound vesicles prominently displayed throughout the cytoplasm. The vesicles were analyzed by immunoelectron microscopy (IEM) and by the accumulation of internalized nonspecific membrane tracer, and thus identified as late endosomes and lysosomes. The accumulation of endosomes and lysosomes in response to the expression of Nef was a consistent finding, observed with several different viral isolates and human T cell lines.
The Nef protein, encoded by HIV and simian immunodeficiency virus (SIV), is essential for viral pathogenesis. The seminal studies of Kestler et al. (1) revealed that the expression of a functional SIVmac239 Nef protein is crucial for high viral titers and the induction of disease in rhesus macaques. This finding is underscored by the recent description of HIV-1 positive individuals, described as “non-progressors,” who were found to be infected with viruses carrying deletions within the nef genes (2, 3). However, because the nef gene product is not essential for growth of the virus in vitro, its precise role in viral pathogenesis has been difficult to study. In vitro studies have variously demonstrated that Nef has effects on viral transcription, replication and infectivity, cellular activation, cellular kinases, the intracellular accumulation of envelope glycoprotein, and the down-regulation of cell surface CD4 (reviewed in refs. 4 and 5).
The ability to modulate cell surface expression of CD4 is one of the most well characterized functions attributed to the Nef protein. The CD4 glycoprotein serves as a cofactor for T cell activation via its interaction with class II major histocompatibility complex (MHC) proteins (6, 7), and is also a cellular receptor for HIV-1 and the related SIV (8). Down-modulation of cell surface CD4 in response to Nef expression has been reproduced in a number of lymphoid and nonlymphoid cell types, using nef genes derived from a variety of HIV-1 and SIV strains (4). Amino-terminal myristoylation of the Nef protein is important for its effect on CD4 (9, 10), presumably by localizing Nef to cellular membranes. We (11) and others (9, 12) have previously shown that expression of SIV and HIV Nef in various cell systems induces the degradation of endogenous CD4, such that cell surface CD4 is rapidly internalized and targeted to degradative compartments. The mechanism by which Nef effects the degradation of CD4 is not known, but may involve a direct interaction between Nef and the cytoplasmic domain of CD4, as has been indicated in vitro (13, 14).
In this report we describe a novel effect of Nef on human T cells. We show that Nef-expressing T cells display a dramatically altered subcellular morphology compared with control cells. The cytoplasm of Nef-expressing cells includes a large number of vesicles, which contain proteins characteristic of late endosomes and lysosomes. In addition, the vesicles accumulate internalized cationized ferritin (CF), demonstrating that they function along the endosomal pathway. All of the Nef-expressing T cell lines examined displayed elevated levels of endosomes and lysosomes, indicating that the effect seen was not restricted to a particular viral isolate or lymphocytic cell line. The accumulation of degradative vesicles observed in the presence of Nef correlates with increased degradation of CD4 in Nef-expressing cells.
MATERIALS AND METHODS
Cell Lines.
GP+ENV-AM12 cells expressing SIVmac239 nef in the Moloney murine leukemia virus-based vector LXSN (15) were obtained from Bryan Cullen (Duke University, Durham, NC), and used for transduction (16) of the SupT1 T cell line. The following cell lines stably expressing Nef from the LXSN vector were generously supplied by Bryan Cullen (16): CEM-SS cells expressing SIVmac239 nef, CEM-SS cells transduced with the chloramphenicol acetyltransferase (CAT) gene, Jurkat cells expressing SIVmac239 nef, and CEM-SS cells expressing the HIV-1NL43 nef gene. CEM-SS cells expressing the consensus sequence of HIV-1 nef in the LXSN vector were established by transduction using virus-containing supernatant from PA317 cells transiently transfected with HIV-1cons nef (pLConsNefSN) or with an antisense control construct, HIV-1cons fen (pLConsFenSN), obtained from Diane Shugars (University of North Carolina, Chapel Hill) (17). Cells were subcloned by limiting dilution, and selected and maintained in RPMI 1640 medium plus 10% fetal calf serum (FCS) plus 0.8–1.0 mg/ml G418.
Immunological Reagents.
The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health: antiserum to recombinant HIV-1 Nef. A rabbit antiserum to SIV Nef was a gift from R. Desrosiers (New England Regional Primate Center, Southborough, MA). The mAb OKT4 (anti-CD4) was collected as culture supernatant from the hybridoma cell line (American Type Culture Collection). Mouse anti-human lysosome-associated membrane protein (LAMP) 2 (H4B4) mAb was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine (Baltimore) and the Department of Biology, University of Iowa (Iowa City), under contract N01-HD-6-2915 from the National Institute of Child Health and Human Development. Rabbit anti-cathepsin D sera were gifts from J. Mort (McGill University, Montreal) and K. von Figura (University of Göttingen, Germany). The rabbit anti-bovine cation-independent mannose 6-phosphate receptor (CI-M6PR) antiserum was a gift from W. Brown (Cornell University, Ithaca, NY). Secondary reagents used for IEM were goat anti-mouse IgG AuroProbe EM 10-nm colloidal gold (Amersham) and protein A-10-nm colloidal gold.
Immunoblotting.
Cells were lysed as described in ref. 11. The supernatant was collected and solubilized in an equal volume of 2× Laemmli sample buffer (20% glycerol/0.125 M Tris, pH 6.8/6% SDS/10% 2-mercaptoethanol/bromophenol blue). Proteins were resolved on 10% SDS/PAGE gels and transferred to nitrocellulose. After transfer the membrane was blocked for 1 hr as described in ref. 16 and hybridized overnight at 4°C with the indicated anti-Nef reagents. Anti-Nef binding was visualized using 125I-labeled goat anti-rabbit IgG and autoradiography.
Indirect Immunofluorescent Staining.
Cells were stained and analyzed as described in ref. 11.
Conventional EM.
Samples were prepared and analyzed as described in ref. 18.
Uptake of CF.
Cells were washed twice with ice-cold serum-free RPMI 1640 medium and resuspended at 5 × 106 cells per ml. CF (Sigma) was added to a final concentration of 0.5 mg/ml and the cells were incubated for 10 min on ice to allow binding. Samples were then warmed to 37°C and incubated for 3 hr. The viability of the cells was >70% after a 3-hr incubation. Samples were washed twice with serum-free media, and the cells resuspended at 106/ml in RPMI 1640 medium plus 10% FCS before culturing overnight at 37°C, followed by fixation for EM.
IEM.
Samples were prepared as described in ref. 18, with the following modifications. Cells were fixed with 4% paraformaldehyde in 150 mM Pipes (pH 7.0), at room temperature for 1 hr. Incubations with primary and secondary reagents were performed for 45 min.
RESULTS
Stable Expression of Functionally Active SIV and HIV-1 Nef Proteins in Human T Lymphoblastoid Cell Lines.
To study the effects of the Nef protein on human T lymphocytes, we established stable cell lines expressing SIV or HIV-1 Nef. The human leukemic T cell lines CEM-SS and Jurkat, and the non-Hodgkin’s T cell lymphoma SupT1 were transduced with the LXSN retroviral vector containing SIVmac239 nef. The SIVmac239 nef isolate was chosen because it had been shown to be essential for the induction of disease in rhesus macaques, demonstrating its biological activity in vivo (1). To also include cells expressing HIV-1 Nef, CEM-SS cells were transduced with LXSN retroviral vectors containing either an HIV-1 nef consensus sequence or the HIV-1NL43 nef gene. The HIV-1 nef consensus sequence was derived by Shugars et al. (17) by comparison of nef sequences found in HIV-1 infected patients, whereas the NL43 viral isolate is a highly cytopathic laboratory adapted strain. As controls, CEM-SS cells were transduced with a CAT control construct or an HIV-1 nef antisense construct, denoted HIV-1 fen.
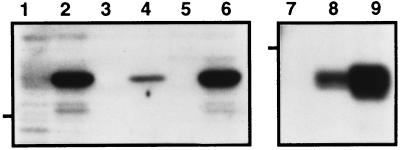
Expression of SIV or HIV-1 Nef proteins in the various cell lines was confirmed by immunoblot analysis (Fig. 1), and by immunoprecipitations of metabolically labeled cells (data not shown). Immunoblots of cell lysates, prepared from equivalent numbers of cells, revealed the presence of immunoreactive proteins of the appropriate approximate molecular weights using specific SIV (lanes 1–6) or HIV-1 (lanes 7–9) Nef antisera. It is well established that the expression of functional Nef proteins leads to the reduction of cell surface levels of CD4 (reviewed in ref. 4). Thus, to confirm that the Nef proteins in our cell lines were functionally active, we measured CD4 surface levels by flow cytometry (Table 1). In all cases, the Nef-expressing cell lines displayed significantly reduced levels of cell surface CD4 (60–95% reduction) compared with nontransduced parental cells.
Figure 1.
Immunoblot analysis of Nef-transduced human T cell lines. Cell lysates were prepared from CEM-SS/cat (lane 1), CEM-SS/SIVmac239 Nef (lane 2), Jurkat (lane 3), Jurkat/SIVmac239 Nef (lane 4), SupT1 (lane 5), SupT1/SIVmac239 Nef (lane 6), CEM-SS/HIVfen (lane 7), CEM-SS/HIV-1NL43 Nef (lane 8), and CEM-SS/HIV-1cons Nef (lane 9), and resolved on 10% SDS/PAGE (7.5 × 105 cell equivalents per lane). The 32-kDa SIV (lanes 1–6) and 27-kDa HIV (lanes 7–9) Nef isolates were detected by blotting with specific rabbit antisera and visualized with 125I-labeled goat anti-rabbit IgG and autoradiography. The 29-kDa molecular weight marker is indicated for each gel.
Table 1.
CD4 surface expression by flow cytometry on control and Nef-expressing human T cell lines
| Cell line | Total mean fluorescence
|
|
|---|---|---|
| P3 | OKT4 | |
| CEM-SS | 4.7 | 188.4 |
| CEM-SS/cat | 5.4 | 105.9 |
| CEM-SS/HIVfen | 10.4 | 178.4 |
| CEM-SS/SIV Nef | 4.7 | 48.5 |
| CEM-SS/HIV-1NL43 Nef | 4.9 | 21.1 |
| CEM-SS/HIV-1cons Nef | 7.0 | 14.0 |
| Jurkat | 5.3 | 40.9 |
| Jurkat/SIV Nef | 3.0 | 16.9 |
| SupT1 | 5.5 | 265.7 |
| SupT1/SIV Nef | 5.0 | 40.9 |
Indirect immunofluorescent staining was performed using the anti-CD4 mAb OKT4 and the mouse IgG1 control mAb P3X63Ag8 (ATCC TIB9; unknown specificity). The results from one representative experiment are shown; staining was repeated with similar results in at least four independent experiments.
Expression of SIV or HIV-1 Nef Results in the Accumulation of Cytoplasmic Vesicles in Human T Cell Lines.
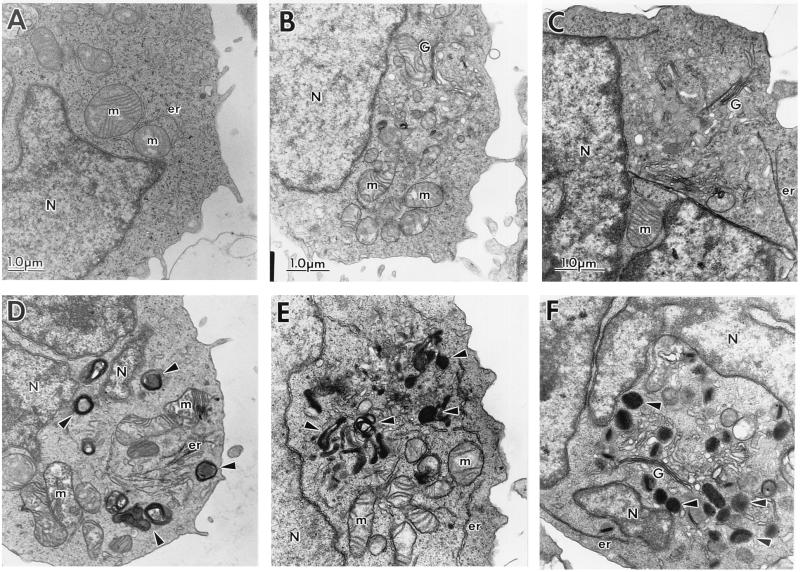
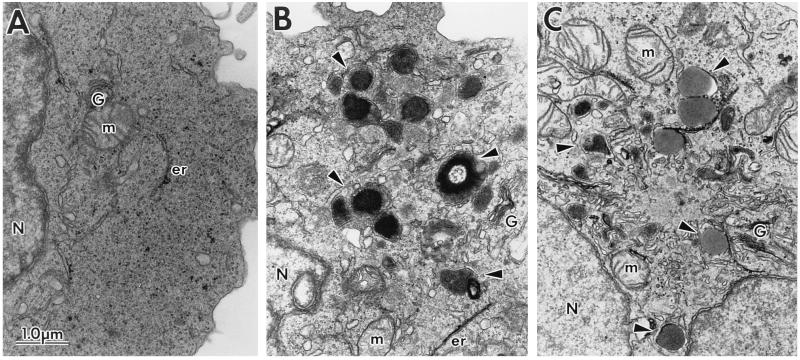
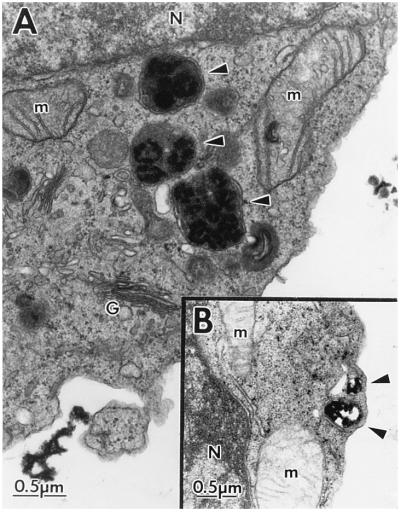
The subcellular morphology and ultrastructure of the human T cell lines in the presence of the SIVmac239 Nef (Fig. 2) or HIV-1 Nef (Fig. 3) proteins were examined by EM. Electron micrographs of clonal populations of CEM-SS cells transduced with the CAT control construct (A) or SIVmac239 nef (D); parent Jurkat cells (B) and Jurkat cells expressing SIVmac239 Nef (E); SupT1 parent cells (C) and clonal SupT1 cells expressing SIVmac239 Nef (F) are shown in Fig. 2. Electron micrographs of the CEM-SS HIV-1 fen control cells (A) and clonal CEM-SS cells expressing HIV-1NL43 Nef (B) or HIV-1cons Nef (C) are shown in Fig. 3. The most noticeable morphologic feature of Nef-expressing cells was the impressive array of cytoplasmic vesicles (representative structures are marked by arrows). Cells (50\N100) were examined for each cell type, and vesicles were present in nearly every cell section. The vesicles ranged in size from 0.2 to 0.8 μm; some contained homogeneous intravesicular material, whereas others were more heterogeneous in nature. They were surrounded by electron-dense membranes of varying thickness. The heterogeneous vesicles contained multivesicular or membranous material, and sometimes electron-dense cores. In some cases the vesicles were too electron-dense to reveal any distinct intravesicular morphology (for example see Figs. 2 F and 3 B). The appearance of this enlarged vesicular compartment correlated with the expression of the Nef protein, in that control cells were largely devoid of vesicles. Cytoplasmic vesicles could only be found occasionally in cells transduced with control constructs or in nontransduced parent cell lines. Of note, the various cell lines differed in their levels of SIV Nef expression based on cell equivalents (Fig. 1). Quantitative evaluation of HIV-1 Nef expression was not possible since the relative reactivity of the Nef antisera to the two different nef isolates is not known. However, all of the Nef-expressing cell lines displayed similar morphological changes. This was also true for several additional Nef-expressing CEM-SS and SupT1 subclones examined (data not shown). Because of the complex intracellular morphology found in Nef-expressing cells, we considered the possibility that these cells were metabolically more active than control cells. However, the Nef-expressing cells did not demonstrate increased proliferation rates compared with control cells, as measured by incorporation of [3H]thymidine (data not shown).
Figure 2.
Electron micrographs of the subcellular morphology of control and SIVmac239 Nef-expressing human T cells. SIVmac239 Nef-expressing and control human T cell lines were fixed and sectioned for examination by EM. Shown are control CEM-SS (CEM-SS/cat, A) and CEM-SS/SIVmac239 Nef cells (D); Jurkat (B) and Jurkat/SIVmac239 Nef cells (E); SupT1 (C) and SupT1/SIVmac239 Nef cells (F). The magnification of each pair is indicated by the scale bars in A–C. Representative intracellular structures are marked: G, Golgi complex; m, mitochondria; N, nucleus; er, endoplasmic reticulum. Arrowheads note representative vesicles in Nef-expressing cells.
Figure 3.
Electron micrographs of the subcellular morphology of control and HIV-1 Nef-expressing CEM-SS cells. CEM-SS cells transduced with HIVfen (A), expressing HIV-1NL43 Nef (B) or HIV-1cons Nef (C) were fixed and sectioned for visualization by EM. G, Golgi; m, mitochondria; er, endoplasmic reticulum; N, nucleus. Arrowheads mark representative vesicles in Nef-expressing cells.
It is evident from these experiments that human T cell lines expressing the HIV-1 or SIV Nef proteins display an abundance of cytoplasmic vesicles of similar size and appearance, showing that this effect is neither cell type specific nor a unique feature of a particular viral isolate of nef. Additionally, the phenotype seen does not simply result from decreased CD4 expression, as CEM-SS/cat cells (Fig. 2A) have reduced levels of CD4 (see Table 1), yet morphologically resemble parental or CEM-SS/fen control cells. Our data indicate that altered subcellular morphology results from Nef expression.
Nef-Induced Vesicles Display Characteristic Features of Endosomes and Lysosomes.
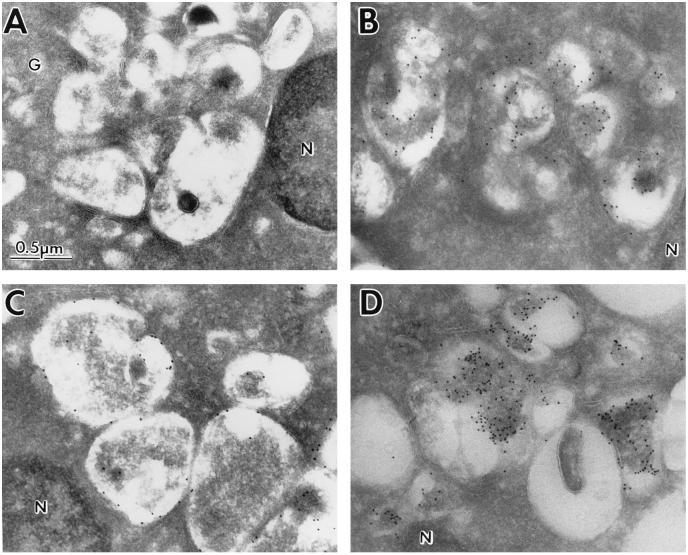
Inasmuch as Nef-expressing cells rapidly degrade newly synthesized CD4 protein (9, 11, 12), we used IEM to investigate whether the vesicles seen in Nef-expressing cells contained proteins characteristic of degradative compartments (Fig. 4). Cryosections were prepared from HIV-1NL43 Nef-expressing CEM-SS cells, and immunogold labeling was performed using antibodies to two endosomal/lysosomal proteins: cathepsin D and LAMP 2 (Fig. 4 B and C, respectively). Cathepsin D is a proteolytic enzyme that can be detected in the lumen of both late endosomes and lysosomes by IEM (19–21). LAMP 2 is a membrane-bound glycoprotein also found in late endosomes and lysosomes (22–24). Both of these proteins were present in the vesicles characteristic of Nef-expressing cells, but not in surrounding areas. As a control, cell sections were also incubated with secondary antibody alone (Fig. 4A), demonstrating that the staining was specific. Identical results were obtained with Jurkat or CEM-SS cells expressing SIVmac239 Nef (data not shown). In addition to these studies, we also examined HIV-1cons Nef-expressing CEM-SS cells using an antisera to the cation-independent mannose 6-phosphate receptor (CI-M6PR). CI-M6PR-expression was examined because CI-M6PR is localized to endosomes, but not lysosomes (19, 20, 25), thus allowing for a more precise identification of the vesicles in Nef-expressing cells. In contrast to the results using antibodies to cathepsin D or LAMP 2, only some vesicles labeled abundantly with antibodies to CI-M6PR, leaving others unlabeled (Fig. 4D). Taken together, our data indicate that the vesicles present in Nef-expressing cells include both late endosomes and lysosomes based on reactivity with antibodies to three diagnostic proteins.
Figure 4.
IEM of cytoplasmic vesicles in Nef-expressing CEM-SS cells using endosomal and lysosomal markers. EM was performed on cryosections of CEM-SS cells expressing HIV-1NL43 Nef stained with no primary reagent (A), anti-cathepsin D (B), or anti-LAMP 2 (C), followed by secondary 10-nm colloidal gold conjugates. CEM-SS cells expressing HIV-1cons Nef were stained with anti-CI-M6PR followed by 10-nm colloidal gold conjugates (D). G, Golgi complex; N, nucleus.
Vesicles functioning along the endosomal pathway can also be identified by analysis of internalized endocytic markers such as CF. Ferritin is a large iron storage protein which, in its cationized form, binds nonspecifically to the negatively charged cell membrane. During a 30–60 min incubation at 37°C, it is taken up via the endocytic pathway and can subsequently be detected by EM in endosomes and lysosomes (18, 19). In the experiments shown here, CF was bound to control and HIV-1NL43 Nef-expressing CEM-SS cells at 4°C, followed by an overnight incubation at 37°C to allow internalization and accumulation. The cells were then sectioned and visualized by EM (Fig. 5). CF accumulated in large vesicles in HIV-1NL43 Nef-expressing cells (Fig. 5A). The vesicles are similar to those seen in all Nef-expressing cell lines, indicating that these structures participate in the endosomal/lysosomal trafficking pathway. Control CEM-SS/cat cells were also able to accumulate CF into large vesicles (Fig 5B). Vesicles were often located close to the plasma membrane (B), but could also be found in the interior of the cytoplasm. Jurkat and CEM-SS cells expressing SIVmac239 Nef were also examined for CF uptake and showed accumulation of internalized CF in large cytoplasmic vesicles, similar to those shown in Fig. 5A (data not shown). In all experiments, less CF was visible inside control cells, as compared with Nef-expressing cells, suggesting that control cells internalize CF less efficiently than Nef-expressing cells. Similar results were obtained when CF (or neutral red) uptake was measured over a time course (data not shown). In conclusion, the populations of vesicles found in all Nef-expressing cell lines appear functionally equivalent, based on protein content as well as the accumulation of the endocytic marker CF.
Figure 5.
Accumulation of internalized CF in cytoplasmic vesicles in control and Nef-expressing CEM-SS cells. HIV-1NL43 Nef-expressing CEM-SS (A) and control CEM-SS/cat (B) cells were incubated with CF for 10 min at 4°C, warmed to 37°C, and incubated overnight. The samples were fixed, sectioned, and examined by EM. G, Golgi complex; M, mitochondria; N, nucleus.
DISCUSSION
The experiments described in this report reveal a novel property of the Nef protein. We have demonstrated by EM that expression of either SIV or HIV-1 Nef in three different human T cell lines induces the formation of cytoplasmic vesicles. The vesicles morphologically resemble endosomes and lysosomes as described in other cell types (26–29). In addition, the Nef-induced vesicles all contain cathepsin D and LAMP 2, and accumulate internalized CF, indicating that they function along the endosomal/lysosomal pathway. Interestingly, only a subset of the vesicles labeled with antibodies to CI-M6PR, a marker for endosomes, but not lysosomes (19, 20, 25). This result indicates that both endosomes and the more acidic lysosomes accumulate in Nef-expressing cells. We conclude that the expression of various isolates of HIV-1 or SIV Nef in different cell types consistently results in the expansion of an endosomal/lysosomal compartment.
There are precedents for the alteration of intracellular compartmentalization in response to the expression of a particular protein. For example, expression of von Willebrand factor, a component of platelet plug formation, is specific to endothelial cells and megakaryocytes, yet the transfection of von Willebrand factor cDNA into other cell types induces the formation of large storage organelles, which accumulate this factor (30). Another example is the MHC class II compartment, which functions to accumulate newly synthesized MHC class II together with endocytosed peptide and to proteolyse the invariant chain. Strikingly, transfection of MHC class II into human embryonic kidney cells, which do not normally express class II proteins, is sufficient to induce the formation of the MHC class II compartment (31). The mechanism by which the expression of MHC class II molecules leads to formation of this compartment is not known, but may involve the aggregation of class II in endocytic vesicles (31). It remains to be determined whether Nef acts similarly to aggregate cellular proteins and thus induces the formation of endosomal and lysosomal vesicles, or if the primary sequence of Nef encodes the information dictating vesicle accumulation. Cells have been shown to up-regulate degradative compartments in response to increased rates of endocytosis. For instance, increased endocytosis leads to the formation of multivesicular endosomal bodies in neutrophils (27). Because it has been previously shown that Nef induces CD4 endocytosis (9), this event may precipitate the formation of degradative vesicles. This is consistent with preliminary IEM studies, wherein Nef appears to accumulate at the plasma membrane (data not shown). Alternatively, it is possible that the Nef protein induces the accumulation of endosomes and lysosomes independently of endocytosis, for example by effects on the process of autophagy (32, 33). Moreover, it is not evident from our studies whether the endosomal/lysosomal vesicles in Nef-expressing cells represent newly formed structures, or the expansion of a preexisting compartment.
From our data, it seems reasonable to hypothesize that Nef-induced CD4 degradation occurs in the degradative compartment up-regulated in Nef-expressing cells. We have not been able to detect CD4 in the late endosomal and lysosomal vesicles found in Nef-expressing cells by EM. This is consistent with the finding that CD4 in Nef-expressing cells can be detected only in early, but not late, endosomes by indirect immunofluorescence microscopy (34), and may reflect the denaturation of CD4 epitopes in the more mature degradative compartment described here. We have previously shown that the degradation of CD4 in Nef-expressing cells can be inhibited by treating cells with lysosomotropic agents, demonstrating that the Nef-induced degradation of CD4 occurs in lysosomes (11). In addition, we have shown by inhibitor studies that CD4 degradation is dependent on cathepsin D (data not shown), consistent with the hypothesis that CD4 is degraded in the type of vesicles described herein.
Several roles for the expanded endosomal and lysosomal compartment found in Nef-expressing cells may be envisaged. First, the primary function of the up-regulated degradative compartment may be to efficiently degrade CD4 in infected cells. This could be important for viral propagation, since clearance of CD4 from the cell surface may act to prevent superinfection (35). However, the endosomal/lysosomal compartment in Nef-expressing cells may serve to degrade other cellular proteins. It has been reported that Nef can reduce the cell surface expression of interleukin 2 receptor (CD25) (36), although the expression of most cell surface markers examined is unaffected by Nef (16). Third, viral components may be accumulated and processed in the endosomal and lysosomal vesicles observed in Nef-expressing cells. For example, an acidic post-Golgi compartment has been implicated in the processing of the envelope gp160 precursor into mature gp120/gp41 heterodimers (37, 38). If the endosomal/lysosomal vesicles serve in this capacity, they must be able to carry out functions other than protein degradation. Such an arrangement would not be without precedent. The multivesicular granules found in RNK-16 cells display features of both endocytic vesicles and secretory granules (18); the MHC class II compartment similarly intersects the degradative and biosynthetic pathways (39, 40). Finally, the endosomal/lysosomal vesicles may act as degradative compartments for viral proteins. For instance, only a small percentage of the gp160 precursor produced is processed in HIV-1 infected cells, with the majority of envelope glycoprotein being degraded in an acidic compartment (38), presumably due to incorrect folding of the newly synthesized protein. The expression of Nef might serve to expand the degradative compartment in infected cells to provide a mechanism to efficiently eliminate superfluous viral proteins, and thus maintain cellular functions. The continued investigation of the endosomal/lysosomal compartment in Nef-expressing cells promises to provide new insights in the role of Nef in viral pathogenesis.
Acknowledgments
We wish to thank Bryan Cullen and Diane Shugars for cell lines and reagents, and Witte Koopmann and Jennifer Brogdon for critical reading of the manuscript. This work was supported by National Institutes of Health (NIH) Pre-Doctoral Training Grant 5T32CA09058 (A.S.), NIH Grant AI38696 and American Cancer Society Junior Faculty Research Award (C.D.), and an Investigator Award in Immunology from the Cancer Research Institute (C.D.)
Footnotes
Abbreviations: CAT, chloramphenicol acetyltransferase; CF, cationized ferritin; CI-M6PR, cation-independent mannose 6-phosphate receptor; LAMP, lysosome-associated membrane protein; MHC, major histocompatibility complex; SIV, simian immunodeficiency virus; EM, electron microscopy; IEM, immunoelectron microscopy.
References
- 1.Kestler H W, III, Ringler D L, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 3.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, et al. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 4.Cullen B R. Virology. 1994;205:1–6. doi: 10.1006/viro.1994.1613. [DOI] [PubMed] [Google Scholar]
- 5.Trono D. Cell. 1995;82:189–192. doi: 10.1016/0092-8674(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 6.Doyle C, Strominger J L. Nature (London) 1987;330:256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- 7.Janeway C A., Jr Semin Immunol. 1991;3:153–160. [PubMed] [Google Scholar]
- 8.Sattentau Q J, Clapham P R, Weiss R A, Beverley P C, Montagnier L, Alhalabi M F, Gluckman J C, Klatzmann D. AIDS. 1988;2:101–105. doi: 10.1097/00002030-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 10.Guy B, Riviere Y, Dott K, Regnault A, Kierny M P. Virology. 1990;176:413–425. doi: 10.1016/0042-6822(90)90011-f. [DOI] [PubMed] [Google Scholar]
- 11.Sanfridson A, Cullen B R, Doyle C. J Biol Chem. 1994;269:3917–3920. [PubMed] [Google Scholar]
- 12.Rhee S S, Marsh J W. J Virol. 1994;68:5156–5163. doi: 10.1128/jvi.68.8.5156-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris M P G, Neil J C. J Mol Biol. 1994;241:136–142. doi: 10.1006/jmbi.1994.1483. [DOI] [PubMed] [Google Scholar]
- 14.Rossi F, Gallina A, Milanes G. Virology. 1996;217:397–403. doi: 10.1006/viro.1996.0130. [DOI] [PubMed] [Google Scholar]
- 15.Garcia J V, Miller A D. AIDS Res Hum Retroviruses. 1994;10:47–52. doi: 10.1089/aid.1994.10.47. [DOI] [PubMed] [Google Scholar]
- 16.Benson R E, Sanfridson A, Ottinger J S, Doyle C, Cullen B R. J Exp Med. 1993;177:1561–1566. doi: 10.1084/jem.177.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shugars D C, Smith M S, Glueck D H, Nantermet P V, Seillier-Moiseiwitsch F, Swanstrom R. J Virol. 1993;67:4639–4650. doi: 10.1128/jvi.67.8.4639-4650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burkhardt J K, Hester S, Lapham C K, Argon Y. J Cell Biol. 1990;111:2327–2340. doi: 10.1083/jcb.111.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geuze H J, Stoorvogel W, Strous G J, Slot J W, Bleekemolen J E, Mellman I. J Cell Biol. 1988;107:2491–2501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 21.Radons J, Biewusch U, Grassel S, Geuze H J, Hasilik A. Biochem J. 1994;302:581–586. doi: 10.1042/bj3020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J W, Chen G L, D’Souza M P, Murphy T L, August J T. Biochem Soc Symp. 1986;51:97–112. [PubMed] [Google Scholar]
- 23.Chen J W, Murphy T L, Willingham M C, Pastan I, August J T. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mane S M, Marzella L, Bainton D F, Holt V K, Cha Y, Hildreth J E K, August J T. Arch Biochem Biophys. 1989;268:360–378. doi: 10.1016/0003-9861(89)90597-3. [DOI] [PubMed] [Google Scholar]
- 25.Brown W J, Goodhouse J, Farquhar M G. J Cell Biol. 1986;103:1235–1247. doi: 10.1083/jcb.103.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becich M J, Mahklouf S, Barnziger J U. Eur J Cell Biol. 1991;55:83–93. [PubMed] [Google Scholar]
- 27.Berger M, Wetzler E, August J T, Tartakoff A M. J Clin Invest. 1994;94:1113–1125. doi: 10.1172/JCI117426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chronopoulos S, Laird D W, Ali-Khan Z. J Pathol. 1994;173:361–369. doi: 10.1002/path.1711730412. [DOI] [PubMed] [Google Scholar]
- 29.Schneider P. Arch Toxicol. 1992;66:23–33. doi: 10.1007/BF02307266. [DOI] [PubMed] [Google Scholar]
- 30.Wagner D D, Saffaripour S, Bonfanti R, Sadler J E, Cramer E M, Chapman B, Mayyadas T N. Cell. 1991;64:403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]
- 31.Calafat J, Nijenhuis M, Janssen H, Tulp A, Dusseljee S, Wubbolts R, Neefjes J. J Cell Biol. 1994;126:967–978. doi: 10.1083/jcb.126.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn W A., Jr J Cell Biol. 1990;110:1935–1945. doi: 10.1083/jcb.110.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunn W A., Jr J Cell Biol. 1990;110:1923–1933. doi: 10.1083/jcb.110.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz O, Dautry-Varsat A, Goud B, Maréchal V, Subtil A, Heard J-M, Danos O. J Virol. 1995;69:528–533. doi: 10.1128/jvi.69.1.528-533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeGuern M, Levy J A. Proc Natl Acad Sci USA. 1992;89:363–367. [Google Scholar]
- 36.Greenway A L, McPhee D A, Grgacic E, Hewish D, Lucantoni A, Macreadie I, Azad A. Virology. 1994;198:245–256. doi: 10.1006/viro.1994.1027. [DOI] [PubMed] [Google Scholar]
- 37.Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk H-D, Garten W. Nature (London) 1992;360:358–361. doi: 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- 38.Willey R L, Bonifacino J S, Potts B J, Martin M A, Klausner R D. Proc Natl Acad Sci USA. 1988;85:9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amigorena S, Drake J R, Webster P, Mellman I. Nature (London) 1994;369:113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- 40.Tulp A, Verwoerd D, Dobberstein B, Ploegh H L, Pieters J. Nature (London) 1994;369:120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]