Abstract
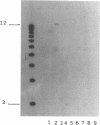
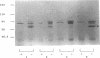
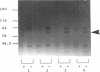
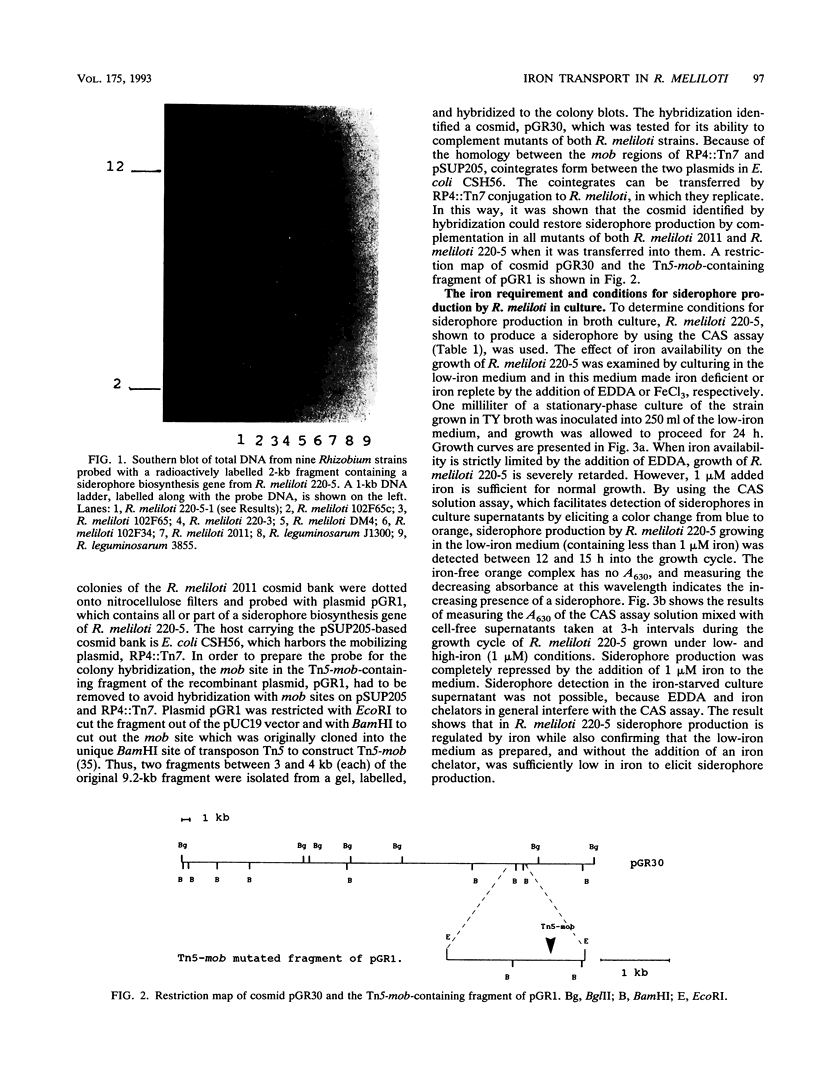
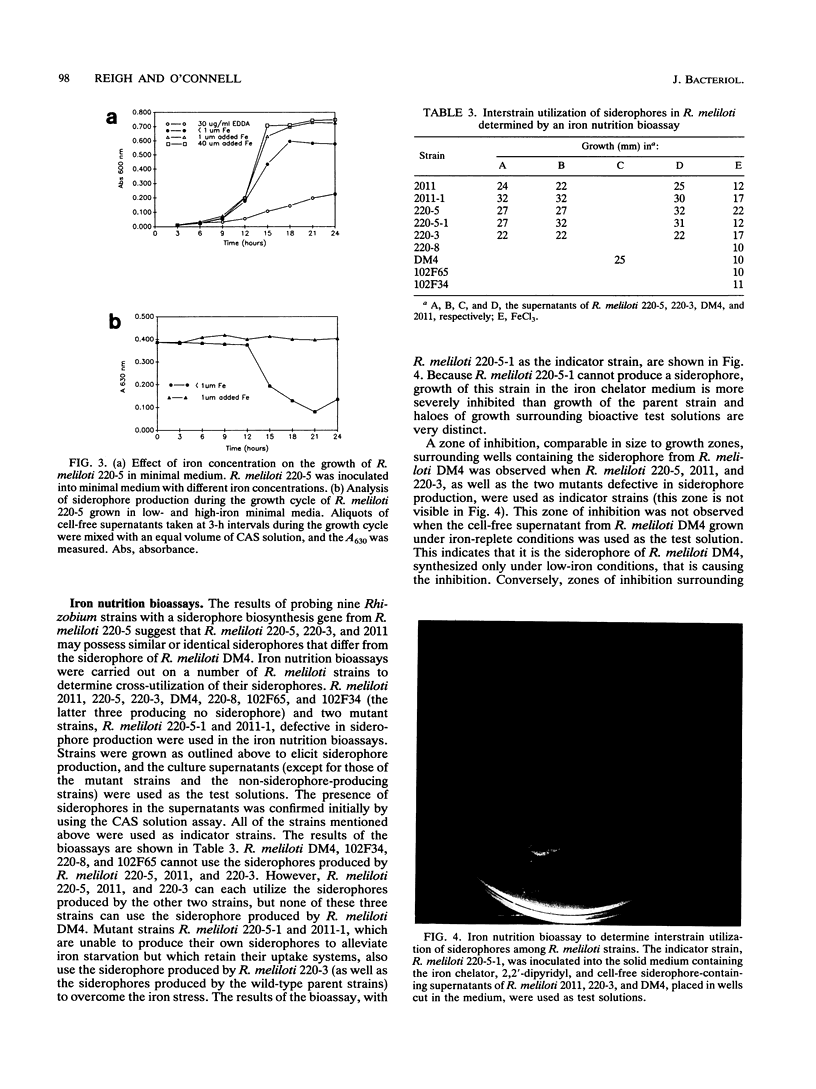
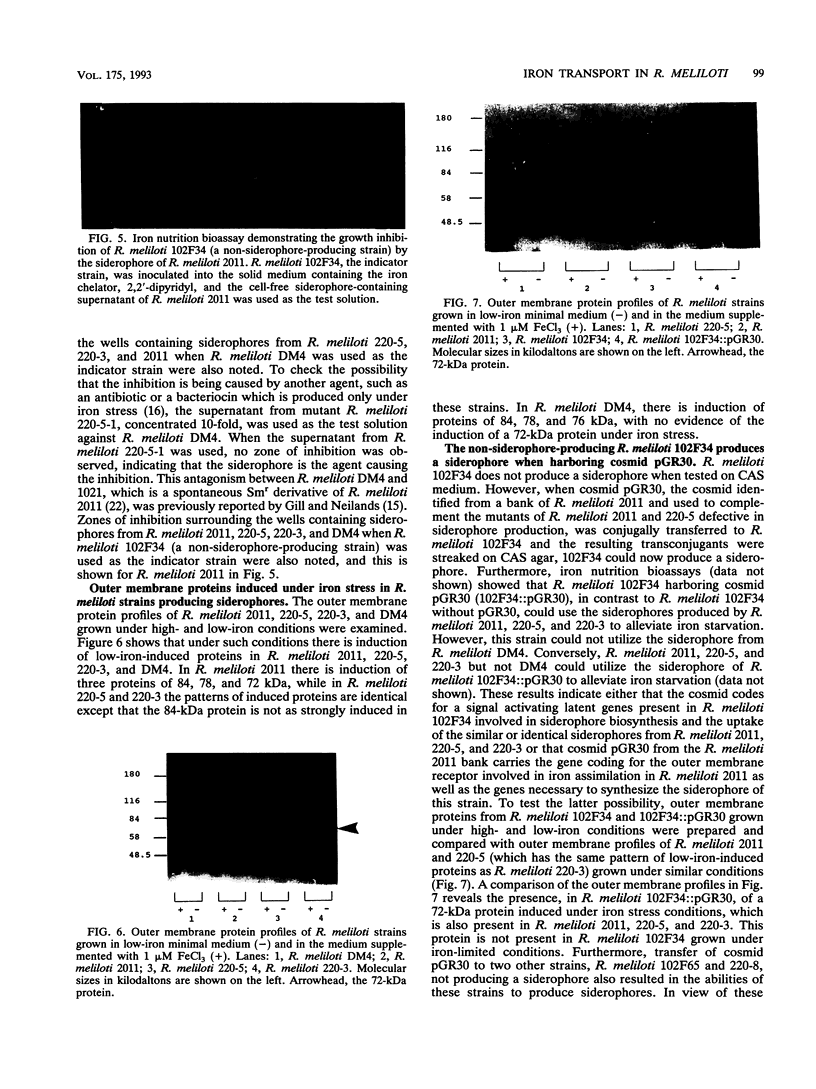
A universal chemical assay used to detect the production of siderophores in a range of Rhizobium strains showed that production is strain specific. Iron nutrition bioassays carried out on Rhizobium meliloti strains to determine cross-utilization of their siderophores showed that R. meliloti 2011, 220-5, and 220-3 could each use the siderophores produced by the other two but not the siderophore produced by R. meliloti DM4 (and vice versa). Mutants of R. meliloti 2011 and 220-5 defective in siderophore production were isolated by Tn5-mob mutagenesis. The Tn5-mob-containing EcoRI fragment of mutant R. meliloti 220-5-1 was cloned into pUC19. By using this fragment as a probe, the presence of a homologous region was observed in R. meliloti 2011 and 220-3 but not in R. meliloti DM4. A complementing cosmid from a gene bank of R. meliloti 2011 was identified by using the same probe. Introduction of this cosmid into R. meliloti 102F34, a strain not producing a siderophore, resulted in the ability of this strain to produce a siderophore and also in the ability to utilize the siderophores produced by R. meliloti 2011, 220-5, and 220-3 but not the siderophore produced by R. meliloti DM4. A comparative analysis of the outer membrane proteins prepared from iron-deficient cultures of R. meliloti 102F34 and 102F34 harboring the cosmid revealed the presence, in the latter, of a low-iron-induced outer membrane protein corresponding to a low-iron-induced protein in R. meliloti 2011, 220-5, and 220-3. This protein is not present in R. meliloti DM4. The results suggest that R. meliloti 2011, 220-5, and 220-3 produce siderophores that are identical or sufficiently similar in structure to be transported by the membrane transport system of each strain while also indicating that utilization of a particular siderophore is correlated with the presence of specific outer membrane proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames-Gottfred N. P., Christie B. R., Jordan D. C. Use of the Chrome Azurol S Agar Plate Technique To Differentiate Strains and Field Isolates of Rhizobium leguminosarum biovar trifolii. Appl Environ Microbiol. 1989 Mar;55(3):707–710. doi: 10.1128/aem.55.3.707-710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyer J. S., Leong J. Iron transport-mediated antagonism between plant growth-promoting and plant-deleterious Pseudomonas strains. J Biol Chem. 1986 Jan 15;261(2):791–794. [PubMed] [Google Scholar]
- Clarke L., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli: specific complementation of argininosuccinate lyase (argH) mutations. J Mol Biol. 1978 Apr 25;120(4):517–532. doi: 10.1016/0022-2836(78)90351-0. [DOI] [PubMed] [Google Scholar]
- Enard C., Diolez A., Expert D. Systemic virulence of Erwinia chrysanthemi 3937 requires a functional iron assimilation system. J Bacteriol. 1988 Jun;170(6):2419–2426. doi: 10.1128/jb.170.6.2419-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. M., Bruderer T., Hennecke H. Essential and non-essential domains in the Bradyrhizobium japonicum NifA protein: identification of indispensable cysteine residues potentially involved in redox reactivity and/or metal binding. Nucleic Acids Res. 1988 Mar 25;16(5):2207–2224. doi: 10.1093/nar/16.5.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P. R., Jr, Neilands J. B. Cloning a genomic region required for a high-affinity iron-uptake system in Rhizobium meliloti 1021. Mol Microbiol. 1989 Sep;3(9):1183–1189. doi: 10.1111/j.1365-2958.1989.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Gill P. R., Jr, Warren G. J. An iron-antagonized fungistatic agent that is not required for iron assimilation from a fluorescent rhizosphere pseudomonad. J Bacteriol. 1988 Jan;170(1):163–170. doi: 10.1128/jb.170.1.163-170.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L., Meidl E. J., Plessner O. Citrate as a siderophore in Bradyrhizobium japonicum. J Bacteriol. 1990 Jun;172(6):3298–3303. doi: 10.1128/jb.172.6.3298-3303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A. W., Beringer J. E. Identification of the rhizobium strains in pea root nodules using genetic markers. J Gen Microbiol. 1975 Apr;87(2):343–350. doi: 10.1099/00221287-87-2-343. [DOI] [PubMed] [Google Scholar]
- Magazin M. D., Moores J. C., Leong J. Cloning of the gene coding for the outer membrane receptor protein for ferric pseudobactin, a siderophore from a plant growth-promoting Pseudomonas strain. J Biol Chem. 1986 Jan 15;261(2):795–799. [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K. D., Johnston A. W., Chen J. W., John T. R. A Rhizobium leguminosarum mutant defective in symbiotic iron acquisition. J Bacteriol. 1990 Feb;172(2):670–677. doi: 10.1128/jb.172.2.670-677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Siderophores of bacteria and fungi. Microbiol Sci. 1984 Apr;1(1):9–14. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Rioux C., Jordan D. C., Rattray J. B. Colorimetric determination of catechol siderophores in microbial cultures. Anal Biochem. 1983 Aug;133(1):163–169. doi: 10.1016/0003-2697(83)90238-5. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Simon R. High frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-Mob transposon. Mol Gen Genet. 1984;196(3):413–420. doi: 10.1007/BF00436188. [DOI] [PubMed] [Google Scholar]
- Skorupska A., Choma A., Deryło M., Lorkiewicz Z. Siderophore containing 2,3-dihydroxybenzoic acid and threonine formed by Rhizobium trifolli. Acta Biochim Pol. 1988;35(2):119–130. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stuart S. J., Greenwood K. T., Luke R. K. Hydroxamate-mediated transport of iron controlled by ColV plasmids. J Bacteriol. 1980 Jul;143(1):35–42. doi: 10.1128/jb.143.1.35-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weger L. A., van Arendonk J. J., Recourt K., van der Hofstad G. A., Weisbeek P. J., Lugtenberg B. Siderophore-mediated uptake of Fe3+ by the plant growth-stimulating Pseudomonas putida strain WCS358 and by other rhizosphere microorganisms. J Bacteriol. 1988 Oct;170(10):4693–4698. doi: 10.1128/jb.170.10.4693-4698.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weger L. A., van Boxtel R., van der Burg B., Gruters R. A., Geels F. P., Schippers B., Lugtenberg B. Siderophores and outer membrane proteins of antagonistic, plant-growth-stimulating, root-colonizing Pseudomonas spp. J Bacteriol. 1986 Feb;165(2):585–594. doi: 10.1128/jb.165.2.585-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]