Abstract
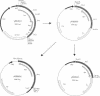
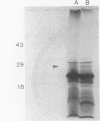
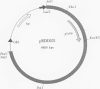
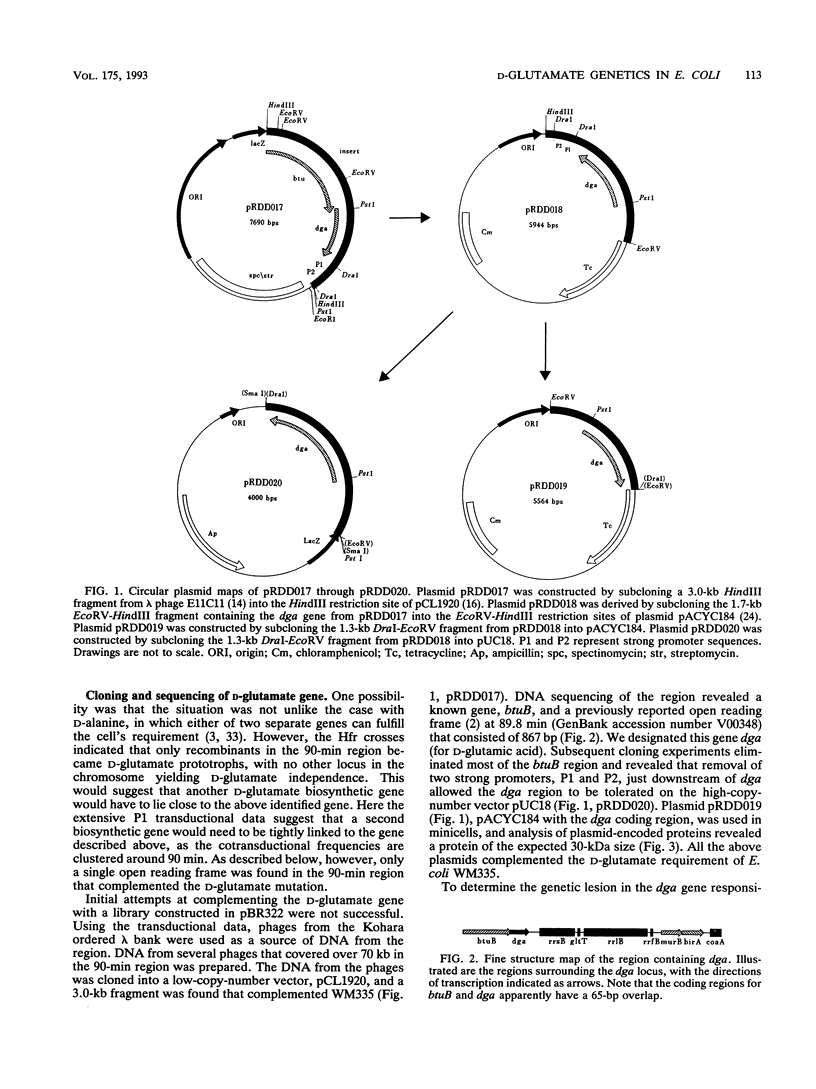
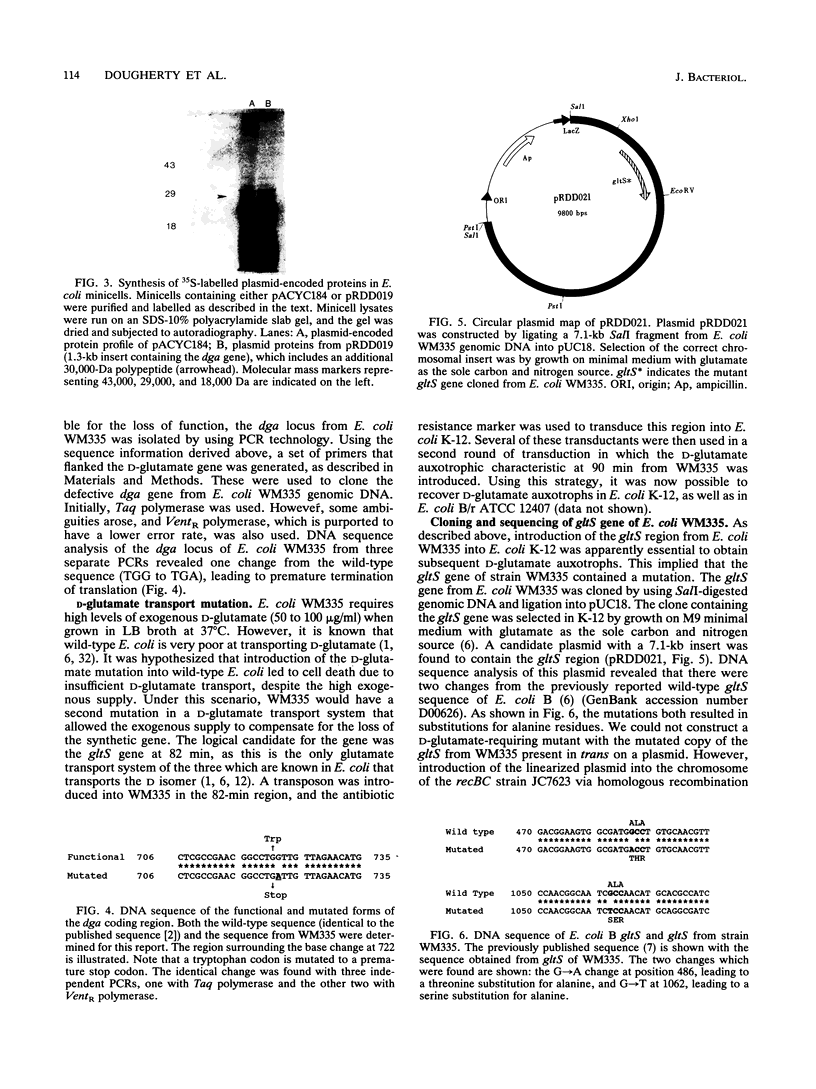
D-Glutamic acid is an essential component of bacterial cell wall peptidoglycan in both gram-positive and gram-negative bacteria. Very little is known concerning the genetics and biochemistry of D-glutamate production in most bacteria, including Escherichia coli. Evidence is presented in this report for the roles of two distinct genes in E. coli WM335, a strain which is auxotrophic for D-glutamate. The first gene, which restores D-glutamate independence in WM335, was mapped, cloned, and sequenced. This gene, designated dga, is a previously reported open reading frame, located at 89.8 min on the E. coli map. The second gene, gltS, is located at 82 min. gltS encodes a protein that is involved in the transport of D- and L-glutamic acid into E. coli, and the gltS gene of WM335 was found to contain two missense mutations. To construct D-glutamate auxotrophs, it is necessary to transfer sequentially the mutated gltS locus, and then the mutated dga locus into the recipient. The sequences of the mutant forms of both dga and gltS are also presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth I. R., Kleppang K. E., Kempsell K. E. A genetic locus for the GltII-glutamate transport system in Escherichia coli. J Gen Microbiol. 1989 Oct;135(10):2767–2774. doi: 10.1099/00221287-135-10-2767. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Bugg T. D., Walsh C. T. Intracellular steps of bacterial cell wall peptidoglycan biosynthesis: enzymology, antibiotics, and antibiotic resistance. Nat Prod Rep. 1992 Jun;9(3):199–215. doi: 10.1039/np9920900199. [DOI] [PubMed] [Google Scholar]
- Deguchi Y., Yamato I., Anraku Y. Molecular cloning of gltS and gltP, which encode glutamate carriers of Escherichia coli B. J Bacteriol. 1989 Mar;171(3):1314–1319. doi: 10.1128/jb.171.3.1314-1319.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi Y., Yamato I., Anraku Y. Nucleotide sequence of gltS, the Na+/glutamate symport carrier gene of Escherichia coli B. J Biol Chem. 1990 Dec 15;265(35):21704–21708. [PubMed] [Google Scholar]
- Doublet P., van Heijenoort J., Mengin-Lecreulx D. Identification of the Escherichia coli murI gene, which is required for the biosynthesis of D-glutamic acid, a specific component of bacterial peptidoglycan. J Bacteriol. 1992 Sep;174(18):5772–5779. doi: 10.1128/jb.174.18.5772-5779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K., Kadner R. J. Nucleotide sequence of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985 Mar;161(3):904–908. doi: 10.1128/jb.161.3.904-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- KURAMITSU H. K., SNOKE J. E. The biosynthesis of D-amino acids in Bacillus licheniformis. Biochim Biophys Acta. 1962 Jul 30;62:114–121. doi: 10.1016/0006-3002(62)90496-1. [DOI] [PubMed] [Google Scholar]
- Kalman M., Gentry D. R., Cashel M. Characterization of the Escherichia coli K12 gltS glutamate permease gene. Mol Gen Genet. 1991 Mar;225(3):379–386. doi: 10.1007/BF00261677. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lerner C. G., Inouye M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990 Aug 11;18(15):4631–4631. doi: 10.1093/nar/18.15.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., Wijsman H. J., van Zaane D. Properties of a D-glutamic acid-requiring mutant of Escherichia coli. J Bacteriol. 1973 May;114(2):499–506. doi: 10.1128/jb.114.2.499-506.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Flouret B., van Heijenoort J. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1109–1117. doi: 10.1128/jb.151.3.1109-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T., Matsuzawa H., Matsuhashi M., Sugino Y. Cell wall peptidoglycan mutants of Escherichia coli K-12: existence of two clusters of genes, mra and mrb, for cell wall peptidoglycan biosynthesis. J Bacteriol. 1972 Nov;112(2):950–958. doi: 10.1128/jb.112.2.950-958.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Discotto L. F., Dougherty T. J. Cloning and identification of the Escherichia coli murB DNA sequence, which encodes UDP-N-acetylenolpyruvoylglucosamine reductase. J Bacteriol. 1992 Mar;174(5):1690–1693. doi: 10.1128/jb.174.5.1690-1693.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W. J., Jackowski S. coaA and rts are allelic and located at kilobase 3532 on the Escherichia coli physical map. J Bacteriol. 1992 Mar;174(5):1705–1706. doi: 10.1128/jb.174.5.1705-1706.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B., GOMEZ C. G., HOUSEWRIGHT R. D. Transamination of D-amino acids by Bacillus subtilis. J Bacteriol. 1955 Mar;69(3):357–362. doi: 10.1128/jb.69.3.357-362.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B., MOLNAR D. M. D-Amino acid transamination in bacillus anthracis. J Bacteriol. 1955 Oct;70(4):420–426. doi: 10.1128/jb.70.4.420-426.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizawa K., Asano S., Masu Y., Kuramitsu S., Kagamiyama H., Tanaka H., Soda K. The primary structure of thermostable D-amino acid aminotransferase from a thermophilic Bacillus species and its correlation with L-amino acid aminotransferases. J Biol Chem. 1989 Feb 15;264(5):2450–2454. [PubMed] [Google Scholar]
- Wallace B., Yang Y. J., Hong J. S., Lum D. Cloning and sequencing of a gene encoding a glutamate and aspartate carrier of Escherichia coli K-12. J Bacteriol. 1990 Jun;172(6):3214–3220. doi: 10.1128/jb.172.6.3214-3220.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. T. Enzymes in the D-alanine branch of bacterial cell wall peptidoglycan assembly. J Biol Chem. 1989 Feb 15;264(5):2393–2396. [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]