Abstract
αA-crystallin (αA) and αB-crystallin (αB) are among the predominant proteins of the vertebrate eye lens. In vitro, the α-crystallins, which are isolated together as a high molecular mass aggregate, exhibit a number of properties, the most interesting of which is their ability to function as molecular chaperones for other proteins. Here we begin to examine the in vivo functions of α-crystallin by generating mice with a targeted disruption of the αA gene. Mice that are homozygous for the disrupted allele produce no detectable αA in their lenses, based on protein gel electrophoresis and immunoblot analysis. Initially, the αA-deficient lenses appear structurally normal, but they are smaller than the lenses of wild-type littermates. αA−/− lenses develop an opacification that starts in the nucleus and progresses to a general opacification with age. Light and transmission electron microscopy reveal the presence of dense inclusion bodies in the central lens fiber cells. The inclusions react strongly with antibodies to αB but not significantly with antibodies to β- or γ-crystallins. In addition, immunoblot analyses demonstrate that a significant portion of the αB in αA−/− lenses shifts into the insoluble fraction. These studies suggest that αA is essential for maintaining lens transparency, possibly by ensuring that αB or proteins closely associated with this small heat shock protein remain soluble.
The ocular lens is principally characterized by its high degree of transparency, which is essential for its sole function of refracting incident light and focusing it onto the retina. Lens clarity derives from the abundant, water-soluble proteins called crystallins that are tightly packed to produce a smooth gradient of refractive index from the center to the periphery of the lens. If this gradient is disrupted by localized aggregations of proteins, a cataract results (1). Development of the encapsulated lens occurs by the differentiation of peripheral epithelial cells into elongated fiber cells that accumulate in concentric layers and lose their nuclei and other organelles (2, 3). As a result of this process, crystallins in the center of the lens cannot turn over and must remain stable and soluble throughout the life of the organism.
Three classes of crystallins are present in vertebrate lenses: α, β, and γ. α-Crystallins compose up to 50% of the total protein mass of the mammalian lens and are isolated under physiologic conditions as a high-molecular mass aggregate of ≈800,000 Da (3). There are two different α-crystallin polypeptides, αA-crystallin (αA) and αB-crystallin (αB), which are ≈60% identical in amino acid sequence and are encoded by separate, unlinked genes. Expression of αA is lens-preferred; only small amounts of the protein are detectable in other tissues, including spleen, thymus, and retina (4–6). αB on the other hand, while most abundant in the lens, is present at significant levels in a variety of tissues (7, 8). α-Crystallins are members of the small heat shock protein family, as revealed initially by sequence similarity to the Drosophila small heat shock proteins (9) and, more recently, by experiments showing that αB is inducible by heat shock or hypertonic stress (10, 11).
Our knowledge of α-crystallin function in the lens is mostly inferential, derived from attempts to elucidate the properties of α-crystallin proteins in vitro and then relate those properties to in vivo functions. For example, it has been demonstrated that α-crystallins are highly resistant to thermal denaturation, a finding that is consistent with the perdurability of these proteins in the central fiber cells of the lens (12, 13). Furthermore, it was shown by Horwitz (14) that α-crystallins function in vitro as chaperones, preventing heat-induced aggregation of numerous proteins and assisting in the renaturation of chemically denatured proteins. α-Crystallin has also been shown to associate with a variety of cytoskeletal proteins, including actin (15–17), vimentin (18), desmin (19), and lens beaded filament proteins (18, 20), and has been detected along with cytoskeletal elements in cytoplasmic inclusion bodies in several human diseases (21). In addition, Kantorow and Piatigorsky (22) demonstrated that purified α-crystallin has autokinase activity. Finally, there is recent evidence that α-crystallin can bind to DNA in gel-retardation assays (23). These findings suggest that α-crystallin has multiple roles in the lens. Here we describe experiments to directly examine the in vivo functions of α-crystallin by creating a targeted disruption of the mouse αA gene. Lenses of mice that are homozygous for the disrupted αA allele have normal gross structure but are smaller than wild-type lenses. αA−/− mice develop a progressive lens opacification that becomes apparent several weeks after birth. A likely contributory cause of this opacity is revealed by immunohistochemical and Western blot analyses, which demonstrate the presence of dense inclusion bodies that contain insoluble αB in lens fiber cells. These experiments provide the first evidence, to our knowledge, that α-crystallin is necessary for maintaining lens clarity and that αA is essential for maintaining solubility of lenticular αB in vivo.
METHODS
Generation of αA Gene Knockout Mice.
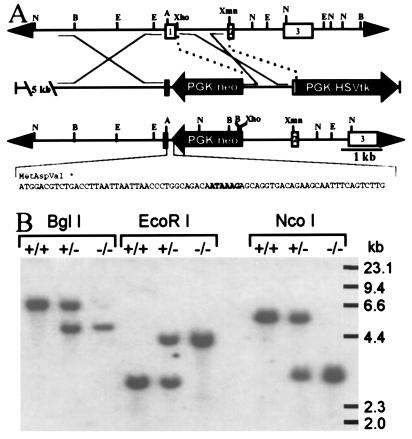
A λ phage clone spanning 16 kb of the αA locus was isolated from a 129Sv mouse genomic library (Stratagene). A targeting vector (see Fig. 1A) was constructed by placing the following pieces of DNA, in the given order, into the plasmid pBluescript (Promega): a 9-kb NotI/AatII restriction fragment encompassing the 5′ flanking region and first nine nucleotides of the αA coding region, the phosphoglycerate kinase/neomycin phosphotransferase (PGK/neo) expression cassette from the vector pPNT (24) in opposite transcriptional orientation to the αA gene, a 1.3-kb XhoI/XmnI restriction fragment containing most of the first intron and part of the second exon of the αA gene in the same orientation as the αA promoter fragment, and the PGK/herpes simplex virus thymidine kinase expression cassette of pPNT in the same orientation as the αA sequences. In addition, to ensure that no protein-encoding transcripts could be produced by the disrupted allele, a double-stranded oligonucleotide containing translation termination codons in all three reading frames as well as a copy of the αA polyadenylylation signal was inserted between the 5′ αA sequence and the PGK/neo cassette.
Figure 1.
Targeted disruption of the mouse αA gene. (A) Strategy used to disrupt the αA gene. (Top) Normal αA locus. (Middle) Targeting vector. (Bottom) Disrupted αA locus. The sequence of an oligonucleotide inserted between the 5′ αA sequences and PGK/neo containing multiple stop codons in all three reading frames and a polyadenylylation signal (enclosed in box) is shown beneath a diagram of the targeted allele. Depicted restriction sites include NcoI (N), BglI (B), EcoRI (E), AatII (A), XhoI, and XmnI. HSVtk, herpes simplex virus thymidine kinase. (B) Southern blot analysis of genomic DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) αA knockout mice. Liver DNA (15 μg) digested with BglI, EcoRI, and NcoI was hybridized with an XmnI/XhoI restriction fragment encompassing most of the first intron and the beginning of exon 2 (see A).
Linearized knockout plasmid DNA (30 μg) was electroporated into 4 × 107 J1 embryonic stem cells derived from 129Sv mice. Cell lines established from 192 colonies surviving double selection with the neomycin analogue G418 and ganciclovir were analyzed by PCR for homologous recombination at the αA locus. Correctly targeted embryonic stem cells, confirmed by Southern blot analysis, were injected into C57BL/6 blastocysts and then implanted into pseudopregnant CD1 female mice. Chimeric males were mated to 129Sv or B6D2F1 females, and heterozygous offspring identified by PCR were interbred to produce mice homozygous for the αA knockout allele.
Transmission Electron Microscopy.
Eyes were fixed by immersion for at least 24 hr at room temperature in a solution of 2.5% glutaraldehyde and 6% sucrose, buffered to pH 7.2 with 50 mM sodium cacodylate. Specimens were postfixed in OsO4 buffered with 150 mM sodium-potassium phosphate (pH 7.4), embedded, sectioned, and stained for electron microscopy. They were examined using a JEM 100B electron microscope (JEOL).
Immunohistochemistry and Western Blot Analysis.
Mouse eyes were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned onto silanated slides. Immunohistochemistry of eye sections was performed as described (25) using Vectastain immunoperoxidase detection reagents and VIP substrate (Vector Laboratories). Polyclonal antibodies to bovine α-, β-, and γ-crystallins were kindly provided by Samuel Zigler (National Eye Institute).
For protein blot analysis, isolated lenses were weighed and then homogenized in buffer containing 0.1 M sodium phosphate (pH 7.4) and 0.1 M NaCl. Homogenate portions containing equal wet weights of lens tissue were divided into soluble and insoluble fractions by centrifugation at 13,000 × g at 4°C for 20 min. Pellets were solubilized in volumes of SDS loading buffer proportional to the wet weight of the lens from which they were derived. Proteins from soluble and insoluble fractions were electophoretically separated in a 12.5% SDS/polyacrylamide gel and transferred onto nitrocellulose using the PhastSystem (Pharmacia Biotech).
The blots were blocked in Superblock (Pierce) supplemented with 0.1% Tween 20 for 3 hr at room temperature with gentle agitation. They were then incubated with gentle agitation overnight at 4°C with the primary antibody in 1/10 strength Milk Diluent/Blocking Solution Concentrate (Kirkegaard & Perry Laboratories). Polyclonal antisera to recombinant human αA and αB were generous gifts from Joseph Horwitz (Jules Stein Eye Institute, University of California at Los Angeles School of Medicine, Los Angeles) and were used at 1:500 dilution. Chemiluminescent detection of the bound antibody was done with the Western Light Kit (Tropix, Bedford, MA) according to the manufacturer’s instructions except, that the wash step after incubation with the secondary antibody was extended to 3 hr at room temperature with agitation and six buffer changes.
RESULTS
Phenotypic Characterization of Knockout Mice and Evaluation of αA Protein Expression.
The mouse αA gene was disrupted by inserting a PGK/neo expression cassette into the first exon so as to completely prevent the production of a functional transcript from this locus (Fig. 1). Lines of knockout mice were established from two independent lines of targeted embryonic stem cells. Southern blot analysis (Fig. 1B) of the resulting mice confirmed that the expected recombination event had occurred. In both lines, αA−/− mice are produced at normal Mendelian ratios from matings between αA+/− mice and exhibit no obvious mutant phenotypes at birth.
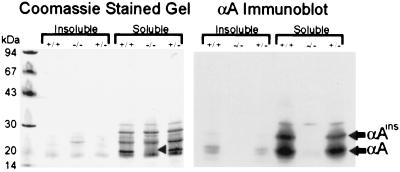
To verify that no αA protein is produced by the αA−/− mice, soluble and insoluble fractions of lens homogenates from αA−/−, αA+/−, and αA+/+ 7-week-old mice were analyzed by SDS/PAGE and immunoblotting with antiserum raised against recombinant human αA. The major band corresponding to αA is not evident in the αA−/− lane of the Coomassie blue-stained gel (Fig. 2 Left). Similarly, both αA and αAinsert, a variant protein resulting from alternative splicing of rodent αA RNA (26), are undetectable in the αA−/− lens immunoblots (Fig. 2 Right). Two-dimensional gel analysis and Western blotting (not shown) also failed to detect any αA or αAins in the lenses of knockout mice. This antiserum exhibited a small degree of cross-reactivity with a ≈30-kDa lens protein in one- and two-dimensional immunoblots of all the mice tested, but this does not affect the detection of authentic αA species. These data suggest that the intended alteration of the αA gene was successful in eliminating αA from the lens.
Figure 2.
Gel electrophoresis and immunoblot analysis of lens proteins. Lens homogenates from αA+/+, αA−/−, and αA+/− mice were separated into soluble and insoluble fractions and subjected to SDS/PAGE. Each lane contains protein from the equivalent of 2.5 μg of lens wet weight. Coomassie staining of the gel (Left) shows the absence of a prominent αA band in the αA−/− lens (arrowhead). Immunoblot analysis of a duplicate gel with antiserum to recombinant human αA (Right) confirms the absence of αA in the αA−/− lenses. Bands corresponding to αA and αAinsert are identified. Weak cross-reactivity of this antiserum with a ≈30-kDa lens protein is observed in all three samples.
The external morphologies of whole eyes and dissected lenses from the αA−/− animals appear normal. αA−/− eyeballs, however, are smaller than wild type. Lenses from these eyes also weigh 25–35% less than those of αA+/+ littermates, and their axial and equatorial dimensions are ≈15% smaller than wild-type lenses. αA+/− lenses weigh 10–15% less than wild-type lenses, and the dimensions of the lens and eye are ≈5% smaller than wild type. It is unclear whether the size difference is due to a decreased number of cells in the lens or to a smaller volume per cell. Although not a rigorous method, counting the nucleated fiber cells in sections through the center of the lens suggested that the cell number was not significantly different between αA+/+ and αA−/− lenses (data not shown).
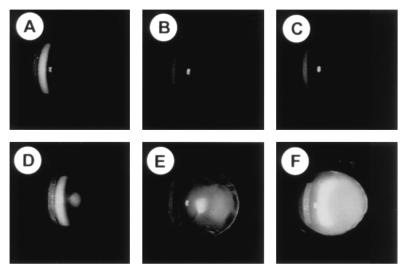
Eyes of αA+/+ and αA−/− mice of several ages were examined by slit lamp biomicroscopy (Fig. 3). By 7 weeks of age, a mild cataract is evident in the lenses of αA−/− mice (Fig. 3D) compared with wild-type mice (Fig. 3A) and progresses with age to a dense opacity (Fig. 3 E and F). By 10 weeks of age, a bilateral, uniform lens opacity is apparent without the aid of instrumentation in αA-deficient mice.
Figure 3.
Lens opacity in an αA-deficient mouse. Eyes were dilated and examined by slit lamp. (A–C) Wild-type mice ages 7, 10, and 20 weeks. (D–F) αA−/− mice ages 7, 10, and 18 weeks. Normal reflection of the slit lamp from the surface of the cornea and lens are visible in all panels. Light scattering within the lens, as evinced by a white haze in the photograph, is significantly higher in the αA−/− mice than in wild-type mice at each age. Mild cataract is seen in the 7-week-old αA−/− lens and a dense opacity is evident in the 18-week-old lens.
Histological Analyses of Knockout Lenses.
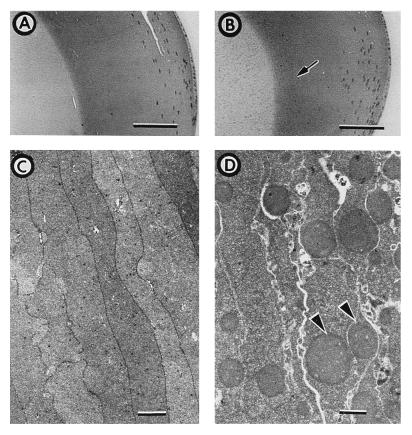
Examination of histological sections from αA−/− lenses reveals the presence of weakly staining spherical bodies of 1–3 μm in the nuclear and inner cortical regions (Fig. 4B). These bodies are not observed in αA+/+ (Fig. 4A) or αA+/− lenses (not shown). The inclusion bodies are not present in epithelial or nucleated fiber cells; their boundary of appearance in the inner cortex, indicated with an arrow in Fig. 4B, is spatially separated from the nucleated fiber cells.
Figure 4.
Histological and transmission electron microscopic analysis of normal and αA-deficient lenses. Methacrylate-embedded eye sections from 11-week-old αA+/+ (A) and αA−/− (B) sibling mice were stained with hematoxylin and eosin. Weakly staining spherical bodies are observed in the nuclear and inner cortical areas of the αA−/− lens; the tip of the arrow in B marks the right boundary of the area containing the spherical bodies. (A and B, Bar = 100 μm.) Transmission electron micrographs of αA+/− (C) and αA−/− (D) lens inner cortex from 38-week-old sibling mice. Electron opaque spherical bodies (arrowheads) are evident in αA−/− lenses. (C and D, Bar = 1 μm.)
Transmission electron microscopy (Fig. 4D) demonstrates that the bodies are intracellular, not enclosed by membranes, are composed of homogenous, electron-opaque material and that a single fiber cell can contain multiple inclusion bodies. Electron micrographs of αA+/+ (not shown) and αA+/− (Fig. 4C) lenses are similar to each other, neither showing any trace of inclusion bodies. We therefore conclude that formation of the inclusion bodies occurs only in the absence of αA.
Immunohistochemical Examination of Lenses.
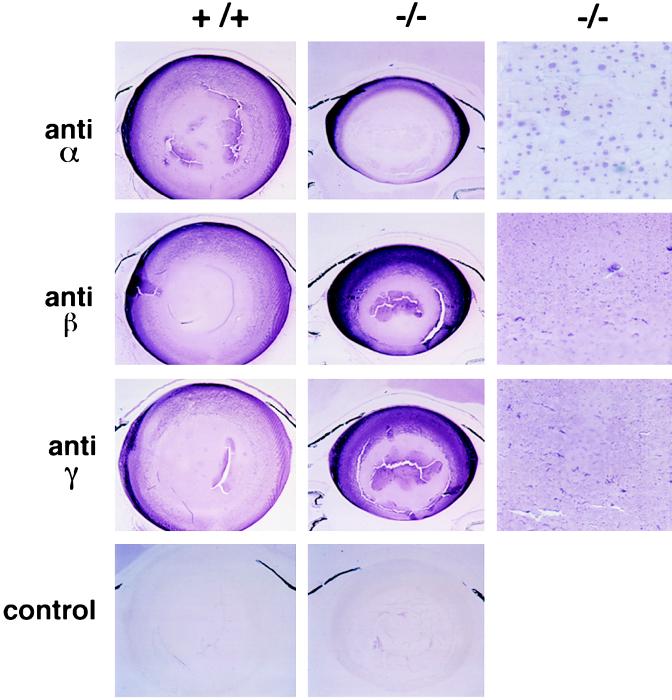
Lo (27) reported the presence of similarly sized inclusions, which he referred to as “droplets,” in electron micrographs of rat cold cataracts (a phase separation phenomenon observed in cooled lenses of immature rodents that is readily reversible upon rewarming). He demonstrated by immunostaining that γ-crystallins were the predominant components in those droplets and that α- and β-crystallins were also present (27). We used the same three anti-crystallin antibodies used by Lo on lenses of 7-week-old αA−/− and αA+/+ mice. Fig. 5 reveals that only the antibody against α-crystallin reacts strongly with the inclusion bodies in the αA−/− mice. Two additional antisera specific for αB (raised against recombinant human αB and the C-terminal peptide of human αB) gave identical results (not shown), confirming that the inclusions in the αA−/− lenses contain significant amounts of αB. In the αA−/− lenses that were labeled with the anti-β and anti-γ antibodies, the inclusion bodies do not stain above the background of uniform immunoreactivity (Fig. 5), suggesting that they contain little, if any, β- or γ-crystallin. We believe that the stippled pattern of staining with the anti-β and anti-γ observed in both αA+/+ and αA−/− lenses is the result of sample processing. The stipples are smaller and more irregularly shaped than the inclusion bodies.
Figure 5.
Immunohistochemistry of αA+/+ and αA−/− lenses with anti-crystallin antibodies. Sections of lenses cut parallel to the optic axis are shown with anteriors of the lenses toward the top of the figure. αA+/+ and αA−/− lenses were labeled with antibodies to bovine α-, β-, or γ-crystallin (anti-α, anti-β, or anti-γ) or with secondary antibody in the absence of a primary antibody (control). (×24 or ×300.)
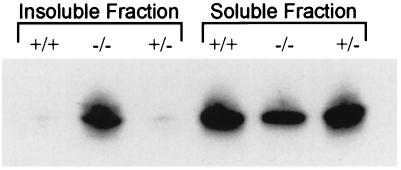
Based on the immunocytochemical analysis and the observation that the inclusion bodies can be pelleted even at 800 × g in 10 min (data not shown), the ratio of insoluble to soluble αB should be higher in the αA-deficient lenses than in normal lenses. To test this hypothesis, proteins from the soluble and insoluble fractions of lens homogenates were analyzed by Western blot analysis with an antibody specific to αB (Fig. 6). These data reveal that the relative amount of αB in the insoluble fraction of αA−/− lenses is much greater than that of αA+/+ or αA+/− lenses and that αB in the soluble fraction of αA−/− lenses is less than that of αA+/+ and αA+/− lenses (Fig. 6). Similar results were obtained with a second αB-specific antiserum (data not shown). The relative amounts of αB in αA−/− and αA+/+ lenses appear to be the same based on quantitation of two-dimensional protein gels of total lens protein (data not shown). Therefore, absence of αA leads to a shift in αB from the soluble to the insoluble fraction, where it is a major constituent of the observed inclusion bodies.
Figure 6.
Immunoblot analysis of soluble and insoluble lens proteins. Lens homogenates from αA+/+, αA−/−, and αA+/− mice were separated into soluble and insoluble fractions and subjected to immunoblot analysis with a polyclonal antibody to recombinant human αB. Soluble and insoluble proteins representing 2.5 μg of lens wet weight were analyzed.
DISCUSSION
We have generated knockout mice that produce no detectable αA protein in their lenses. Our principal finding from these animals is that, in the absence of αA, apparently normal levels of αB are insufficient to maintain transparency of the lens. It has been proposed that the αA and αB subunits are structurally equivalent based on the ability of α-crystallin to form a variety of homo- or heteropolymeric aggregates containing very different proportions of the two subunits (28). However, our data demonstrate that lenticular complexes of αB formed in the absence of αA are mostly insoluble and are localized in large, dense cytoplasmic inclusion bodies. Proteins other than αB may also be present in the inclusions, but immunostaining indicates that the inclusions do not contain large amounts of the other major lens proteins, β- and γ- crystallin. This differentiates them from the inclusions reported for cold cataracts in rats which contain all three classes of crystallins (27). Clearly, further work will need to be done to elucidate what, if any, other proteins are contained in these inclusion bodies. αB is also present in many nonlenticular tissues that do not express αA, but it has not been reported to form large insoluble masses in any of those tissues under normal physiological conditions, nor has it been reported to form insoluble bodies in vitro. αB, however, has been shown to be a component of inclusion bodies in some human pathological conditions such as Rosenthal fibers in Alexander disease, cortical Lewy bodies in Lewy body disease, and Mallory bodies in alcoholic liver disease (21). These disease-related inclusion bodies differ somewhat from the inclusion bodies in the αA−/− lenses in that αB is distributed nonuniformly in Rosenthal fibers and Mallory bodies and is detectable in only a subset of cortical Lewy bodies (21). It is possible that the amount of αB in the lens exceeds a threshold value above which αA is required to maintain its solubility or that other proteins substitute for the function of αA in different tissues. In this regard, αB in muscle cells is found to be localized in the Z-lines (19, 29) and has been shown to bind weakly to desmin, a protein localized at the Z-lines (19). Alternatively, the insolubility of αB in the αA-deficient lenses may result from promiscuous association with other proteins that normally interact with αA.
That gross lens morphology is normal in the αA−/− mice is interesting in light of reports that α-crystallin associates with and modulates components of the cytoskeleton. Lens fiber cells contain unique cytoskeletal structures called beaded filaments. Two lens-specific intermediate filament-like proteins, CP49 and CP115, purified from bovine lenses will polymerize in vitro to form 10-nm filaments (20). However, beaded filaments resembling those seen in vivo are formed only when the two proteins coassemble in the presence of α-crystallin (20). Moreover, α-crystallin has been shown to inhibit assembly of vimentin (18), accelerate actin polymerization and reduce actin depolymerization (17), and protect against stress-induced aggregation of actin. Additionally, α-crystallin has been shown to bind to both actin and filamentous desmin in cardiac muscle cells (16, 19, 30). These data suggest that α-crystallin is intimately associated with the lens cytoskeletal network, either as a structural component or as a chaperone for other cytoskeletal proteins.
The cytoskeletal network of bovine lens cells undergoes extensive remodeling during fiber cell differentiation and maturation with vimentin in immature fiber cells gradually being replaced with beaded filaments in more mature cells (31). Vimentin expression was shown to cease at a discreet boundary within the bovine lens cortex (31), in a location similar to the boundary we observe as the beginning of inclusion body formation in αA−/− lenses. It is possible that formation of the αB-containing inclusion bodies is in some way related to breakdown of the vimentin network and cytoskeletal remodeling in the maturing lens fiber cell. This hypothesis receives tangential support from a study demonstrating that the presence of αB in human disease-related inclusion bodies correlates with the presence of intermediate filaments in those inclusions (21). We are currently analyzing the cytoskeletons of αA−/− lenses to identify any changes in the cellular architecture that result from the absence of αA.
Horwitz’s (14) discovery that α-crystallin functions in vitro as a chaperone was a major advancement in our understanding of α-crystallin function. The in vivo data presented here are consistent with Horwitz’s findings in that changes in the solubility and intracellular distribution of αB in the lens could result from the loss of αA chaperone activity. We have also knocked out the αB gene, and we have recently bred mice lacking both αA and αB. These mice will provide a much broader understanding of how α-crystallin functions in the lens as a chaperone, a structural protein, a kinase, or perhaps even a regulatory protein. Finally, we propose that the lens opacification manifested by the αA knockout mice might serve as a model for studying the progression and treatment of cataracts and that identifying all of the components of the inclusion bodies and determining the mechanisms of their formation may lead to a greater understanding of human diseases exhibiting αB-containing inclusion bodies.
Acknowledgments
We thank Sing-Ping Huang and Alex Grinberg for their help in getting the embryonic stem cell culture technology started; Rudolph Jaenisch for providing the embryonic stem cells; Victor Tubylowicz and Paul Love for providing the pPNT plasmid; Samuel Zigler and Joseph Horwitz for providing antibodies; Joram Piatigorsky for his continued enthusiastic support for this work; and Paul Russell, Kenneth Mitton, Vasanth Rao, and Santa Tumminia for helpful discussions. We thank members of the National Eye Institute central transgenic facility, Steven Lee, Susan DiCamillo, and Michael Murr, and the National Eye Institute Histology Unit, Mary Alice Crawford and Nicole Newman, for their expert help.
Footnotes
Abbreviations: αA, αA-crystallin; αB, αB-crystallin; neo, neomycin phosphotransferase; PGK, phosphoglycerate kinase.
References
- 1.Benedek G B. Appl Opt. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- 2.Piatigorsky J. Differentiation (Berlin) 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 3.de Jong W W. In: Molecular and Cellular Biology of the Eye Lens. Bloemendal H, editor. New York: Wiley; 1981. pp. 221–278. [Google Scholar]
- 4.Kato K, Shinohara H, Goto S, Inaguma Y, Morishita R, Asano T. J Biol Chem. 1992;267:7718–7725. [PubMed] [Google Scholar]
- 5.Srinivasan A N, Nagineni C N, Bhat S P. J Biol Chem. 1992;267:23337–23341. [PubMed] [Google Scholar]
- 6.Deretic D, Aebersold R H, Morrison H D, Papermaster D S. J Biol Chem. 1994;269:16853–16861. [PubMed] [Google Scholar]
- 7.Bhat S P, Nagineni C N. Biochem Biophys Res Commun. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- 8.Dubin R A, Wawrousek E F, Piatigorsky J. Mol Cell Biol. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingolia T D, Craig E A. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klemenz R, Frohli E, Steiger R H, Schafer R, Aoyama A. Proc Natl Acad Sci USA. 1991;88:3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasgupta S, Hohman T C, Carper D. Exp Eye Res. 1992;54:461–470. doi: 10.1016/0014-4835(92)90058-z. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz J, McFall-Nagai M, Ding L L, Yaron O. In: The Lens: Transparency and Cataract. Duncan G, editor. Rijswijk, The Netherlands: Eurage; 1986. pp. 227–240. [Google Scholar]
- 13.Maiti M, Kono M, Chakrabarti B. FEBS Lett. 1988;236:109–114. doi: 10.1016/0014-5793(88)80295-3. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz J. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopalakrishnan S, Takemoto L. Curr Eye Res. 1992;11:929–933. doi: 10.3109/02713689209033490. [DOI] [PubMed] [Google Scholar]
- 16.Chiesi M, Longoni S, Limbruno U. Mol Cell Biochem. 1990;97:129–136. doi: 10.1007/BF00221054. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Spector A. Eur J Biochem. 1996;242:55–66. doi: 10.1111/j.1432-1033.1996.0056r.x. [DOI] [PubMed] [Google Scholar]
- 18.Nicholl I D, Quinlan R A. EMBO J. 1994;13:945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennardini F, Wrzosek A, Chiesi M. Circ Res. 1992;71:288–294. doi: 10.1161/01.res.71.2.288. [DOI] [PubMed] [Google Scholar]
- 20.Carter J M, Hutcheson A M, Quinlan R A. Exp Eye Res. 1995;60:181–192. doi: 10.1016/s0014-4835(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 21.Lowe J, McDermott H, Pike I, Spendlove I, Landon M, Mayer R J. J Pathol. 1992;166:61–68. doi: 10.1002/path.1711660110. [DOI] [PubMed] [Google Scholar]
- 22.Kantorow M, Piatigorsky J. Proc Natl Acad Sci USA. 1994;91:3112–3116. doi: 10.1073/pnas.91.8.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietrowski D, Durante M J, Liebstein A, Schmitt-John T, Werner T, Graw J. Gene. 1994;144:171–178. doi: 10.1016/0378-1119(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 24.Tybulewicz V L, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 25.Larsson R I. Immunocytochemistry: Theory and Practice. Boca Raton, FL: CRC; 1988. [Google Scholar]
- 26.King C R, Piatigorsky J. Cell. 1983;32:707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- 27.Lo W K. Proc Natl Acad Sci USA. 1989;86:9926–9930. doi: 10.1073/pnas.86.24.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Augusteyn R C, Ellerton H D, Putilina T, Stevens A. Biochim Biophys Acta. 1988;957:192–201. doi: 10.1016/0167-4838(88)90272-5. [DOI] [PubMed] [Google Scholar]
- 29.Atomi Y, Yamada S, Strohman R, Nonomura Y. J Biochem (Tokyo) 1991;110:812–822. doi: 10.1093/oxfordjournals.jbchem.a123665. [DOI] [PubMed] [Google Scholar]
- 30.Longoni S, Lattonen S, Bullock G, Chiesi M. Mol Cell Biochem. 1990;97:121–128. doi: 10.1007/BF00221053. [DOI] [PubMed] [Google Scholar]
- 31.Sandilands A, Prescott A R, Carter J M, Hutcheson A M, Quinlan R A, Richards J, FitzGerald P G. J Cell Sci. 1995;108:1397–1406. doi: 10.1242/jcs.108.4.1397. [DOI] [PubMed] [Google Scholar]