Abstract
The c-Jun NH2-terminal kinase (JNK) group of mitogen-activated protein kinases is stimulated in response to a wide array of cellular stresses and proinflammatory cytokines. Mice lacking individual members of the Jnk family (Jnk1, Jnk2, and Jnk3) are viable and survive without overt structural abnormalities. Here we show that mice with a compound deficiency in Jnk expression can survive to birth, but fail to close the optic fissure (retinal coloboma). We demonstrate that JNK initiates a cytokine cascade of bone morphogenetic protein-4 (BMP4) and sonic hedgehog (Shh) that induces the expression of the paired-like homeobox transcription factor Pax2 and closure of the optic fissure. Interestingly, the role of JNK to regulate BMP4 expression during optic fissure closure is conserved in Drosophila during dorsal closure, a related morphogenetic process that requires JNK-regulated expression of the BMP4 ortholog Decapentaplegic (Dpp).
Keywords: MAP kinase, JNK, bone morphogenetic protein-4, sonic hedgehog, Pax2, coloboma
The c-Jun NH2-terminal kinase (JNK) subfamily of mitogen-activated protein kinases is encoded by three related genes (Davis 2000; Weston and Davis 2002). Jnk1 and Jnk2 are expressed ubiquitously during development, whereas Jnk3 is primarily expressed in the brain and to a lesser extent in the heart and testis. Mice lacking individual members of the Jnk family are viable (Yang et al. 1997, 1998; Dong et al. 1998; Sabapathy et al. 1999a). However, mice lacking both of the ubiquitously expressed JNK isoforms (JNK1 and JNK2) die during midgestation with neural tube closure defects and brain abnormalities (Kuan et al. 1999; Sabapathy et al. 1999b). These defects are associated with regional and developmental stage-specific alterations in apoptosis in the developing nervous system (Kuan et al. 1999; Sabapathy et al. 1999b). Neural tube closure is a morphogenetic process that involves apoptosis, cell migration, and cell proliferation; it is therefore possible that during development JNK not only contributes to apoptosis regulation, but may also play a crucial role in other morphogenetic processes.
Components of the JNK signaling pathway are highly conserved between mammals and insects (Ip and Davis 1998). The Drosophila JNK ortholog Basket (DJNK) is necessary for both dorsal and thorax closure (Martin-Blanco 1997; Ip and Davis 1998; Zeitlinger and Bohmann 1999). The signaling mechanism that controls dorsal closure has been studied by genetic analysis in Drosophila. This signaling pathway requires the expression of the transforming growth factor-β (TGF-β)-like gene decapentaplegic (dpp) in the cells that form the leading edge of the epidermis during dorsal closure. This pattern of dpp expression is induced by the JNK signaling pathway and is mediated by AP-1 (DJun and DFos) and Ets (Aop) family transcription factors (Riesgo-Escovar et al. 1996; Sluss et al. 1996; Hou et al. 1997; Riesgo-Escovar and Hafen 1997; Sluss and Davis 1997; Zeitlinger and Bohmann 1999).
The purpose of this study was to examine the role of the JNK signaling pathway in mammalian development. We report that JNK is essential for closure of the optic fissure, a morphogenetic process that resembles dorsal and thorax closure in Drosophila. Interestingly, this function of JNK involves the induced expression of bone morphogenetic protein-4 (BMP4), the mammalian ortholog of Dpp. Thus, the JNK signaling pathway plays similar roles in both insects and mammals to control the developmentally regulated expression of BMP4/Dpp. We show that BMP4 acts to initiate a cytokine cascade that induces the expression of Sonic Hedgehog (Shh) and subsequently the expression of the paired-like homeobox transcription factor Pax2 and closure of the optic fissure.
Results
JNK is essential for eye development in mice
To examine the role of JNK in development, we investigated the effect of compound Jnk mutations in the C57BL/6 strain background. Mice lacking both of the ubiquitously expressed JNK isoforms (JNK1 and JNK2) died during midgestation with neural tube closure defects and brain abnormalities (Kuan et al. 1999; Sabapathy et al. 1999b). We therefore examined the consequence of the expression of a single allele of the ubiquitously expressed JNK isoforms. Mice with a single allele of Jnk1 (Jnk1−/+ Jnk2−/−) did not present an obvious developmental phenotype. In contrast, mice with a single allele of Jnk2 (Jnk1−/− Jnk2−/+) were born with the predicted Mendelian frequency, but 80% of these mice died within 48 h after birth. At embryonic day 18.5 (E18.5), these mutant embryos exhibited a number of developmental defects in the eye including severe lens abnormality and retinal coloboma (Fig. 1A,B).
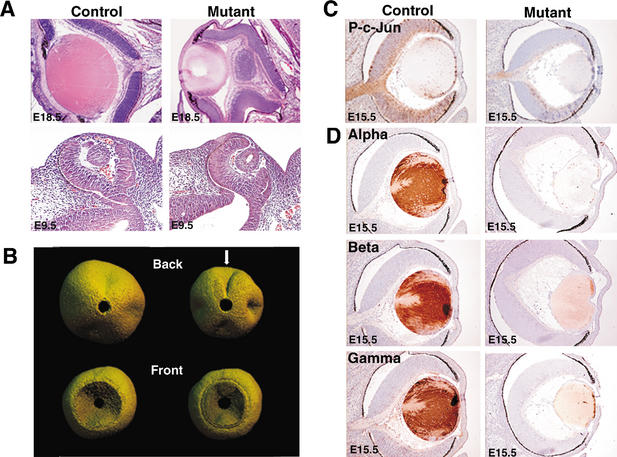
Figure 1.
Mutant Jnk1−/−Jnk2−/+ embryos exhibit decreased JNK activity and eye abnormalities. (A) Hematoxylin- and eosin-stained sections of E18.5 and E9.5 eyes indicate that mutant (Jnk1−/−Jnk2−/+) embryos exhibit lens defects and retinal coloboma. (B) Three-dimensional digital microanalysis of retinas from E18.5 embryos. The arrow indicates coloboma at the back of the mutant eye. (C) Immunohistochemical staining of Ser-63-phosphorylated c-Jun as an in situ measurement of JNK protein kinase activity in vivo. Phosphorylated c-Jun is present in the optic nerve, lens, and retina of control embryos (brown) but is markedly reduced in the mutant eyes. (D) Mutant E15.5 lenses are smaller than lenses from control littermates and have decreased levels of αA-, β-, and γ-crystallin (brown). Control studies demonstrated that no immunohistochemistry staining was detected in control tissues incubated with normal IgG plus the appropriate secondary antibodies. Throughout this study, Jnk1+/+Jnk2−/+ embryos were used as littermate controls, and in all cases these results were consistent with those observed in wild-type C57BL/6 embryos of the same age. Ocular anomalies including defective lens cell development have been reported in a small percentage of C57BL/6 mice (Favor et al. 1996; Eccles and Schimmenti 1999); however, the phenotype reported here is genotype-specific and is not similar to wild-type mice.
JNK phosphorylates c-Jun on sites in the activation domain (Davis 2000; Weston and Davis 2002). We therefore used immunohistochemical staining of phosphorylated c-Jun as an in situ indicator of the biological activity of JNK in vivo (Yang et al. 1997). Phosphorylated c-Jun was present in the optic nerve, lens, and retina of control embryos, but was markedly reduced in the JNK-deficient mutant (Jnk1−/− Jnk2−/+) embryos (Fig. 1C). A small amount of phosphorylated c-Jun was detected in the mutant optic nerve and this may be attributed to either Jnk3, or to the single remaining allele of Jnk2. Together, these data demonstrate that the JNK signaling pathway is severely attenuated in the JNK-deficient mutant (Jnk1−/− Jnk2−/+) embryos.
The JNK-deficient embryos exhibited multiple eye defects including open eyelids, small lenses, and retinal coloboma (Fig. 1A). Although open eyelids have been associated with eye deformities, we observed ocular pathology in the JNK-deficient embryos prior to E16.5, the time when mouse eyelids close during gestation (Fig. 1A). Thus, the open eyelid phenotype does not contribute to the failure to close the optic fissure, a morphogenetic process that occurs at E11.5. However, it is likely that the open eyelids may contribute to additional ocular pathologies late in development.
JNK is required for normal development of the lens
Mutant embryos exhibited smaller lenses with decreased expression of α-, β-, and γ-crystallin compared with littermate control embryos (Fig. 1C). The decreased crystallin expression may result from at least two defects in the JNK-deficient embryos. First, AP-1 proteins have been shown to regulate a diverse population of crystallin genes in several different species (Piatigorsky 1992; Tomarev et al. 1994; McDermott et al. 1997; Klok et al. 1998). For example, the AP-1 protein c-Jun plays a role in directly regulating αA-crystallin gene expression in the mouse (Ilagan et al. 1999). It is therefore likely that JNK/c-Jun directly influences crystallin expression in mutant embryos. Second, the transcription factors Sox2 and Pax6 are widely implicated in the regulation of crystallin gene expression (Cvekl et al. 1994, 1995; Richardson et al. 1995; Gopal-Srivastava et al. 1996; Chow and Lang 2001) and can functionally cooperate to bind DNA (Kamachi et al. 2001). The expression of these two transcription factors was decreased in JNK-deficient embryos. In control embryos, Sox2 was expressed strongly in the retina, but this expression was markedly reduced in mutant embryos (Fig. 2C). Similarly, Pax6 protein and mRNA were expressed strongly in the retina and the lens epithelium in control embryos, and a marked reduction (but not total ablation) of both Pax6 mRNA and protein expression was observed in the JNK-deficient embryos (Fig. 2D).
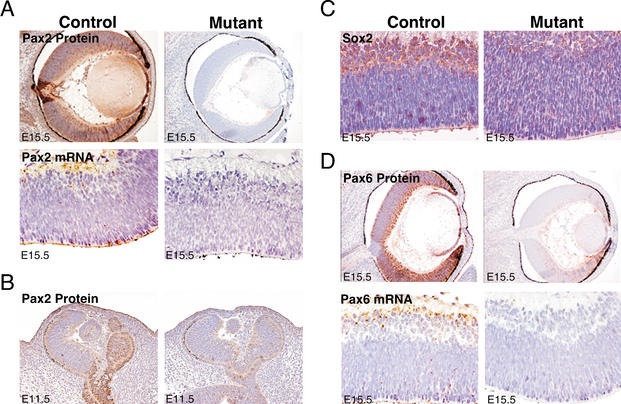
Figure 2.
JNK is necessary for the expression of the Pax2, Sox2, and Pax6 transcription factors in embryonic eyes. (A) Mutant E15.5 eyes express decreased amounts of Pax2 protein, as determined by immunohistochemistry, and Pax2 mRNA, as determined by in situ hybridization, compared with littermate controls (brown). (B) Mutant E11.5 eyes express decreased amounts of Pax2 protein (brown). (C) Mutant E15.5 eyes express decreased amounts of Sox2 protein (brown). (D) Mutant eyes express decreased amounts of Pax6 protein and Pax6 mRNA compared with littermate controls (brown). No staining was detected in control tissues incubated with either normal IgG plus the appropriate secondary antibodies (immunohistochemistry) or the relevant sense probes (in situ hybridization; data not shown).
Reduced Pax6 expression results in a number of ocular phenotypes, including the mouse mutant Small eye (Hogan et al. 1986; Hill et al. 1991) and human Aniridia patients (Hill et al. 1991). Homozygous deletion of Pax6 is embryonic lethal in mice; these embryos lack eyes and a nose, and also have some brain defects (Hill et al. 1991). The eyes of the Jnk mutant embryos were smaller than littermate controls, consistent with a reduction (but not complete loss) of Pax6 expression (Fig. 1). However, reduced Pax6 expression by itself does not fully account for the defective lens development observed in JNK-deficient embryos because, although Pax6 can increase the expression of a number of crystallin genes (Cvekl et al. 1994, 1995; Richardson et al. 1995; Gopal-Srivastava et al. 1996; Chow and Lang 2001), it is established that Pax6 functions as a repressor of β-crystallin gene expression (Duncan et al. 1998). In contrast, studies of JNK-deficient embryos demonstrated that decreased expression of Pax6 is associated with decreased β-crystallin expression (Fig. 1C). It is therefore likely that the reduced crystallin expression observed in JNK-deficient embryos is a consequence of the combined alterations in Pax6, Sox2, and c-Jun.
JNK is essential for closure of the optic fissure
JNK-deficient embryos exhibited retinal coloboma (Fig. 1A,B). Coloboma describes a failure of the optic cleft to close during eye development (Kaufmann 1999). Retinal coloboma was apparent in JNK-deficient mice during midgestation and was associated with major eye defects at E18.5 (Fig. 1A,B). In humans, coloboma occurs in ∼1:10,000 births (http://www.rnib.com). Human coloboma can affect the retina, iris, lens, eyelid, or optic nerve and is often associated with renal malformations (known as renal coloboma syndrome; Eccles and Schimmenti 1999). Coloboma can be caused by mutations in the Pax2 gene, and at least eight different Pax2 mutations have been reported in humans (Eccles and Schimmenti 1999). Similarly, mice lacking the Pax2 gene display both coloboma and deformities of the kidney, brain, ear, and urogenital system (Torres et al. 1995, 1996; Favor et al. 1996). Pax2 heterozygous mutants exhibit exencephaly at E11.5 (Torres et al. 1996) and, consistent with these observations, homozygous JNK-deficient embryos also exhibit exencephaly (Kuan et al. 1999; Sabapathy et al. 1999b). Interestingly, JNK-deficient embryos had significantly reduced expression of Pax2 in the eye at E15.5 and also at E11.5, the time when closure of the optic fissure occurs (Kaufmann 1999), whereas control embryos exhibited strong expression throughout the lens and retina, with particularly high levels in the optic nerve (Fig. 2A,B).
The effects of Pax2 deficiency and JNK deficiency on mouse development share some similarities. For example, Pax2-deficient embryos show altered expression of Shh at the basis of the diencephalon at E9.5 (Torres et al. 1996). Similarly, JNK deficiency also caused markedly altered Shh expression at the basis of the diencephalon in E9.5 embryos (Fig. 3A). However, differences between the phenotype of Pax2-deficient and JNK-deficient embryos are apparent. Thus, Pax2-deficient embryos exhibit only ipsilateral optic tracts as a result of agenesis of the optic chiasma (Torres et al. 1996), but this was not observed in JNK-deficient embryos (data not shown). Furthermore, in Pax2 mutant mice, the retinal pigmentation does not stop at the eyestalk limit, but extends abnormally into the stalk region (Torres et al. 1996); this is also not observed in JNK-deficient embryos. The failure of JNK1/JNK2-deficient embryos to mimic the effects of Pax2 deficiency on the optic chiasma may reflect a role for JNK3, or may indicate that Pax2 has some functions that are independent of JNK.
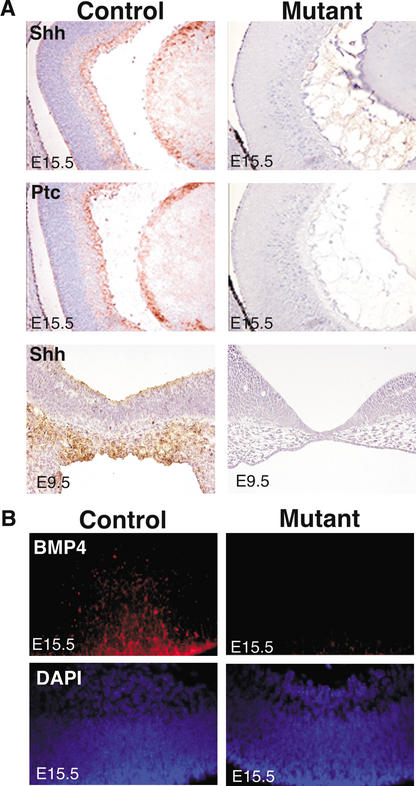
Figure 3.
JNK is essential for the expression of Sonic Hedgehog (Shh), Patched-1 (Ptc), and bone morphogenic protein-4 (BMP4) in the developing eye. (A) Mutant E15.5 retinas have decreased levels of Shh and Ptc mRNA (brown) compared with controls, as determined by in situ hybridization. There is also decreased Shh expression in the diencephalon of E9.5 mutant embryos compared with control littermates. (B) Embryonic eyes were hybridized with BMP4 riboprobes, visualized with Texas Red (red) and counterstained with DAPI (blue). A significant reduction in BMP4 expression was detected in mutant retinas compared with control littermates. No staining was detected in control tissues stained with the relevant Shh, Ptc, or BMP4 sense probes.
JNK regulates Sonic Hedgehog expression in the retina
We investigated the mechanism that regulates Pax2 expression, and therefore coloboma formation, in JNK-deficient mutant embryos. The Drosophila Hedgehog protein and the mammalian homolog Shh are important for pattern formation during retinal development (Zhang et al. 2000; Neumann 2001). Indeed, homozygous deletion of the Shh gene in mice causes embryonic lethality, neural tube closure defects, and cyclopia (Chiang et al. 1996). Shh has been reported to influence Pax2 expression during eye morphogenesis (Zhang and Yang 2001). We therefore examined Shh expression in wild-type and JNK-deficient embryos. Strong expression of Shh was observed in the retina and lens of control murine embryos, but no expression of Shh was detected in JNK-deficient eyes (Fig. 3A). Interestingly, we found that a small percentage of JNK-deficient mutant embryos also exhibited cyclopia (data not shown). To confirm the lack of Shh signaling in the JNK-deficient embryos, we examined the expression of the Shh membrane receptor and reporter gene Patched-1 (Ptc; Fig. 3A; Marigo and Tabin 1996). The pattern of Ptc expression was very similar to Shh in wild-type embryonic eyes, but Ptc and Shh were absent in the JNK-deficient embryonic eyes, indicating a lack of both Shh expression and activity.
JNK regulates BMP4 expression in the retina
Studies of Drosophila demonstrate that JNK is required for embryonic epithelial cell sheet movements during dorsal closure and thorax closure, and for epithelial planar polarity (Ip and Davis 1998). During Drosophila dorsal closure, DJNK is necessary for expression of the TGF-β family protein Dpp in the cells that form the leading edge of the lateral epithelial cell sheet (Ip and Davis 1998). To determine whether JNK can regulate the mammalian Dpp ortholog BMP4, we examined the expression of BMP4 in control and mutant embryonic eyes. Interestingly, BMP4 was expressed in control retinas but was not detected in JNK-deficient mutant eyes (Fig. 3B). Mouse embryos homozygous for null mutations in Bmp4 die between E6.5 and E10 (Winnier et al. 1995; Lawson et al. 1999). Moreover, several defects have been described in heterozygous Bmp4 mutants, including eye defects, skeletal abnormalities, cystic kidneys, and urinary tract anomalies (Dunn et al. 1997; Miyazaki et al. 2000). The similar ocular phenotypes of mice lacking JNK, BMP4, Shh, and Pax2 imply that these proteins may be regulated by a similar mechanism during eye development.
Biochemical complementation of JNK deficiency with BMP4 in retinal explant cultures
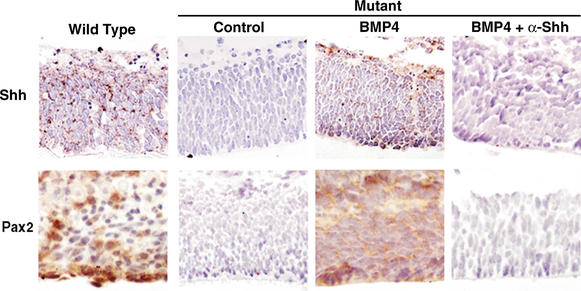
BMP4 has been implicated in the regulation of Shh expression in the mouse (Zhang et al. 2000). To test whether BMP4 regulates the expression of Shh and Pax2 in the eye, we examined retinal explant cultures. Consistent with in vivo results, strong expression of both Shh and Pax2 was detected in control retinas, but not in mutant retinas (Fig. 4). When the mutant retinas were cultured in the presence of BMP4 for 48 h, induced expression of both Shh and Pax2 was detected, indicating that BMP4 is sufficient to cause expression of Shh and Pax2, and that the BMP4–Shh–Pax2 pathway is intact in the JNK-deficient mutant embryonic eyes. In contrast, no BMP4-stimulated expression of Shh or Pax2 in the mutant retinas was detected in the absence or presence of an antagonistic antibody to Shh (Fig. 4). Similarly, when control retinas were cultured in the presence of BMP4 plus the antagonistic antibody to Shh, there was a dramatic decrease in both Shh and Pax2 expression (data not shown). These data imply that BMP4 induces the expression of Shh and Pax2 in mutant retinas, and that Shh is upstream of Pax2 expression. This signaling cascade is initiated by JNK and is absent in JNK-deficient retinas.
Figure 4.
Bone morphogenetic protein-4 (BMP4) is sufficient to induce a cascade of Sonic hedgehog (Shh) and Pax2 expression in retinal explant cultures. Retinas were dissected from E17.5 embryos and cultured in the presence of vehicle control, 50 ng/mL BMP4, or 50 μg/mL anti-Shh antibody 5E1 that acts as an Shh antagonist, for 48 h. Consistent with the in vivo data, Shh mRNA was detected in wild-type retinas (brown), but not in the mutant retinas treated with vehicle control. When mutant retinas were cultured in the presence of BMP4, there was a strong induction of Shh mRNA and Pax2 protein. However, when mutant retinas were cultured in the presence of BMP4 plus the Shh antagonist antibody, Shh and Pax2 were not detected. Similar results were obtained for Patched- 1 (Ptc) expression (data not shown). No staining was detected in control tissues stained with the relevant Shh or Ptc sense probes, or with control antibodies (data not shown).
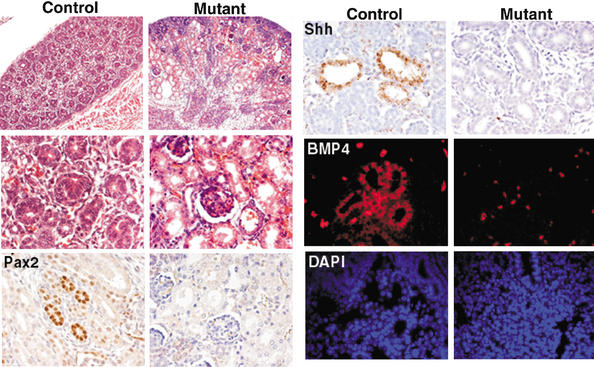
JNK is essential for the BMP4–Shh–Pax2 signaling pathway in the renal epithelium
JNK is necessary for the expression of BMP4, Shh, and Pax2 in the kidney (Fig. 5). During kidney development, Pax2 is required for the specification and differentiation of the renal epithelium in both mouse and human (Rothenpieler and Dressler 1993; Keller et al. 1994; Torres et al. 1995; Eccles and Schimmenti 1999; Ostrom et al. 2000). Similarly, Shh regulates proliferation and differentiation of kidney mesenchymal cells (Yu et al. 2002), and BMP4 is necessary for the morphogenesis of the kidney and urinary tract (Miyazaki et al. 2000; Raatikainen-Ahokas et al. 2000; Martinez et al. 2002). Consistent with these observations, kidneys from JNK-deficient mutant embryos had deformed renal epithelial cells and nephrons (Fig. 5). These defects in the renal epithelium could explain why the mutant mice die within 48 h after birth. There was no difference in the size of kidney, number of nephrons/S-shaped bodies, or degree of uteric branching in E15.5 kidneys in control and mutant embryos (data not shown). In the normal kidney, specific expression of Pax2, Shh, and BMP4 in the renal epithelial cells was detected, indicating that these proteins could regulate each other in the same BMP4–Shh–Pax2 cascade as that during retinal development (Fig. 5). However, markedly decreased expression of BMP4, Shh, or Pax2 in JNK-deficient embryonic kidneys was observed, consistent with the possible role of JNK as an initiator of this signaling cascade.
Figure 5.
JNK is required for the expression of Pax2, Sonic Hedgehog (Shh), and bone morphogenic protein-4 (BMP4) in the developing kidney. Hematoxylin- and eosin-stained E18.5 embryonic kidneys indicate that mutant kidneys contain enlarged tubules (low magnification; upper left panels) and have deformed renal epithelium and nephrons (high magnification; middle left panels). A high level of Pax2 protein (brown) and Shh mRNA (brown) was detected in renal epithelial cells in control E18.5 kidneys, but not in mutant kidneys. BMP4 mRNA (red) was also detected in control kidneys, counterstained with DAPI (blue), but not in mutant kidneys. Autofluorescence from erythrocytes was observed in control tissue, mutant tissue, and in control tissue hybridized with the sense probe. This autofluorescence was easily distinguished from the specific BMP4 staining observed in control tissues, but not in mutant kidneys or control kidneys stained with the sense probe.
Discussion
JNK-induced expression of BMP4/Dpp initiates a morphogenetic process that is conserved between insects and mammals
Genetic analysis of Drosophila demonstrates that the DJNK signaling pathway is required during midembryogenesis for dorsal closure, a morphogenetic process in which the lateral epidermal cells spread and migrate to cover the amnioserosa and form the dorsal surface of the embryo (Ip and Davis 1998). This morphogenetic process is mediated by the DJNK-dependent expression of the TGF-β-related molecule Dpp by the cells that form the leading edge of the epithelium. Here we demonstrate a role for JNK in a similar morphogenetic process during the development of the mammalian eye. Loss of JNK function in mice causes failure to close the optic fissure, resulting in retinal coloboma.
JNK is necessary for the expression of Dpp during dorsal closure in Drosophila (Ip and Davis 1998). We show that the mammalian ortholog of Dpp (BMP4) is also under the control of JNK. In vitro studies using retinal explant cultures indicate that the function of BMP4, in part, is to induce the expression of Shh in a cytokine cascade that leads to the expression of the paired-like homeobox transcription factor Pax2. The absence of this JNK-induced cytokine cascade in JNK-deficient embryos causes retinal coloboma.
JNK is required for lens development
BMP4 expression is severely reduced in JNK-deficient embryonic eyes (Fig. 3B). It was therefore possible that JNK deficiency and BMP4 deficiency may cause similar ocular defects. It is established that BMP4 is necessary for lens induction, apoptosis, and the development of primary lens fiber cells (Furuta and Hogan 1998; Trousse et al. 2001; Faber et al. 2002). Indeed, lens induction is absent in Bmp4−/− mouse embryos and this is associated with reduced expression of Sox2, but not Pax6 (Furuta and Hogan 1998). In contrast to the very severe effects of BMP4 deficiency on lens induction, JNK-deficient embryos exhibited a deformed lens (Fig. 1). The more moderate lens phenotype caused by JNK deficiency compared with that caused by BMP4 deficiency may be accounted for by the presence of a low level of JNK activity present in the JNK-deficient (Jnk1−/− Jnk2−/+) embryonic eyes (Fig. 1C). Studies using a conditional allele of JNK will be required to confirm this conclusion because JNK homozygous null embryos die during early development.
The altered lens development in JNK-deficient mice may result from the combined effects of at least three different defects. First, JNK deficiency causes a marked decrease in AP-1 activity (Ventura et al. 2003), a transcription factor that is important for crystallin gene expression (Piatigorsky 1992; Tomarev et al. 1994; McDermott et al. 1997; Klok et al. 1998; Ilagan et al. 1999). Second, the reduced crystallin expression observed in the JNK-deficient lens may result, in part, from reduced expression of BMP4, a cytokine that is necessary for the expression of Sox2 (Furuta and Hogan 1998), a transcription factor that mediates crystallin gene expression (Kamachi et al. 2001). Indeed, Sox2 expression is reduced in JNK-deficient embryos (Fig. 2C). Third, the JNK-deficient embryos express low levels of Pax6 (Fig. 2D), a transcription factor that can collaborate with Sox2 to increase crystallin gene expression (Cvekl et al. 1994, 1995; Richardson et al. 1995; Gopal-Srivastava et al. 1996; Duncan et al. 1998; Chow and Lang 2001; Kamachi et al. 2001). This defect in Pax6 expression is independent of BMP4 (Furuta and Hogan 1998). Although Pax6 is phosphorylated by two members of the MAPK family (ERK and p38), it is not phosphorylated by JNK (Mikkola et al. 1999); JNK-dependent Pax6 regulation may therefore be indirect. Recently, it has been established that Pax6 expression is regulated by BMP7 and fibroblast growth factor (FGF; Faber et al. 2001). It is therefore possible that altered BMP7 or FGF signaling may contribute to the decreased expression of Pax6 in JNK-deficient embryos.
The Pax2 transcription factor is a target of the JNK signal transduction pathway
It is striking that the effects of Pax2 deficiency are similar to those caused by JNK deficiency. For example, both of these mutations cause failure of optic fissure closure (coloboma) and renal epithelial cell necrosis (Figs. 1, 5; Favor et al. 1996). Furthermore, both mutations alter the expression of Shh at the basis of the diencephalon in E9.5 embryos (Fig. 3A; Torres et al. 1996). These similar phenotypes are most likely accounted for by the observation that Pax2 expression was markedly reduced in the eyes and kidney epithelium of JNK-deficient mice (Figs. 2, 5). A further contributing factor may be that JNK can phosphorylate Pax2 (Cai et al. 2002). However, because the level of Pax2 mRNA and protein in JNK-deficient eyes is extremely low (Fig. 2), the role of altered Pax2 phosphorylation is unclear.
Although there are significant similarities between Pax2 deficiency and JNK deficiency, differences between JNK deficiency and Pax2 deficiency were apparent. For example, Pax2-deficient mutants exhibit only ipsilateral optic tracts as a result of agenesis of the optic chiasma (Torres et al. 1996) and display severe defects in the development of the urogenital tract (Torres et al. 1995), but these phenotypes were not observed in JNK-deficient embryos. The failure of JNK deficiency to mimic these phenotypes may be caused by the residual low level of JNK activity in the JNK-deficient (Jnk1−/+ Jnk2−/−) embryos (Fig. 1C). Alternatively, the role of JNK to regulate Pax2 expression may be restricted to a limited number of tissues, including the developing eye and renal epithelium.
Conclusions
JNK plays a conserved role in morphogenetic processes during embryonic development in mammals and insects. In Drosophila, JNK is required for dorsal closure. Here we demonstrate that JNK is required for a similar morphogenetic process in mice: closure of the optic fissure. The role of JNK in insects is mediated by regulated expression of Dpp. In mice, the role of JNK is mediated by the regulated expression of the Dpp ortholog BMP4. Conservation of the developmental role of JNK in mammals and insects to regulate the expression of the morphogen Dpp/BMP4 indicates that JNK is likely to be critical for embryonic morphogenesis in many organisms.
Materials and methods
Mice
Mice were generated (Yang et al. 1997, 1998) and backcrossed to C57BL/6 (Jackson Laboratories) for 10 generations. The mice were housed in a facility accredited by the American Association for Laboratory Animal Care and the animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Massachusetts Medical School.
Morphology and histology
Embryos from timed matings were fixed in 4% paraformaldehyde for 24 h prior to processing and embedding in paraffin wax following a standard protocol. Sections were cut at 4 μm and stained with hematoxylin and eosin. Three-dimensional digital microanalysis of E18.5 eyes and kidneys was visualized using ResView software (Resolution Sciences).
Immunohistochemistry
Sections were stained with the following antibodies: Phospho-c-Jun (Cell Signaling), Pax2 (Zymed), Pax6 (Developmental Studies Hybridoma Bank), and antibodies to crystallin (obtained from S. Zigler, National Eye Institute). Immune complexes were detected using a biotinylated secondary antibody (Biogenex), streptavidin-conjugated horseradish peroxidase (Biogenex), and the substrate 3,3′-diaminobenzene (Vector Labs) followed by brief counter-staining with Mayer's hematoxylin (Sigma).
In situ hybridization
Digoxigenin-labeled sense or antisense BMP4 riboprobes were transcribed from the linearized plasmid (Miyazaki et al. 2000). The probe was hybridized overnight at 52°C in a humid chamber, visualized using biotinylated antidigoxigenin antibody followed by incubation with streptavidin conjugated to Texas red (Vector Laboratories) and mounted in Vectashield mountant containing DAPI (Vector Laboratories). Digoxigenin-labeled antisense oligoprobes were generated as follows: Pax2: 5′-TCTAATGTGGGCAGGATCAGTG-3′, 5′-CTGTCGCTT GTATTCGGCAATC-3′, 5′-AAAGGCTGCTGAACTTTGGT CC-3′, 5′-GCTGAATCTCCAAGCCTCATTG-3′, 5′-GCTTTG CAGTGCATATCCATCG-3′, 5′-CTGGACTTGACTTCATCA AGCC-3′. Pax6: 5′-AGACACCACCAAGCTGATTCAC-3′, 5′-GTCTCGGATTTCCCAAGCAAAG-3′, 5′-TCATCCGAGTCTT CTCCGTTAG-3′, 5′-TACTGAAGCTGCTGCTGATAGG-3′, 5′-GGTCCTTGGTTCTAGTCCATTC-3′, 5′-GGTATCATAACTC CGCCCATTC-3′. Shh: 5′-GTCTTTGCACCTCTGAGTCATC-3′, 5′-TCGACCCTCATAGTGTAGAGAC-3′, 5′-GGATTCATA GTAGACCCAGTCG-3′, 5′-TCACGTAGAAGACCTTCTTGG C-3′, 5′-GAGCACCCGGTTGATGAGAATG-3′, 5′-CATTCCC AAGGGATGCATGGTC-3′. Ptc: 5′-ACCTGTCTCCGTGATAA GTTCC-3′, 5′-AGGCATAGGCAAGCATCAGTAG-3′, 5′-ACT TGAATCACCCTGCTGACAC-3′, 5′-CCTCCAGCATGACAT ACTTCAC-3′, 5′-CAGAACCAGTCCATTGAGAACC-3′, 5′-TA TGACCTCCACCTTTGAGTCC-3′. Corresponding sense oligoprobes were also prepared. Oligoprobes were hybridized overnight at 37°C in a humid chamber. The signal was enhanced using a Tyramide Amplification System (Biogenex) followed by incubation with streptavidin-conjugated horseradish peroxidase (Biogenex) for 15 min. The amplified product was developed with 3,3′-diaminobenzidine (Vector Laboratories), and counterstained briefly with Mayers' hematoxylin (Sigma).
Retinal explant cultures
Embryonic retinas were dissected and cultured on 13-mm Nuclepore membranes (Whatman) in medium containing 45% Hams F-12 (Invitrogen), 45% Dulbecco's modified Eagle's medium (Invitrogen), 10% fetal bovine serum (HyClone), 100 units/mL penicillin–streptomycin (Invitrogen), and 2 mM L-glutamine (Invitrogen) for 48 h. Where stated, retinas were cultured in the presence of 50 ng/mL BMP4 (R&D Systems) or 50 μg/mL of the anti-Shh monoclonal antibody 5E1 (Developmental Studies Hybridoma Bank, Iowa). Retinas were fixed in 4% paraformaldehyde for 24 h prior to processing, embedding in paraffin wax, and immunohistochemical or in situ hybridization analysis.
Acknowledgments
We thank M. Dyer for advice concerning retinal explant cultures, B. Hogan for the BMP4 probe, S. Zigler for the antibodies to crystallin proteins, J. Reilly and L. Block for expert technical assistance, and K. Gemme for administrative assistance. These studies were supported by a grant from the National Cancer Institute. R.A.F. and R.J.D. are investigators of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL Roger.Davis@umassmed.edu; FAX (508) 856-3210.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1087303.
References
- Cai Y, Lechner MS, Nihalani D, Prindle MJ, Holzman LB, Dressler GR. Phosphorylation of Pax2 by the c-Jun N-terminal kinase and enhanced Pax2-dependent transcription activation. J Biol Chem. 2002;277:1217–1222. doi: 10.1074/jbc.M109663200. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Sax CM, Bresnick EH, Piatigorsky J. A complex array of positive and negative elements regulates the chicken α A-crystallin gene: Involvement of Pax-6, USF, CREB and/or CREM, and AP-1 proteins. Mol Cell Biol. 1994;14:7363–7376. doi: 10.1128/mcb.14.11.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Sax CM, Li X, McDermott JB, Piatigorsky J. Pax-6 and lens-specific transcription of the chicken δ 1-crystallin gene. Proc Natl Acad Sci. 1995;92:4681–4685. doi: 10.1073/pnas.92.10.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Haynes JI, II, Cvekl A, Piatigorsky J. Dual roles for Pax-6: A transcriptional repressor of lens fiber cell-specific beta-crystallin genes. Mol Cell Biol. 1998;18:5579–5586. doi: 10.1128/mcb.18.9.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997;188:235–247. doi: 10.1006/dbio.1997.8664. [DOI] [PubMed] [Google Scholar]
- Eccles MR, Schimmenti LA. Renal-coloboma syndrome: A multi-system developmental disorder caused by PAX2 mutations. Clin Genet. 1999;56:1–9. doi: 10.1034/j.1399-0004.1999.560101.x. [DOI] [PubMed] [Google Scholar]
- Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA. Fgf receptor signaling plays a role in lens induction. Development. 2001;128:4425–4438. doi: 10.1242/dev.128.22.4425. [DOI] [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang RA. Bmp signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–3737. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, Senft E, Wurst W, Blanquet V, Grimes P, Sporle R, et al. The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc Natl Acad Sci. 1996;93:13870–13875. doi: 10.1073/pnas.93.24.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes & Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal-Srivastava R, Cvekl A, Piatigorsky J. Pax-6 and alphaB-crystallin/small heat shock protein gene regulation in the murine lens. Interaction with the lens-specific regions, LSR1 and LSR2. J Biol Chem. 1996;271:23029–23036. doi: 10.1074/jbc.271.38.23029. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Horsburgh G, Cohen J, Hetherington CM, Fisher G, Lyon MF. Small eyes (Sey): A homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J Embryol Exp Morphol. 1986;97:95–110. [PubMed] [Google Scholar]
- Hou XS, Goldstein ES, Perrimon N. Drosophila Jun relays the Jun amino-terminal kinase signal transduction pathway to the Decapentaplegic signal transduction pathway in regulating epithelial cell sheet movement. Genes & Dev. 1997;11:1728–1737. doi: 10.1101/gad.11.13.1728. [DOI] [PubMed] [Google Scholar]
- Ilagan JG, Cvekl A, Kantorow M, Piatigorsky J, Sax CM. Regulation of alphaA-crystallin gene expression. Lens specificity achieved through the differential placement of similar transcriptional control elements in mouse and chicken. J Biol Chem. 1999;274:19973–19978. doi: 10.1074/jbc.274.28.19973. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)—From inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes & Dev. 2001;15:1272–1286. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann M. The atlas of mouse development. London, UK: Academic Press; 1999. [Google Scholar]
- Keller SA, Jones JM, Boyle A, Barrow LL, Killen PD, Green DG, Kapousta NV, Hitchcock PF, Swank RT, Meisler MH. Kidney and retinal defects (Krd), a transgene-induced mutation with a deletion of mouse chromosome 19 that includes the Pax2 locus. Genomics. 1994;23:309–320. doi: 10.1006/geno.1994.1506. [DOI] [PubMed] [Google Scholar]
- Klok EJ, van Genesen ST, Civil A, Schoenmakers JG, Lubsen NH. Regulation of expression within a gene family. The case of the rat gammaB- and gammaD-crystallin promoters. J Biol Chem. 1998;273:17206–17215. doi: 10.1074/jbc.273.27.17206. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes & Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo V, Tabin CJ. Regulation of patched by sonic hedgehog in the developing neural tube. Proc Natl Acad Sci. 1996;93:9346–9351. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco E. Regulation of cell differentiation by the Drosophila Jun kinase cascade. Curr Opin Genet Dev. 1997;7:666–671. doi: 10.1016/s0959-437x(97)80015-9. [DOI] [PubMed] [Google Scholar]
- Martinez G, Mishina Y, Bertram JF. BMPs and BMP receptors in mouse metanephric development: In vivo and in vitro studies. Int J Dev Biol. 2002;46:525–533. [PubMed] [Google Scholar]
- McDermott JB, Cvekl A, Piatigorsky J. A complex enhancer of the chicken beta A3/A1-crystallin gene depends on an AP-1-CRE element for activity. Invest Ophthalmol Vis Sci. 1997;38:951–959. [PubMed] [Google Scholar]
- Mikkola I, Bruun JA, Bjorkoy G, Holm T, Johansen T. Phosphorylation of the transactivation domain of Pax6 by extracellular signal-regulated kinase and p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:15115–15126. doi: 10.1074/jbc.274.21.15115. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CJ. Pattern formation in the zebrafish retina. Semin Cell Dev Biol. 2001;12:485–490. doi: 10.1006/scdb.2001.0272. [DOI] [PubMed] [Google Scholar]
- Ostrom L, Tang MJ, Gruss P, Dressler GR. Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Dev Biol. 2000;219:250–258. doi: 10.1006/dbio.2000.9618. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens crystallins. Innovation associated with changes in gene regulation. J Biol Chem. 1992;267:4277–4280. [PubMed] [Google Scholar]
- Raatikainen-Ahokas A, Hytonen M, Tenhunen A, Sainio K, Sariola H. BMP-4 affects the differentiation of metanephric mesenchyme and reveals an early anterior-posterior axis of the embryonic kidney. Dev Dyn. 2000;217:146–158. doi: 10.1002/(SICI)1097-0177(200002)217:2<146::AID-DVDY2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Richardson J, Cvekl A, Wistow G. Pax-6 is essential for lens-specific expression of zeta-crystallin. Proc Natl Acad Sci. 1995;92:4676–4680. doi: 10.1073/pnas.92.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesgo-Escovar JR, Hafen E. Drosophila Jun kinase regulates expression of decapentaplegic via the ETS-domain protein Aop and the AP-1 transcription factor DJun during dorsal closure. Genes & Dev. 1997;11:1717–1727. doi: 10.1101/gad.11.13.1717. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar JR, Jenni M, Fritz A, Hafen E. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes & Dev. 1996;10:2759–2768. doi: 10.1101/gad.10.21.2759. [DOI] [PubMed] [Google Scholar]
- Rothenpieler UW, Dressler GR. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993;119:711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- Sabapathy K, Hu Y, Kallunki T, Schreiber M, David JP, Jochum W, Wagner EF, Karin M. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr Biol. 1999a;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- Sabapathy K, Jochum W, Hochedlinger K, Chang L, Karin M, Wagner EF. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev. 1999b;89:115–124. doi: 10.1016/s0925-4773(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Sluss HK, Davis RJ. Embryonic morphogenesis signaling pathway mediated by JNK targets the transcription factor JUN and the TGF-beta homologue decapentaplegic. J Cell Biochem. 1997;67:1–12. [PubMed] [Google Scholar]
- Sluss HK, Han Z, Barrett T, Davis RJ, Ip YT. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes & Dev. 1996;10:2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Duncan MK, Roth HJ, Cvekl A, Piatigorsky J. Convergent evolution of crystallin gene regulation in squid and chicken: The AP-1/ARE connection. J Mol Evol. 1994;39:134–143. doi: 10.1007/BF00163802. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- Trousse F, Esteve P, Bovolenta P. Bmp4 mediates apoptotic cell death in the developing chick eye. J Neurosci. 2001;21:1292–1301. doi: 10.1523/JNEUROSCI.21-04-01292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J-J, Kennedy NJ, Lamb J, Flavell RA, Davis RJ. JNK is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol. 2003;23:2871–2882. doi: 10.1128/MCB.23.8.2871-2882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes & Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- Yang DD, Conze D, Whitmarsh AJ, Barrett T, Davis RJ, Rincon M, Flavell RA. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–585. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- Zeitlinger J, Bohmann D. Thorax closure in Drosophila: Involvement of Fos and the JNK pathway. Development. 1999;126:3947–3956. doi: 10.1242/dev.126.17.3947. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Yang XJ. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol. 2001;233:271–290. doi: 10.1006/dbio.2000.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Z, Zhao X, Yu X, Hu Y, Geronimo B, Fromm SH, Chen YP. A new function of BMP4: Dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development. 2000;127:1431–1443. doi: 10.1242/dev.127.7.1431. [DOI] [PubMed] [Google Scholar]