Abstract
CPEB is an mRNA-binding protein that stimulates polyadenylation-induced translation of maternal mRNA once it is phosphorylated on Ser 174 or Thr 171 (species-dependent). Disruption of the CPEB gene in mice causes an arrest of oogenesis at embryonic day 16.5 (E16.5), when most oocytes are in pachytene of prophase I. Here, we show that CPEB undergoes Thr 171 phosphorylation at E16.5, but dephosphorylation at the E18.5, when most oocytes are entering diplotene. Although phosphorylation is mediated by the kinase aurora, the dephosphorylation is due to the phosphatase PP1. The temporal control of CPEB phosphorylation suggests a mechanism in which CPE-containing mRNA translation is stimulated at pachytene and metaphase I.
Keywords: Oocyte; mRNA translation; CPEB, meiosis; prophase I
Oocytes of probably all metazoans synthesize and store mRNA that is destined for translation during re-entry into the meiotic divisions (oocyte maturation) or after fertilization. One mechanism that controls translation in early development is cytoplasmic polyadenylation (Richter 2000; Mendez and Richter 2001). Several dormant mRNAs have short poly(A) tails, which, when elongated in response to an exogenous cue (e.g., hormonal stimulation or fertilization), stimulate translation. In Xenopus oocytes, in which most of the molecular details of this process have been defined, progesterone stimulation of maturation induces the activation of aurora (Eg2; Andresson and Ruderman 1998; Littlepage et al. 2002), a kinase that phosphorylates Ser 174 of CPEB, a sequence-specific RNA binding protein (Mendez et al. 2000a, 2002). Phospho-CPEB, which is bound to the 3′ untranslated region (UTR) cytoplasmic polyadenylation element (CPE), then associates with CPSF (cleavage and polyadenylation specificity factor), possibly helping it stably bind the AAUAAA, a nearby cis element that is also necessary for polyadenylation (Dickson et al. 1999; Mendez et al. 2000b). CPSF in turn attracts poly(A) polymerase to the end of the mRNA. Another factor, maskin, mediates polyadenylation and translational activation. Maskin interacts with both CPEB and the cap binding factor eIF4E (Stebbins-Boaz et al. 1999), a configuration that excludes eIF4G from interacting with eIF4E, which is necessary to form an initiation complex on CPE-containing mRNAs. Translational repression is alleviated when the newly elongated poly(A) tail associates with poly(A) binding protein (PABP), a factor that helps eIF4G displace maskin from and itself bind to eIF4E, thereby initiating translation (Cao and Richter 2002).
Because many of the events described above also occur in mouse oocytes, disruption of the CPEB gene would be expected to inhibit meiotic maturation. Surprisingly, meiotic progression in CPEB knockout (KO) mice was prevented not during entry into metaphase I but during the earlier pachytene to diplotene transition in prophase I (Tay and Richter 2001). Oocytes of CPEB KO animals fail to polyadenylate and translate the CPE-containing synaptonemal complex proteins (SCPs) 1 and 3 mRNAs. Consequently, synaptonemal complexes are not formed, and possibly as a result, the oocytes and ovaries are resorbed (Tay and Richter 2001).
Aurora-catalyzed phosphorylation is necessary for the polyadenylation-inducing activity of CPEB during oocyte maturation (Mendez et al. 2000a; Hodgman et al. 2001), and thus, this posttranslational modification would be expected to also occur during pachytene. However, the inactivity of CPEB during the prolonged diplotene stage at the end of prophase I suggests that it is silenced during this time, perhaps by dephosphorylation. To investigate these possibilities, we addressed whether CPEB is phosphorylated on Thr 171, the aurora phosphorylation site, during pachytene. By using a phospho-specific antibody, we show that CPEB is phosphorylated on this site at embryonic day 16.5 (E16.5; when most oocytes are in pachytene), but not at E14.5 (most oocytes in leptotene-zygotene) or E18.5 (most oocytes in diplotene). Although the kinase aurora is present at E16.5 and E18.5, it catalyzes CPEB phosphorylation at the earlier time period. At E18.5, the phosphatase PP1 dephosphorylates CPEB, thereby rendering it and CPE-mediated mRNA translation inactive until oocyte maturation. These data suggest a mechanism whereby polyadenylation-induced translation can be stimulated and subsequently inactivated at different phases of meiosis. The results also suggest that the activities of both aurora and PP1 are tightly regulated during prophase I progression.
Results and Discussion
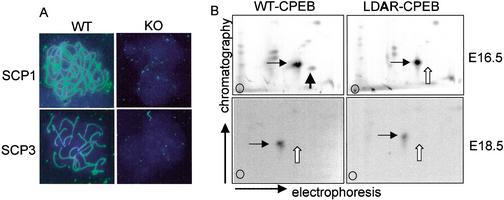
Disruption of the CPEB gene in mice results in the cessation of oocyte meiosis at pachytene and female sterility. At pachytene, homologous chromosome synapsis is maintained by the SC, a large multiprotein structure that is regulated at least in part at the translational level. mRNAs encoding two components of the SC, SCPs 1 and 3, contain CPEs in their 3′ UTRs and are not polyadenylated or translated in CPEB KO mice (Tay and Richter 2001). The requirement of CPEB for SC formation is shown in Figure 1A, in which E16.5 oocytes from wild-type and CPEB KO mice were immunostained for SCP1 and SCP3. Although both proteins were clearly evident in wild-type oocytes, neither was detected in the KO oocytes, thus establishing the necessity of CPEB for SC formation. During oocyte maturation (MI), CPEB activity is controlled by aurora-mediated phosphorylation (Ser 174 in Xenopus, Thr 171 in the mouse; Mendez et al. 2000a; Hodgman et al. 2001). To assess whether CPEB might be phosphorylated on this site at pachytene in the mouse, extracts were prepared for E16.5 embryonic ovaries and, for comparison, E18.5 embryonic ovaries. The extract was supplemented with γ32P-ATP and either wild-type recombinant CPEB or a mutant CPEB with an alanine substitution in the aurora phosphorylation site (LDAR). After incubation, the CPEB proteins were resolved by SDS-PAGE, blotted onto a membrane, digested with trypsin, and processed for two-dimensional phospho-peptide mapping. Figure 1B shows that although WT CPEB was phosphorylated in the E16.5 ovary extract (cf. wild type and mutant for loss of a phospho-peptide, white arrow), it was not phosphorylated in the E18.5 extract (cf. white arrows; the horizontal arrow denotes a reference phospho-peptide). Therefore, E16.5 ovary extracts, but not E18.5 ovary extracts, contain a kinase capable of phosphorylating CPEB on a residue that is essential to promote polyadenylation-induced translation.
Figure 1.
CPEB activity during prophase I. (A) E16.5 oocyte chromatin from wild-type and CPEB knockout animals was immunostained for SCP1 and SCP3. The filaments in the wild-type oocytes are synaptonemal complexes, which are absent in the knockout oocytes. (B) Extracts prepared from wild-type E16.5 and E18.5 ovaries were supplemented with recombinant CPEB containing a wild-type or mutated aurora phosphorylation site, together with γ32P-ATP. After incubation, CPEB was gel isolated, digested with trypsin, and analyzed by two-dimensional phospho-peptide mapping. The horizontal arrow denotes a reference peptide that is present in all panels. The white vertical arrow denotes the phospho-peptide containing the aurora phosphorylation site, which, although present when the E16.5 ovary extract was used as the kinase source (cf. wild-type and mutant CPEB proteins), was absent when the E18.5 ovary extract was the kinase source.
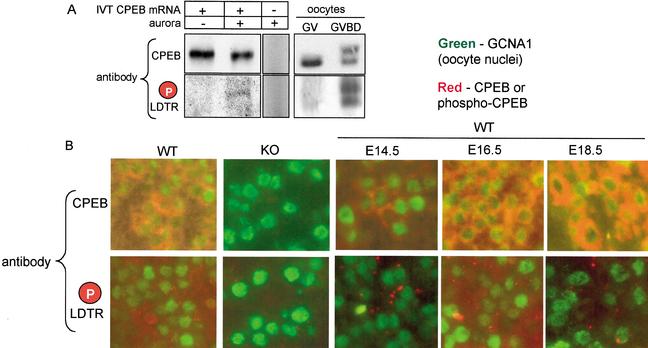
To examine whether endogenous CPEB is phosphorylated during prophase I progression, we generated antibody against the mouse CPEB LDT*R (the asterisk denotes phosphate) peptide. This antibody is specific for phospho-LDTR as determined by its ability to recognize CPEB synthesized in a reticulocyte lysate (IVT CPEB mRNA) only when the lysate is supplemented with recombinant aurora (Fig. 2A). The relative weakness of the phospho-CPEB band probably reflects a low amount of CPEB that was phosphorylated in vitro. This antibody also recognizes CPEB in mature (GVBD) oocytes (Fig. 2A), which we have shown previously is a stage when CPEB is phosphorylated by aurora and by cdc2, the latter of which retards its electrophoretic mobility (Mendez et al. 2000a, 2002; Tay et al. 2000; Hodgman et al. 2001).
Figure 2.
CPEB phosphorylation is regulated during prophase I progression. (A) CPEB mRNA was translated in a reticulocyte lysate, some of which was then supplemented with recombinant aurora. The proteins were then Western blotted and probed with general CPEB antibody or phospho-specific antibody. Some lysate that was not primed with any RNA was supplemented with aurora and then probed with both CPEB antibodies. GV and GVBD oocytes were also probed with the general and phospho-specific CPEB antibodies. Although only nonphosphorylated CPEB is present in GV stage oocytes (Tay et al. 2000; Hodgman et al. 2001), aurora-phosphorylated CPEB is present after GVBD. Some CPEB is additionally phosphorylated by cdc2 at this time, which slows its electrophoretic mobility (Mendez et al. 2000; Tay et al. 2000). (B) Ovaries from E16.5 wild-type and CPEB knockout animals were fixed, embedded, sectioned, and immunostained with general CPEB antibody or the phospho-specific antibody. The sections were also immunostained for GCNA1, a marker for oocyte nuclei. Note that although both the general and phospho-specific antibodies were immunoreactive with the wild-type ovaries, there was no signal with the knockout ovaries. Ovaries from wild-type E14.5, E16.5, and E18.5 ovaries were immunostained with the same three antibodies noted above. Although general CPEB immunoreactivity was detected at all three stages, the phospho-specific antibody was immunoreactive only with the E16.5 ovary section.
The phospho-specific and general CPEB antibodies were used to probe paraffin sections of E16.5 ovaries from wild-type and CPEB KO mice. Figure 2B demonstrates that in sections costained with germ cell nuclear antigen 1 (GCNA1), a marker for oocyte nuclei (Enders and May 1994), both phospho-specific and general CPEB antibodies were immunoreactive only with material from wild-type mice, thus demonstrating the specificity of the antibodies for CPEB in fixed and sectioned ovary material. Ovary sections from E14.5, E16.5, and E18.5 embryos were next probed with the antibodies. Although the general CPEB antibody was immunoreactive at all embryonic stages to similar extents, the phospho-specific antibody was immunoreactive most strongly with oocytes from E16.5 ovaries. Thus, endogenous CPEB is phosphorylated at the critical LDTR motif in ovaries that contain mostly pachytene oocytes.
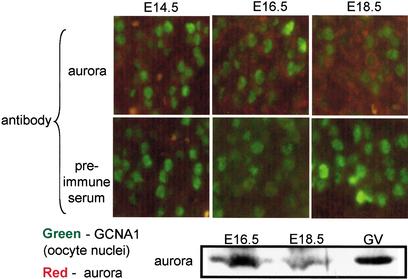
The observation that CPEB is not phosphorylated between diplotene (E18.5) and metaphase I (GVBD) suggests that aurora, the only kinase known to phosphorylate the LDTR motif, is either absent or inactive during this time. To distinguish between these possibilities, an immunocytochemical analysis of this kinase was performed; it shows that aurora is present in oocytes from E14.5 through E18.5. We also prepared E16.5 and E18.5 embryonic ovary extracts and probed them on a Western blot for aurora. Figure 3 shows that this kinase was clearly detected during these embryonic stages, as well as in isolated GV stage oocytes. We therefore conclude that although aurora is present throughout oogenesis, it may be inactive between E18.5 (Fig. 2B) and entry into MI.
Figure 3.
Aurora is present in oocytes during prophase I progression. Wild-type E14.5, E16.5, and E18.5 ovary sections were probed with aurora antibody or preimmune serum, as well as GNCA1 antibody. Compared with preimmune serum, the aurora antibody yielded an immunoreactive signal significantly above background at all stages. To confirm this result, protein wild-type E16.5 and E18.5 ovaries, as well as GV stage oocytes, were Western blotted and probed with aurora antibody.
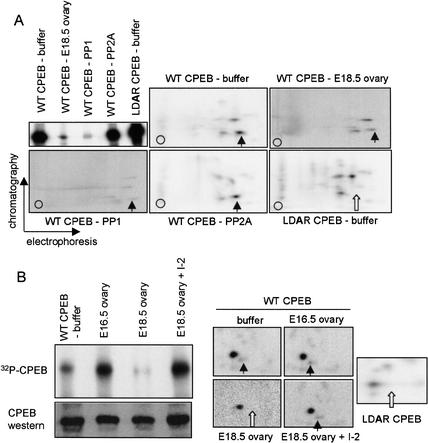
CPEB is present throughout oogenesis, yet its ability to promote polyadenylation-induced translation occurs only at E16.5 (i.e., mostly pachytene oocytes) and at metaphase I. This activity of CPEB coincides with LDTR phosphorylation and implies that dephosphorylation of this site during the diplotene stage helps maintain translational silencing of CPE-containing RNAs at this time. To determine whether a phosphatase present in embryonic diplotene oocytes can indeed dephosphorylate CPEB, we prepared an extract from E18.5 embryonic ovaries. To this extract was added recombinant CPEB that was phosphorylated in vitro with a Xenopus egg extract, which phosphorylates not only the aurora site (Mendez et al. 2000a) but also a few other sites (by cdc2) as well (Mendez et al. 2002). For comparison, an LDAR mutant CPEB was also phosphorylated with the Xenopus egg extract. The phosphorylated CPEB proteins were then incubated with buffer only, the E18.5 ovary extract, or commercial phosphatases PP1 or PP2A. When analyzed by quantitative SDS-PAGE and autoradiography, treatment only with the E18.5 ovary extract and PP1 resulted in a significant decrease in phosphorylated CPEB (Fig. 4A; CPEB stability was unaffected, cf. Fig. 4B). To determine whether specific sites of CPEB phosphorylation were affected by the phosphatases, we performed two-dimensional phospho-peptide mapping (Fig. 4A). A comparison of wild-type CPEB and the LDAR mutant CPEB phospho-peptide maps denotes the aurora-induced phospho-peptide (Fig. 4A, arrow). Although PP2A had no quantitative or qualitative affect on CPEB phosphorylation, treatment with either PP1 or the E18.5 ovary extract resulted in substantially reduced phosphorylation of CPEB at all sites (note that the signals of these two panels were enhanced to show all the phospho-peptides). Moreover, with the E18.5 ovary extract, there was a slight preferential reduction of the aurora-catalyzed phospho-peptide relative to the other phospho-peptides. We conclude that E18.5 ovary extracts contain a phosphatase capable of acting upon the CPEB phosphorylation site that is critical for polyadenylation, and that the phosphatase most likely responsible for this event is PP1. To confirm this result, E18.5 extracts were pre-treated with I-2, a specific inhibitor of PP1, prior to the addition of phospho-CPEB. The I-2 completely abrogated the dephosphorylation of CPEB, including the aurora phosphorylation site (Fig. 4B, one-dimensional SDS gel and two-dimensional phospho-peptide maps; the E18.5 two-dimensional sample was exposed ∼5× longer than the other samples). Figure 4B also shows that E16.5 extracts contain no detectable CPEB phosphatase activity, and demonstrates that the conditions used for the phosphatase assay did not destabilize CPEB.
Figure 4.
Phosphatase activity in E18.5 ovaries. (A) Recombinant histidine-tagged CPEB, containing a wild-type or mutated (LDAR) aurora phosphorylation site, was incubated in a Xenopus egg extract together with γ32P-ATP. The CPEB proteins were then isolated on a nickel-agarose column and incubated with buffer alone, phosphatase PP1, phosphatase PP2A, or an extract derived from E18.5 wild-type ovaries. When analyzed by quantitative SDS-PAGE, only PP1 and the E18.5 ovary extract had the capability of dephosphorylating CPEB. All phosphorylated CPEB proteins were subsequently analyzed by qualitative two-dimensional phospho-peptide mapping. In each panel, the vertical arrow denotes the aurora-catalyzed phospho-peptide, which was absent from the mutant CPEB (white arrow). The signals from the panels denoted E18.5 ovary and PP1 were enhanced to show the individual phospho-peptides, which otherwise would not be detected at the same exposure level as in the other three panels. (B) Phospho-CPEB was added to E16.5 and E18.5 ovary extracts, as well as E18.5 ovary extracts that were preincubated with I-2, a specific inhibitor of PP1. The degree of CPEB dephosphorylation was then monitored by SDS-PAGE and autoradiography, as well as two-dimensional phospho-peptide mapping (arrows are as noted above). Some of the extract was also Western blotted and probed for CPEB.
The results presented here and in other studies (Gebauer et al. 1994; Gebauer and Richter, 1996; Tay et al. 2000; Hodgman et al. 2001; Tay and Richter 2001) suggest a mechanism by which the translation of maternal mRNAs is differentially controlled during murine meiosis (Fig. 5). As oogenesis progresses into pachytene, the kinase aurora is activated, perhaps by phosphorylation (Andresson and Ruderman 1998; Walter et al. 2000; Littlepage et al. 2002). The upstream kinase that phosphorylates aurora is unclear, although some evidence indicates that PKA is involved (Walter et al. 2000). Activated aurora then phosphorylates CPEB Thr 171, which stimulates the polyadenylation and translation of SCP1 and SCP3 mRNAs. The translation of other mRNAs might also be stimulated by CPEB at this time. SCP1 and SCP3 help form the synaptonemal complex, which is necessary for meiotic progression to diplotene. At diplotene, PP1 dephosphorylates and inactivates CPEB, an event that allows key CPE-containing mRNAs such as mos to accumulate but remain translationally dormant. As the fully grown (GV) oocytes begin to mature, aurora again becomes active and phosphorylates CPEB, which in turn induces the polyadenylation and translation of mos (Gebauer et al. 1994), cyclin B (Tay et al. 2000), and other (e.g., see Oh et al. 2000) mRNAs with encoded products that either stimulate maturation (Tay et al. 2000) or lead to cytostatic factor (CSF)-mediated meta-phase II arrest (Colledge et al. 1994; Gebauer et al. 1994; Hashimoto et al. 1994).
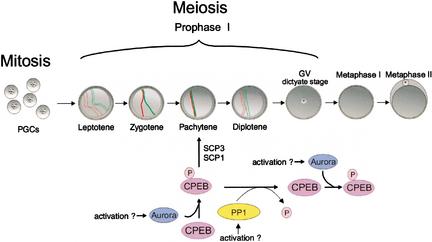
Figure 5.
Model for CPEB activity during meiosis. Primordial germ cells (PGCs), which divide mitotically, enter meiosis in the genital ridge and progress to pachytene, at which time CPEB is phosphorylated by aurora and activated. Active CPEB then induces the polyadenylation and translation of synaptonemal complex proteins 1 and 3 (SCPs 1 and 3) mRNAs, which are necessary for the formation of the synaptonemal complex. As meiosis progresses to diplotene, PP1 dephosphorylates and inactivates CPEB, thereby preventing the translation of CPE-containing mRNAs during this period. As oocytes subsequently mature, CPEB is again phosphorylated and activated by aurora, which stimulates the translation of mos, cyclin B, and other mRNAs.
Two additional points of upstream CPEB regulation should be considered. First, although aurora, in addition to CPEB, appears to be inactive in E18.5 diplotene oocytes (i.e., no T171 phosphorylation), it is plausible that the kinase is active at this time but its ability to phosphorylate CPEB is overcome by a very active PP1. To investigate this possibility, we repeated the phosphorylation experiment shown in Figure 1 except that some E18.5 ovary extracts were supplemented with I-2, the PP1 inhibitor. Neither I-2-supplemented nor un-supplemented extracts supported T171 phosphorylation (data not shown). Because I-2 inhibits dephosphorylation in the extracts (Fig. 4), we attribute the lack of T171 phoshorylation to inactive aurora rather than overriding PP1 activity. It is also interesting to note that PP1 has been suggested to inactivate aurora as well (Katayama et al. 2001; Murnion et al. 2001; Sugiyama et al. 2002). Perhaps PP1 acts on a CPEB and aurora-containing complex to inactivate these proteins simultaneously at diplotene. Second, we infer that PP1, which is present in mouse oocytes (Smith et al. 1998), is also regulated; it is inactive during E16.5 (pachytene) when CPEB phosphorylation is robust but active at E18.5 (diplotene) to dephosphorylate CPEB. PP1 activity is regulated by a number of modulator proteins (Cohen 2002), some of which could function during oogenesis.
The results presented above and in Tay and Richter (2001) demonstrate a finely tuned mRNA translational control mechanism at pachytene. Although the only known mRNAs that are regulated by CPEB at this stage are SCP1 and SCP3, it is likely that there are a number of others. For example, mRNAs encoding other components of the SC are prime candidates. So, too are mRNAs for proteins involved in chromosome synapsis such as ATM (Xu et al. 1996) and Spo11 (Baudet et al. 2000; Romanienko and Camerini-Otero, 2000). Irrespective of which mRNAs are regulated by CPEB, it is clear that this protein, together with its upstream and downstream modifiers, is necessary for meiosis in vertebrates.
Materials and methods
Immunostaining and immunoblotting
Immunostaining of synaptonemal complexes was performed according to the method of Knietz et al. (2000) by using SCP1 and SCP3 polyclonal antibodies [gifts from C. Heyting (Molecular Genetics Group, Wageningen University, Wageningen, Netherlands) and C. Hoog (Department of Cell and Molecular Biology, Karolinska Institutet, Stockholm, Sweden), respectively]. The secondary antibody was FITC-conjugated goat anti-rabbit IgG.
Five-micrometer sections from embryonic ovaries from wild-type or CPEB KO mice were fixed in Bouin's fixative for 24 h, embedded in paraffin, and probed with affinity-purified CPEB antibody, affinity-purified phospho-specific antibody generated against a CPEB peptide containing LDTpR antibody, or polyclonal antibody generated against aurora. Immunostaining for GCNA1 was performed with a monoclonal antibody [10D9G11, a gift from G. Enders (Department of Cell Biology, University of Kansas Medical Center, Kansas City, Kansas)]. The CPEB phospho-specific antibody was generated against the sequence RGSRLDTpRPILLSC; the cysteine is not a part of the CPEB sequence, but was added for conjugation to KLH. Rabbit anti-sera generated against this conjugate was affinity-purified with the phospho-peptide. A commercial source (QCB) was used for peptide and antibody synthesis and antibody purification.
CPEB mRNA was translated in a rabbit reticulocyte, a portion of which was incubated with recombinant aurora. The lysates, as well as GV and GVBD stage oocytes, were Western blotted and probed with CPEB antibody or the phospho-specific antibody. Extracts from embryonic ovaries were probed with aurora antibody.
Analysis of CPEB phosphorylation in embryonic ovaries
One-hundred-twenty pairs of microdissected E16.5 or E18.5 embryonic ovaries were incubated in a solution containing 0.25 mg/mL trypsin and 1 mM EDTA for 3 min at room temperature and then were washed four times with 1 mL each of buffer containing 20 mM Hepes at pH 7.4, 10 mM MgCl2, 50 mM KCl, and 1 mM dithiothreitol. The ovaries were then homogenized by using fine forceps, freeze-thawed three times, and centrifuged for 5 min at 4°C. The resulting supernatant was incubated with γ32PATP and recombinant wild-type or mutant CPEB (containing an LDAR motif) proteins for 60 min at 30°C. The 32P-labeled CPEB proteins were then resolved by SDS-PAGE, transferred onto a PVDF membrane, digested with trypsin, and subjected to two-dimensional phospho-peptide mapping as described (Mendez et al. 2000a).
Phosphatase assays
Recombinant histidine-tagged CPEB (wild-type or LDAR mutant) was incubated in a Xenopus egg extract, which contains active aurora (Mendez et al. 2000a), and γ32P-ATP. The resulting phosphorylated CPEB was then purified on a Ni2+ agarose column and used for phosphatase analysis with commercial PP1 (0.5 units in 50 mM Tris at pH 7.5, 0.1 mM EDTA, 5 mM DTT, and 0.01% Brij 35; New England Biolabs), PP2A (0.5 units in 50 mM Tris at pH 8.5, 20 mM MgCl2, and 1 mM DTT; Promega), or E16.5 or E18.5 mouse ovary extracts. The ovary extracts were prepared as described above with phosphatase buffer containing 50 mM Tris at pH 7.5, 2 mM EGTA, 2 mM DTT, and 0.1 mg/mL BSA and were then assayed in either the PP1 or PP2A buffer noted above. The extracts, as well as the commercial phosphatases, were incubated for 60 min at 30°C with the phosphorylated CPEB. In some cases, the PP1 I-2 inhibitor (New England Biolabs) was preincubated with the ovary extract prior to the addition on phospho-CPEB. The products were resolved by 10% SDS-PAGE, visualized by autoradiography, and then blotted, digested with trypsin, and subjected to two-dimensional phospho-peptide mapping. A portion of the extracts was also probed with CPEB antibody on a Western blot.
Acknowledgments
We thank G. Enders, C. Heyting, and C. Hoog for gifts of antibodies. We are also grateful to Q. Cao, Y.-S Huang, R. Mendez, and K. Padmanabhan for their assistance during the course of this work. J.T. was supported by an Institutional Postdoctoral Training Grant from the NIH. This work was supported by grants from the NIH (to J.D.R.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Corresponding author.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1071403.
References
- Andresson T. and Ruderman, J.V. 1998. The kinase Eg2 is a component of the Xenopus oocyte progesterone-activated signaling pathway. EMBO J. 17: 5627–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat F., Manova, K., Yuen, J.P., Jasin, M., and Keeney, S. 2000. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking spo11. Mol. Cell 6: 989–998. [DOI] [PubMed] [Google Scholar]
- Cao Q. and Richter, J.D. 2002. Dissolution of the maskin–eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J. 21: 3852–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P.T. 2002. Protein phosphatase 1—targeted in many directions. J. Cell. Sci. 115: 241–256. [DOI] [PubMed] [Google Scholar]
- Colledge W.H., Carlton, M.B., Udy, G.B., and Evans, M.J. 1994. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature 370: 65–68. [DOI] [PubMed] [Google Scholar]
- Dickson K.S., Bilger, A., Ballantyne, S., and Wickens, M.P. 1999. The cleavage and polyadenylation specificity factor in Xenopus laevis oocytes is a cytoplasmic factor involved in regulated polyadenylation. Mol. Cell. Biol. 19: 5707–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G.C. and May II, J.J. 1994. Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev. Biol. 163: 331–340. [DOI] [PubMed] [Google Scholar]
- Gebauer F. and Richter, J.D. 1996. Mouse cytoplasmic polyadenylylation element binding protein: an evolutionarily conserved protein that interacts with the cytoplasmic polyadenylylation elements of c-mos mRNA. Proc. Natl. Acad. Sci. 93: 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F., Xu, W., Cooper, G.M., and Richter, J.D. 1994. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J. 13: 5712–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N., Watanabe, N., Furuta, Y., Tamemoto, H., Sagata, N., Yokoyama, M., Okazaki, K., Nagayoshi, M., Takeda, N., Ikawa, Y., et al. 1994. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature 370: 68–71. [DOI] [PubMed] [Google Scholar]
- Hodgman R., Tay, J., Mendez, R., and Richter, J.D. 2001. CPEB phosphorylation and cytoplasmic polyadenylation are catalyzed by the kinase IAK1/Eg2 in maturing mouse oocytes. Development 128: 2815–2822. [DOI] [PubMed] [Google Scholar]
- Katayama H., Zhou, H., Li, Q., Tatsuka, M., and Sen, S. 2001. Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase 1 through mitotic cell division cycle. J. Biol. Chem. 276: 46219–46224. [DOI] [PubMed] [Google Scholar]
- Kneitz B., Cohen, P.E., Avdievich, E., Zhu, L., Kane, M.F., Hou Jr., H., Kolodner, R.D., Kucherlapati, R., Pollard, J.W., and Edelmann W. 2000. MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes & Dev. 14: 1085–1097. [PMC free article] [PubMed] [Google Scholar]
- Littlepage L.E., Wu, H., Andresson, T., Deanehan, J.K., Amundadottir, L.T., and Ruderman, J.V. 2002. Identification of phosphorylated residues that affect the activity of the mitotic kinase aurora-A. Proc. Natl. Acad. Sci. 99: 15440–15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R. and Richter, J.D. 2001. Translational control by CPEB: A means to the end. Nature Rev. Mol. Cell Biol. 2: 521–529. [DOI] [PubMed] [Google Scholar]
- Mendez R., Hake, L.E., Andresson, T., Littlepage, L.E., Ruderman, J.V., and Richter, J.D. 2000a. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404: 302–307. [DOI] [PubMed] [Google Scholar]
- Mendez R., Murthy, K.G., Ryan, K., Manley, J.L., and Richter, J.D. 2000b. Phosphorylation of CPEB by Eg2 mediates the recruitment of CPSF into an active cytoplasmic polyadenylation complex. Mol. Cell 6: 1253–1259. [DOI] [PubMed] [Google Scholar]
- Mendez R., Barnard, D., and Richter, J.D. 2002. Differential mRNA translation and meiotic progression require cdc2-mediated CPEB destruction. EMBO J. 21: 1833–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnion M.E., Adams, R.R., Callister, D.M., Allis, C.D., Earnshaw, W.C., and Swedlow, J.R. 2001. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J. Biol. Chem. 276: 26656–26665. [DOI] [PubMed] [Google Scholar]
- Oh B., Hwang, S., McLaughlin, J., Solter, D., and Knowles, B.B. 2000. Timely translation during the mouse oocyte-to-embryo transition. Development 127: 3795–3803. [DOI] [PubMed] [Google Scholar]
- Richter J.D. 2000. The influence of polyadenylation-induced translation on metazoan development and neuronal synaptic function. In: Translational control of gene expression (eds. M.B. Matthews, et al.), pp 785–805. Cold Spring Harbor Laboratory Press, New York.
- Romanienko P.J. and Camerini-Otero, R.D. 2000. The mouse spo11 gene is required for meiotic chromosome synapsis. Mol. Cell 6: 975–987. [DOI] [PubMed] [Google Scholar]
- Smith G.D., Sadhu, A., Mathies, S., and Wolf, D.P. 1998. Characterization of protein phosphatases in mouse oocytes. Dev. Biol. 204: 537–549. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Cao, Q., de Moor, C. H., Mendez, R., and Richter, J.D. 1999. Maskin is a CPEB-associated factor that transiently interacts with elF-4E. Mol. Cell 4: 1017–1027. [DOI] [PubMed] [Google Scholar]
- Sugiyama K., Sugiura, K., Hara, T., Sugimoto, K., Shima, H., Honda, K., Furukawa, K., Yamashita, S., and Urano, T. 2002. Aurora-B-associated protein phosphatases as negative regulators of kinase activation. Oncogene 21: 3103–3111. [DOI] [PubMed] [Google Scholar]
- Tay J. and Richter, J.D. 2001. Germ cell differentiation and synaptonemal complex formation are disrupted in CPEB knockout mice. Dev. Cell 1: 201–213. [DOI] [PubMed] [Google Scholar]
- Tay J., Hodgman, R., and Richter, J.D. 2000. The control of cyclin B1 mRNA translation during mouse oocyte maturation. Dev. Biol. 221: 1–9. [DOI] [PubMed] [Google Scholar]
- Walter A.O., Seghezzi, W., Korver, W., Sheung, J., and Lees, E. 2000. The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene 19: 4906–4916. [DOI] [PubMed] [Google Scholar]
- Xu Y., Ashley, T., Brainerd, E.E., Bronson, R.T., Meyn, M.S., and Baltimore, D. 1996. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes & Dev. 10: 2411–2422. [DOI] [PubMed] [Google Scholar]