Abstract
Protein kinase C (PKC) has been implicated in the Wnt signaling pathway; however, its molecular role is poorly understood. We identified novel genes encoding δ-type PKC in the Xenopus EST databases. Loss of PKCδ function revealed that it was essential for convergent extension during gastrulation. We then examined the relationship between PKCδ and the Wnt pathway. PKCδ was translocated to the plasma membrane in response to Frizzled signaling. In addition, loss of PKCδ function inhibited the translocation of Dishevelled and the activation of c-Jun N-terminal kinase (JNK) by Frizzled. Furthermore, PKCδ formed a complex with Dishevelled, and the activation of PKCδ by phorbol ester was sufficient for Dishevelled translocation and JNK activation. Thus, PKCδ plays an essential role in the Wnt/JNK pathway by regulating the localization and activity of Dishevelled.
Keywords: PKCδ, Xenopus, gastrulation, convergent extension, noncanonical Wnt pathway
During Xenopus gastrulation, mesodermal cells migrate to the inside of the embryo and move along the blastocoel roof. This movement is essential for embryonic morphogenic processes such as the establishment of the three germ layers and body axes. The process involves highly integrated cell movements. One of the important mechanisms for this movement is convergent extension. As convergent extension begins, cells are polarized and aligned mediolaterally; this is followed by the intercalation of these polarized cells. This movement elongates the mesodermal tissue along the anteroposterior axis, producing a driving force for gastrulation movements (Wilson and Keller 1991; Shih and Keller 1992; Wallingford et al. 2002). The regulation of the convergent extension movements is known to involve a noncanonical Wnt signaling pathway.
The Wnts are a family of secreted proteins that regulate many biological processes (Cadigan and Nusse 1997). Functional analyses in Xenopus suggest that the Wnt family can be divided into two functionally distinct groups. The first group of Wnts induces a secondary axis when ectopically expressed in embryos. They activate the canonical Wnt/β-catenin pathway and induce the transcription of target genes such as siamois and Xnr3 (Brannon and Kimelman 1996; Carnac et al. 1996; McKendry et al. 1997). The second group of Wnts, which includes Xwnt5a and Xwnt11, activates the noncanonical Wnt signaling pathway that controls morphogenetic cell movements (Kuhl 2002; Tada et al. 2002). It was shown in zebrafish that mutations in Wnt11/silberbrick and Wnt5a/pipetail inhibit normal gastrulation movements (Rauch et al. 1997; Heisenberg et al. 2000). The noncanonical Wnt pathway branches into two cascades. One is the Wnt/JNK pathway, which involves c-Jun N-terminal kinase (JNK; Boutros et al. 1998; Yamanaka et al. 2002). The other is the Wnt/Ca2+ pathway (Kuhl et al. 2000). In Drosophila, the Wnt/JNK pathway is called the planer cell polarity (PCP) pathway, and it specifies cell polarities in epithelial cells and other types of cells (Adler 2002).
The Wnt signaling pathway is mediated by a seven-transmembrane Wnt receptor, Frizzled, and the signal is transmitted through a cytoplasmic protein, Dishevelled (Dsh), which plays pivotal roles in both the canonical and noncanonical Wnt pathways (Boutros and Mlodzik 1999; Wharton 2003). In Drosophila, Dsh localizes to the membrane, and this localization is required for Dsh function (Axelrod 2001). Xenopus Dsh (Xdsh) is also translocated from the cytoplasm to the plasma membrane in response to a signal generated by some Frizzled receptors (Yang-Snyder et al. 1996; Axelrod et al. 1998; Rothbacher et al. 2000). One such receptor is Xenopus Frizzled7 (Xfz7), which is involved in the noncanonical Wnt pathway (Djiane et al. 2000; Medina and Steinbeisser 2000; Medina et al. 2000). However, the mechanism of this translocation and the activation of Dishevelled is not known. The signal transduction of the canonical pathway seems to be different from that of the noncanonical pathway, because the membrane translocation of Xdsh is not required for the activation of the canonical Wnt pathway (Rothbacher et al. 2000).
Protein kinase C (PKC) is thought to be involved in the noncanonical Wnt signaling pathway, particularly in the Wnt/Ca2+ pathway, for several reasons. Xwnt5a and rat Frizzled2 activate the phosphatidylinositol pathway and increase the intracellular Ca2+ levels in zebrafish embryos (Slusarski et al. 1997a,b). The phosphatidylinositol pathway and Ca2+ levels are closely related to PKC activation. In fact, overexpression of Frizzled causes the translocation of epitope-tagged PKCα from the cytoplasm to the plasma membrane in Xenopus embryos (Sheldahl et al. 1999; Medina et al. 2000). Kuhl et al. (2001) showed that PKCα phosphorylated Dsh in vitro. In addition, the loss of Xfz7 function leads to a defect in tissue separation during Xenopus gastrulation, which is rescued by the overexpression of PKCα (Winkbauer et al. 2001). PKC is also implicated in the Xwnt11 signaling pathway for Xenopus cardiogenesis (Pandur et al. 2002) and in the Dwnt4 pathway for Drosophila ovarian morphogenesis (Cohen et al. 2002).
Although much evidence suggests that PKC is involved in the Wnt signaling pathway, the molecular roles of PKC in this pathway are not well understood. The PKC family is subdivided into three subfamilies: the classical, novel, and atypical PKCs (cPKC, nPKC, and aPKC, respectively). cPKC is activated by Ca2+ and diacylglycerol (DAG), nPKC is activated by DAG but not by Ca2+, and aPKC is not activated by these molecules (Kikkawa et al. 1989; Bell and Burns 1991; Nishizuka 1995; Newton 1997). In Xenopus, cDNAs encoding PKCα and PKCβ, which belong to the cPKC subfamily, have been isolated (Chen et al. 1988), and shown to be involved in neural induction (Otte et al. 1988; Otte and Moon 1992). However, their roles in the regulation of gastrulation movements are not clear. Thus, we searched for novel PKC genes that might have roles in the noncanonical Wnt signaling pathway. Here, we describe the identification and functional analyses of Xenopus PKCδ, which belongs to the nPKC subfamily. We demonstrate that PKCδ is essential for convergent extension, and that PKCδ regulates the function of Dishevelled in the Wnt/JNK pathway.
Results
PKCδ is expressed during Xenopus embryogenesis
Although PKC has been implicated in the noncanonical Wnt signaling pathway, its molecular role is poorly understood. It has been shown that Xwnt5a and rat Frizzled2 trigger the phosphatidylinositol pathway and induce an increase in intracellular Ca2+ (Slusarski et al. 1997a,b). Among the PKC subfamilies, cPKC and nPKC are known to be activated by Ca2+ and/or diacylglycerol (DAG). For this reason, we searched our Xenopus EST database (NIBB XDB, http://Xenopus.nibb.ac.jp) to identify PKC family members that belong to the cPKC or nPKC subfamily. We found that in addition to PKCα and PKCβ, which have already been reported (Chen et al. 1988), the database included two novel cDNAs encoding nPKC family members. The predicted amino acid sequences of these two PKCs had 95% identity, suggesting that these are duplicated genes due to the tetraploidism of Xenopus laevis. As described later, these two genes had indistinguishable activities in the tests we performed. These proteins are the most similar to mammalian δ-type PKC (Fig. 1A,B). Thus, we designated these genes PKCδ1 and PKCδ2. It is known that the N-terminal regulatory domain of PKCs inhibits the kinase activity by masking the catalytic domain, and activators such as DAG release this autoinhibition by binding to the C1 domain (Kemp et al. 1994; Orr and Newton 1994; Nishizuka 1995; Newton 1997). The regulatory domain of Xenopus PKCδ1/2, including the C1 domain, is highly homologous to that of human PKCδ, suggesting that these regulatory mechanisms are conserved. PKCδ is relatively similar to PKCθ and PKCε, which also belong to the nPKC family. This class of PKCs is found not only in vertebrates, but also in sea sponges (GenBank accession no. CAA73557), Aplysia (GenBank accession no. 16975), Hydra (GenBank accession no. CAA72926), Drosophila (GenBank accession no. NP_511171), and nematodes (GenBank accession no. NP_499860). Thus, the nPKCs may have evolutionally conserved regulatory mechanisms and functions distinct from those of other PKC subfamilies.
Figure 1.
PKCδ is expressed during Xenopus embryogenesis. (A) Sequence alignment of Xenopus PKCδ1 and human PKCδ. The DAG-binding C1 domain is underlined. The pseudosubstrate region is indicated by dots. (B) Sequence comparison between Xenopus PKCδ and some human PKC family members. Xenopus PKCδ1 is the most similar to human PKCδ in both the regulatory and the catalytic domains. (C) RT–PCR analysis of PKCδ expression during Xenopus development. Primers whose sequences were common between PKCδ1 and PKCδ2 were used. Stages are according to Nieukoop and Faber (1994). (D) In situ hybridization probing with PKCδ1 and PKCδ2 showing their ubiquitous expression.
To determine the expression patterns during Xenopus development, we performed reverse transcriptase PCR (RT–PCR) using primers whose sequences were common to PKCδ1 and PKCδ2. As shown in Figure 1C, PKCδ was expressed from the two-cell stage through the tadpole stage. In situ hybridization using probes for PKCδ1 and PKCδ2 revealed that they were ubiquitously expressed (Fig. 1D). PKCδ1 and PKCδ2 were strongly expressed in the mesoderm and ectoderm during gastrulation, indicating their possible involvement in the regulation of gastrulation movements.
Overexpression of PKCδ lacking the catalytic domain inhibits gastrulation movements
To test whether PKCδ is involved in the regulation of gastrulation movements, we made an expression construct for PKCδ1 lacking the catalytic domain (PKCδΔC). The N-terminal regulatory domain of PKCs includes pseudosubstrate and C1 domains. The pseudosubstrate domain interacts with the kinase domain and inhibits the catalytic activity (Kemp et al. 1994; Orr and Newton 1994). The C1 domain interacts with DAG and other activators. A mutant lacking the catalytic domain was expected to function as a dominant-negative form by binding to the catalytic domain of a native protein through its pseudosubstrate domain and/or by competitive binding to the activators. RNA encoding PKCδΔC was synthesized in vitro and injected into the two dorsal blastomeres of four-cell embryos. As shown in Figure 2A, PKCδΔC severely inhibited gastrulation movements. Involution of the mesoderm was impaired, and the blastopore remained open or showed delayed closing. The same phenotype was observed in embryos injected with PKCδ2 lacking the catalytic domain (data not shown). The phenotype was rescued by full-length PKCδ1 (Fig. 2A) or full-length PKCδ2 (data not shown), suggesting that PKCδΔC functioned as a dominant-negative mutant of PKCδ. It has been reported that a similar gastrulation-defect phenotype is caused by loss-of-function of the noncanonical Wnt signaling components, such as Xwnt11 (Tada and Smith 2000) and Xfz7 (Djiane et al. 2000).
Figure 2.
Overexpression of PKCδ lacking the catalytic domain inhibits gastrulation movements. (A) PKCδ1 lacking the catalytic domain (PKCδΔC) inhibits gastrulation movements. RNA (100 pg) encoding PKCδΔC was injected into the two dorsal blastomeres of four-cell embryos. PKCδΔC severely inhibited gastrulation movements, and this effect was rescued by the coinjection of 1 ng of RNA encoding full-length PKCδ1. Embryos in the top panels are at the early neurula stage. (B) In situ hybridization of early gastrula embryos probed with a pan-mesodermal marker, Xbra, and dorsal mesodermal markers chordin (chd) and goosecoid (gsc). (Left) Uninjected. (Middle) PKCδΔC RNA (200 pg) was injected into all four blastomeres of four-cell embryos. (Right) In order to trace the cell lineage, mRNA encoding β-galactosidase (β-gal) with a nuclear localization signal was coinjected with PKCδΔC into two dorsal blastomeres of four-cell embryos. Cells expressing β-gal were stained in red. (C) Immunostaining of the notochord and somites in PKCδΔC-injected embryos. (D) The PKCδΔC mutant blocked the elongation of animal cap explants by activin. Activin RNA (0.5 pg) was injected with or without 100 pg of PKCδΔC RNA. (E) The induction of mesodermal markers by activin in animal caps was not affected by PKCδΔC. ui, uninjected; Em, whole embryo; gsc, goosecoid; chd, chordin.
To test whether PKCα or PKCβ has an activity similar to that of PKCδ, we constructed mutant genes encoding PKCα and PKCβ lacking the catalytic domain (PKCαΔC and PKCβΔC, respectively), expecting that they would function as dominant-negative mutants of their respective native forms. We injected the same amount of mRNA encoding PKCαΔC, PKCβΔC, or PKCδΔC into Xenopus embryos. Although comparable levels of the mutant proteins were detected by Western blotting, PKCαΔC and PKCβΔC did not have any effects on gastrulation, unlike PKCδΔC (data not shown). This result suggests that the role in gastrulation movements may be PKCδ-specific.
To determine whether this gastrulation-defective phenotype was caused by a defect in mesodermal differentiation, the expression of mesodermal markers, we used in situ hybridization to examine a pan-mesodermal marker, Xbra, and dorsal mesodermal markers chordin (chd) and goosecoid (gsc). At the gastrula stage, PKCδΔC-injected embryos expressed these markers at the same level as control embryos (Fig. 2B). In tadpoles, the notochord and somites were differentiated in the PKCδΔC-injected embryos, but the extension of these tissues was severely inhibited (Fig. 2C). These results indicated that the phenotype was caused not by a defect in mesoderm differentiation, but by a defect in morphogenetic movements.
We then tested whether PKCδΔC inhibited the elongation of animal caps by activin. In this system, when the dorsal mesoderm is induced in animal cap explants by activin, the explants elongate by convergent extension movements. PKCδΔC expression inhibited the elongation of explants by activin without affecting the induction of mesodermal markers (Fig. 2D,E). A similar effect has been observed as a result of inhibition of the noncanonical Wnt pathway. Our results suggest that PKCδ is also required for the convergent extension.
PKCδ antisense morpholino also blocked gastrulation movements and convergent extension
To confirm that the effects of PKCδΔC were due to the depletion of PKCδ activity, we made antisense morpholino oligonucleotides (MOs) for PKCδ and tested their effects on development. Because the highly homologous PKCδ1 and PKCδ2 are both expressed, MOs for both PKCδ1 and PKCδ2 were prepared. First, we confirmed that these MOs inhibited the translation of mRNA that has the corresponding sequences. As shown in Figure 3A, each MO blocked the production of its respective GFP-tagged PKCδ, but unrelated GFP was not affected.
Figure 3.
PKCδ antisense morpholino also blocked gastrulation movements. (A) Morpholino oligonucleotides (MOs) for PKCδ1 and PKCδ2 inhibited the translation of mRNA that had the corresponding sequences. RNA encoding GFP-tagged PKCδ and unrelated GFP were coinjected with or without each MO. PKCδ1 (left panel) and PKCδ2 MO (right panel) blocked the production of each GFP-tagged PKCδ, but unrelated GFP was not affected. (B) Control MO or PKCδ MO (20 ng each) was injected into four-cell embryos (panels a,b, respectively). The PKCδ MO caused a gastrulation-defective phenotype that was indistinguishable from that of PKCδΔC-injected embryos. This phenotype was rescued by 1 ng of full-length PKCδ1 RNA (panel c). (C) In situ hybridization of early gastrula embryos probed with chordin (chd), goosecoid (gsc), and Xbra. The left and middle panels show 20 ng of control or PKCδ MO was injected into all four blastomeres of four-cell embryos. (Right) To trace the cell lineage, mRNA encoding β-gal with a nuclear localization signal was coinjected with PKCδ MO into two dorsal blastomeres of four-cell embryos. (D) PKCδ MO inhibited the elongation of dorsal marginal zone explants. Twenty nanograms of MO were injected into the two dorsal blastomeres of four-cell embryos.
To inhibit PKCδ synthesis in embryos, the MOs for PKCδ1 and PKCδ2 were mixed at an equimolar ratio and injected into four-cell embryos (the mixed MOs will be referred to as PKCδ MO hereafter). The PKCδ MO caused a gastrulation-defective phenotype that was indistinguishable from that of PKCδΔC-injected embryos (Fig. 3B). This phenotype was efficiently rescued by Flag-tagged PKCδ1, which do not have 5′-UTR binding to the MOs. In addition, MO-injected embryos expressed the mesodermal markers Xbra, chordin, and goosecoid, suggesting that the mesoderm differentiation was not affected (Fig. 3C). The similarity of the phenotypes of the embryos injected with PKCδΔC and PKCδ MO indicated that PKCδΔC functioned as a dominant-negative mutant for PKCδ. To investigate the role of PKCδ in convergent extension, we examined the effect of PKCδ MOs on the elongation of dorsal marginal zone (DMZ) explants. As shown in Figure 3D, the PKCδ MO inhibited the elongation of these explants, supporting the idea that PKCδ may be required for convergent extension.
PKCδ is translocated to the membrane in response to Xfz7 signaling and interacts with Xdsh
The inhibition of convergent extension by the loss of PKCδ function strongly implies that PKCδ plays a role closely related to the noncanonical signaling pathway. It has been shown that Frizzled translocates Xdsh and PKCα from the cytoplasm to the plasma membrane in Xenopus embryos (Axelrod et al. 1998; Sheldahl et al. 1999; Medina and Steinbeisser 2000; Medina et al. 2000). To examine whether Frizzled also regulates the subcellular localization of PKCδ, Flag-tagged PKCδ and myctagged Xdsh were coexpressed with or without Xfz7 in animal cap explants of Xenopus embryos. The localization of the tagged proteins was then observed with a laser-scanning confocal microscope (Fig. 4A). In the absence of Xfz7 mRNA, PKCδ and Xdsh were mainly in the cytoplasm. Interestingly, however, when Xfz7 was coexpressed, they were mostly localized to the plasma membrane. In general, this class of PKC binds to and is activated by DAG that is produced on the membrane upon extracellular signaling. Thus, DAG may be produced by Xfz7 and then localize PKCδ to the membrane, which further implies that PKCδ is involved in the Wnt/Xfz7 pathway.
Figure 4.
PKCδ is localized to the plasma membrane with Xdsh by Xfz7 signaling. (A) Flag-tagged PKCδ RNA (200 pg) and myc-tagged Xdsh RNA (100 pg) were coinjected with or without 500 pg of Xfz7 RNA in the animal cap explants of Xenopus embryos. Their localization was observed by laser-scanning confocal microscopy. (B) Coimmunoprecipitation of PKCδ and Xdsh. Flag-tagged PKCδ and myc-tagged Xdsh were expressed as indicated in HEK293T cells. PKCδ and Xdsh coimmunoprecipitated, indicating that they form a complex. (C) The indicated genes were expressed in HEK293T cells. PMA was added to the medium at a final concentration of 1 μM 2 h before the cell lysate preparation. The addition of PMA did not change the amount of coimmunoprecipitated Xdsh and PKCδ.
The above finding that the cotranslocation of PKCδ and Xdsh from the cytoplasm to the membrane depended on Xfz7 function prompted us to examine whether these proteins might interact with each other. To test this possibility, Flag-tagged PKCδ and myc-tagged Xdsh were expressed in HEK293T cells and immunoprecipitation was performed. As shown in Figure 4B, PKCδ and Xdsh were coimmunoprecipitated. When the Flag and myc tags on PKCδ and Xdsh were exchanged, we obtained essentially the same result (data not shown). These findings indicated that PKCδ and Xdsh form a complex. To test whether the activation of PKCδ by the phorbol ester PMA (phorbol 12–myristate 13–acetate) altered this binding property, we treated transfected HEK293T cells with PMA and performed an immunoprecipitation. PMA is known to be a potent activator for PKCδ and other members of the novel and classical PKC subfamilies (Kikkawa et al. 1989; Bell and Burns 1991; Zhang et al. 1995). As shown in Figure 4C, PMA treatment did not change the amount of coimmunoprecipitated Xdsh and PKCδ. In addition, a kinase-negative mutant of PKCδ was also coimmunoprecipitated with Xdsh in HEK293T lysates (data not shown), indicating that this physical interaction may not depend on the activity of PKCδ. Taken together, these results suggest that PKCδ and Xdsh form a complex, and this complex is translocated to the membrane upon activation of the Xfz7 signal.
PKCδ is required for Xdsh activation by Xfz7 signaling
The precise molecular mechanisms of the membrane localization of Xdsh by Wnt/Xfz7 signaling are not known. To investigate this issue, we next tested whether PKCδ is required for the Xfz7-dependent membrane localization of Xdsh. PKCδ MO was coinjected with myc-tagged Xdsh and Xfz7 mRNAs, and localization of Xdsh to the animal cap cells was observed. As shown in Figure 5A, the coinjection of PKCδ MO blocked the membrane localization of Xdsh in response to Xfz7.
Figure 5.
PKCδ is required for the activation of Xdsh by Xfz7 signaling. (A) Twenty nanograms of PKCδ MO were coinjected with 100 pg of myc-tagged Xdsh and 500 pg of Xfz7 mRNAs, and the localization of Xdsh in animal cap explants was observed. The coinjection of PKCδ MO blocked the membrane localization of Xdsh by Xfz7. (B) Myc-tagged Xdsh and Xfz7 mRNAs were coinjected with PKCδ MO or PKCδΔC mRNA. Animal cap explants were isolated at stage 10, and their extracts were fractionated by SDS-PAGE. Myc-tagged Xdsh protein was detected by Western blotting using an anti-myc antibody. Two bands were detected in all four lanes for Xdsh-injected samples. (C) PKCδ is required for JNK activation by Xfz7. GAL4 DNA-binding domain (DBD)-tagged c-Jun mRNA was injected, and the phosphorylation levels of c-Jun were detected by Western blotting using anti-phosphorylated c-Jun (P-c-Jun) and anti-DBD antibodies (c-Jun). (D) Animal cap explants expressing myc-tagged Xdsh and Flag-tagged PKCδ were treated with PMA, and the localization of Xdsh and PKCδ was observed. Xdsh was translocated to the plasma membrane by PMA. (E) PMA can activate JNK in animal cap explants. Isolated explants were treated with or without 1 μM PMA for 1 h. The JNK activity was detected by an anti-phospho-c-Jun antibody.
Xdsh is a phosphoprotein whose phosphorylated state is elevated (hyperphosphorylated) upon the activation of the noncanonical Wnt signaling pathway (Yanagawa et al. 1995; Willert et al. 1997; Rothbacher et al. 2000; Tada and Smith 2000). This increase in phosphorylation can be monitored by a mobility shift of the Xdsh protein in SDS polyacrylamide gel electrophoresis (SDS-PAGE). To test whether PKCδ affects the phosphorylation state of Xdsh, myc-tagged Xdsh mRNA was coinjected with PKCδ MO or PKCδΔC mRNA into four-cell embryos. Animal cap explants were isolated at around stage 10, and their extracts were subjected to SDS-PAGE (Fig. 5B). The myc-tagged Xdsh protein was detected by Western blotting using an anti-myc antibody. Two bands were detected in the Xdsh-injected samples. In the absence of Xfz7, the lower band was more intense than the upper band. When Xfz7 was coinjected, the upper band became much more intense. This indicated that Xdsh was hyperphosphorylated by Xfz7 signaling. The coinjection of PKCδΔC or PKCδ MO blocked this hyperphosphorylation of Xdsh. These results indicated that PKCδ is required for both the membrane localization and the phosphorylation of Xdsh, suggesting that PKCδ is essential for the signaling from Xfz7 to Xdsh.
If the Xdsh function requires PKCδ activity, the activation of JNK in the noncanonical Wnt pathway should be blocked by the loss of PKCδ function. To examine this possibility, we assayed the JNK activity. GAL4 DNA-binding domain (DBD)-tagged c-Jun mRNA was injected into Xenopus embryos, and the phosphorylation level of c-Jun was assessed by Western blotting using anti-phosphorylated-c-Jun and anti-DBD antibodies. As shown in Figure 5C, the overexpression of PKCδ or Xfz7 alone slightly activated the JNK activity. However, the activity was greatly enhanced by the coexpression of Xfz7 and PKCδ. Moreover, PKCδ MO blocked the activation of JNK by Xfz7. These results indicated that PKCδ is required for the activation of JNK by Xfz7 signaling.
Activation of PKCδ is sufficient for Xdsh translocation and for activation of the JNK pathway
As described above, PKCδ and Xdsh form a complex, and both are translocated to the plasma membrane upon the activation of the noncanonical Wnt pathway. We postulated that PKCδ recruits Xdsh to the membrane in this process. If this is true, the activation of PKCδ might be sufficient for the translocation of Xdsh. To test this possibility, we injected RNAs encoding Flag-tagged PKCδ1 and myc-tagged Xdsh into Xenopus embryos, and the animal caps were explanted and treated with PMA. PMA is a functional analog of DAG that activates PKC on the membrane by binding to the C1 domain. As shown in Figure 5D, both PKCδ and Xdsh were translocated to the plasma membrane by PMA within 15 min. In addition, PMA treatment activated the JNK in the animal cap explants (Fig. 5E). Thus, PKCδ activation is sufficient for Xdsh translocation and the activation of downstream signaling.
The PKCδ loss-of-function phenotype is partially rescued by the overexpression of active MKK7
We have shown that PKCδ is required for the activation of JNK by Xfz7. If the gastrulation-defective phenotype caused by PKCδ MO is due to the blockade of JNK activation, it might be rescued by the overexpression of MKK7, which is known to activate JNK directly. To examine this possibility, we coinjected PKCδ Mo with constitutively active (CA) MKK7 (Yamanaka et al. 2002). Closure of the blastopore of the injected embryos was compared at stage 14, which is when the blastopore in the control embryos was completely closed. PKCδ MO blocked the gastrulation movement in more than 90% of the injected embryos (Fig. 6A). PKCδ and CA MKK7 mRNAs rescued the phenotype completely and partially, respectively, suggesting that MKK7/JNK functions at least in part downstream of PKCδ. This result supported the idea that Xfz7 regulates JNK activity through PKCδ. At the tadpole stage, the embryos rescued by MKK7 showed short trunks and defects in head formation (Fig. 6B). This partial rescue suggests that PKCδ may act not only through the JNK pathway, but in other pathways as well.
Figure 6.
PKCδ loss-of-function is rescued by overexpression of active MKK7. (A) Twenty nanograms of PKCδ MO were coinjected with 1 ng of PKCδ1, and 200 pg of constitutively active (CA) MKK7. The closure of the blastopore of injected embryos was compared at stage 14, when the blastopore in the control embryos was completely closed. PKCδ MO blocked gastrulation movement. PKCδ mRNA rescued the phenotype completely, and CA MKK7 mRNA partially rescued it. (B) At the tadpole stage, embryos coinjected with CA MKK7 showed a partially rescued phenotype. (C) To test whether PKCδ is essential for the canonical Wnt pathway, 100 pg of PKCδΔC or 20 ng of PKCδ MO was coinjected with 10 pg of Xwnt8 into Xenopus embryos. PKCδΔC and PKCδ MO did not inhibit the induction of siamois or Xnr3, indicating that PKCδ does not affect the canonical Wnt signaling pathway.
PKCδ is not essential for the canonical Wnt pathway
We next tested whether PKCδ plays a role in the canonical Wnt pathway. It is known that the ectopic expression of Xwnt8 induces secondary axis formation (Smith and Harland 1991) and marker genes such as siamois and Xnr3 by activating the canonical Wnt pathway in Xenopus embryos (Brannon and Kimelman 1996; Carnac et al. 1996; McKendry et al. 1997). When Xwnt8 was coinjected with PKCδΔC or PKCδ MO, the induction of siamois and Xnr3 by Xwnt8 was not inhibited (Fig. 6C). Furthermore, the secondary axis formation by Xwnt8 was not inhibited by the loss of PKCδ function (data not shown). Therefore, although PKCδ is required for the Wnt/JNK pathway, it may not be necessary for the canonical Wnt pathway, which is independent of the membrane relocalization of Dishevelled (Yang-Snyder et al. 1996; Axelrod et al. 1998; Moriguchi et al. 1999; Rothbacher et al. 2000).
PKCδ regulates the cell shape and intercalative behavior of the mesodermal cells during convergent extension movements
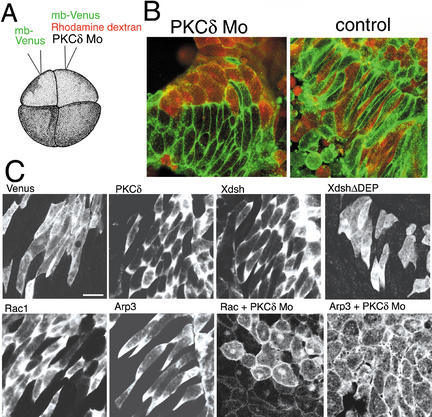
During convergent extension of the mesoderm, cells are polarized and aligned mediolaterally, then intercalated. To test whether PKCδ is required in this process, the convergent extension in DMZ explants was observed microscopically. The procedure was basically according to Wallingford et al. (2000). PKCδ MO, Rhodamine dextran, and mRNA for Venus (a YFP variant; Nagai et al. 2002) fused with the membrane localization signal of K-ras (mb-Venus) were coinjected into one of the two dorsal blastomeres at the four-cell stage. As a control, mb-Venus mRNA alone was injected into the other dorsal blastomere (Fig. 7A). At the gastrula stage, dorsal marginal zone explants were cultured on a cover glass coated with fibronectin. These explants adhered to the surface, and subsequently, convergent extension movements occurred in the mesoderm. In the absence of PKCδ MO, red cells and non-red cells intercalated (Fig. 7B). In the PKCδ MO-injected explants, the non-red cells, which were assumed to lack the MO, were polarized and showed convergent extension movements. In contrast, the red cells (MO-injected cells) were round-shaped, were not polarized, and did not participate in the intercalation, even when they were adjacent to the intercalating cells. Thus, this inhibition by PKCδ MOs appeared to be cell-autonomous. These results indicated that PKCδ is essential for the cell polarization during convergent extension movements.
Figure 7.
PKCδ is required for convergent extension movements. (A) PKCδ MO, Rhodamine dextran, and mRNA for Venus fused with a membrane localization signal (mb-Venus) were coinjected into one of the two dorsal blastomeres at the four-cell stage. As a control, mb-Venus mRNA alone was injected into the other dorsal blastomere of the same embryo. (B) At the gastrula stage, dorsal marginal zone (DMZ) explants were cut and cultured on a cover glass coated with fibronectin, and the convergent extension movements were observed by laser-scanning confocal microscopy. (C) The indicated cDNAs were fused to Venus and expressed in the dorsal mesodermal cells. DMZ explants were cultured and observed as described above. Bar, 50 μm.
To investigate the subcellular localization of PKCδ and Xdsh in the dorsal mesodermal cells, we expressed these proteins tagged with Venus. Interestingly, these were accumulated around the tips of the elongated cells (Fig. 7C). However, Xdsh lacking the DEP domain, which is known to play an important role in the tissue polarity (Axelrod et al. 1998) was almost uniformly distributed (XdshΔDEP). In addition, Rac tagged with Venus is also localized in the same region. The finding that Rac forms a complex with Xdsh (Habas et al. 2003) suggests that Rac may be recruited by Xdsh.
The process of convergent extension movements includes cell shape change and cell movements, suggesting that the regulation of actin polymerization may be crucial for the process. It is known that the Arp2/3 complex is a key component of the assembly of actin filaments and the cell motility (Suetsugu et al. 2002; Weaver et al. 2003). Thus, we tested the localization of the Arp3 tagged with Venus in these cells. As shown in Figure 7C, Arp3 is also localized around the tips of these cells. In addition, Rac and Arp3 were localized almost uniformly on the membrane or the cortical region when PKCδ MO was injected. The Arp2/3 complex may be recruited by PKCδ and its downstream PCP signaling and may regulate the cell polarity, bipolar protrusive activity, and cell motility in these cells.
Discussion
PKCδ is essential for Xdsh function in the noncanonical Wnt pathway
The membrane localization of Xdsh is thought to be an important step for Xdsh activation in the noncanonical Wnt pathway (Moriguchi et al. 1999; Rothbacher et al. 2000; Wallingford et al. 2000; Axelrod 2001). However, the molecular mechanism of this process has not been well understood. In the present study, we identified PKCδ in the Xenopus embryo and investigated its role in the Wnt/JNK pathway. These analyses revealed that PKCδ is essential for the translocation and activation of Xdsh in the Wnt/JNK pathway, based on the following findings: (1) PKCδ was translocated from the cytoplasm to the plasma membrane in response to Xfz7 signaling. (2) The loss of PKCδ function inhibited the membrane localization and hyperphosphorylation of Xdsh. (3) PKCδ was also essential for JNK activation by Xfz7. (4) PKCδ physically interacted with Xdsh. (5) The activation of PKCδ by PMA was sufficient for the translocation of Xdsh and the activation of the JNK pathway. Taking all these results together, we propose the following model of the Xdsh activation mechanism in the noncanonical Wnt/JNK pathway. In the absence of Wnt/Xfz7 signaling, Xdsh and PKCδ localize to the cytoplasm. It is likely that Xdsh and PKCδ form a complex even in the absence of Xfz7 signaling. This was suggested by our finding that the Xdsh-PKCδ complex formation was not dependent on Frizzled signaling or PKCδ activity. However, further investigations may be necessary to confirm this hypothesis. When the receptor is activated, the phosphatidylinositol pathway may be activated and produce the PKC activator, diacylglycerol (DAG). Then, PKCδ, which has a DAG-binding C1 domain, is translocated and recruits Xdsh to the membrane. PKCδ may be activated by DAG, resulting in the hyperphosphorylation of Xdsh. The membrane localization and/or phosphorylation of Xdsh activate the downstream JNK pathway.
How does Frizzled activate PKCδ?
Xenopus PKCδ has a highly conserved C1 domain, which binds to DAG and phorbol esters such as PMA, a functional analog of DAG. PKCδ was translocated to the plasma membrane in animal cap cells in response to both Xfz7 and PMA. These results and other observations suggested that Xfz7 might activate PKCδ through DAG on the plasma membrane, although there is no direct evidence that activation of the Wnt/Frizzled pathway produces DAG. However, heterotrimeric G proteins have been implicated in the Wnt/Frizzled pathway (Liu et al. 1999, 2001). It has been shown that certain heterotrimeric G proteins coupled with seven-transmembrane receptors activate phospholipase C-β, which hydrolyzes phosphatidylinositol phosphate to produce DAG and inositol triphosphate. In addition, Xfz7 function is blocked by pertussis toxin, which inhibits the Gi family (Sheldahl et al. 1999; Winkbauer et al. 2001). Taken together, these findings suggest that Xfz7 probably activates PKCδ through a heterotrimeric G protein that produces DAG. It will be important to determine which G protein is involved in this pathway and whether DAG is produced by G protein function.
Mechanism of activation of the Xdsh/JNK pathway
In this study, we showed that Xdsh and PKCδ form a complex and that the complex formation is not dependent on PKCδ activity. In addition, the activation of PKCδ is sufficient and necessary for the membrane localization of Xdsh in response to Xfz7. These findings suggest that Xfz7 may be involved in the translocation of the PKCδ–Xdsh complex to the plasma membrane through the production of DAG. In other words, PKCδ recruits Xdsh to the membrane in response to Xfz7 signaling. It will be necessary to determine which domain of Xdsh interacts with PKCδ and vice versa. Our preliminary work showed that a C-terminal fragment including the DEP domain of Xdsh coimmunoprecipitated with PKCδ as well as the full-length Xdsh protein (A. Miyakoshi, unpubl.). This is consistent with the fact that this domain of Dishevelled is sufficient for its membrane translocation and function in the PCP pathway (Axelrod et al. 1998; Boutros et al. 1998; Moriguchi et al. 1999; Rothbacher et al. 2000; Wallingford et al. 2000).
The Dishevelled protein is known to be hyperphosphorylated in response to Wnt and Frizzled (Yanagawa et al. 1995; Willert et al. 1997; Rothbacher et al. 2000; Tada and Smith 2000). We showed that the loss of PKCδ function blocked this phosphorylation of Xdsh. It has been shown that the phosphorylation and membrane localization of Xdsh are closely related (Rothbacher et al. 2000). The simplest model is that DAG activates PKCδ on the membrane, and PKCδ phosphorylates Xdsh directly. Kuhl et al. (2001) showed that PKCα phosphorylates Xdsh in vitro. PKCδ may have the similar activity. However, Dishevelled is known to interact with other kinases, such as casein kinases 1 and 2, Par-1, and PAK1/MuSK (Willert et al. 1997; Sun et al. 2001; Vielhaber and Virshup 2001; Luo et al. 2002). PKCδ may regulate such protein kinases and thus indirectly regulate Xdsh phosphorylation. It would be interesting to examine whether PKCδ phosphorylates Xdsh directly, and to elucidate the role of Xdsh phosphorylation in its localization and in the activation of downstream signaling. Determination of the sites in Xdsh that are phosphorylated by Xfz7 signaling awaits further study.
The following three results indicate that PKCδ mediates the activation of JNK by Xfz7: (1) JNK activation by Xfz7 was inhibited by the loss of PKCδ function. (2) The activation of PKCδ by PMA was sufficient for JNK activation. (3) The gastrulation-defective phenotype of PKCδ MO was rescued by active MKK7, which activates JNK. JNK has been implicated in the noncanonical Wnt pathway (Boutros et al. 1998; Yamanaka et al. 2002), but it is still unknown how Xdsh activates the JNK pathway. The membrane localization and/or phosphorylation of Xdsh may enable other proteins such as Rho to interact with Xdsh to activate the JNK cascade. It will be interesting and important to learn how JNK regulates convergent extension movements during gastrulation.
Specificity of PKC in the Wnt pathway
The PKC family is comprised of three subfamilies, cPKC, nPKC, and aPKC. cPKC and nPKC can be activated by DAG and/or Ca2+, both of which may increase upon activation of the Wnt signaling pathway. In addition to PKCδ, which belongs to the nPKC subfamily, two cPKCs, PKCα and PKCβ, are expressed during Xenopus gastrulation. We decided to focus on PKCδ for the following reason. Truncated PKCα, PKCβ, and PKCδ lacking the catalytic domain were constructed and overexpressed in Xenopus embryos, with the assumption that they would function as dominant-negative mutants. PKCδΔC seemed to function as a dominant-negative mutant because the phenotype of Xenopus embryos injected with PKCδΔC was very similar to that caused by the injection of PKCδ MO and was rescued by full-length PKCδ. Of these three mutants, PKCδΔC blocked gastrulation movements very effectively, whereas PKCαΔC or PKCβΔC had little effect, although comparable levels of protein expression were detected by Western blotting. We also made constructs with point mutations at the lysine residue in the ATP-binding domain, which are generally used as dominant-negative mutants for PKCs and other kinases (e.g., see Li et al. 1996). When these forms were overexpressed, the PKCα and PKCβ mutants had no effect on gastrulation (data not shown). These results indicated that PKCα and PKCβ might not play a crucial role in this process. Although PKCδ regulates the Wnt/JNK pathway, as we have shown, it is possible that PKCα and/or PKCβ may mainly be involved in the Wnt/Ca2+ pathway, although we cannot completely exclude the possibility that PKCα and/or PKCβ may also be involved in the Wnt/JNK pathway.
Role of PKCδ in gastrulation movements
Convergent extension is comprised of several steps involving changes in cell morphology and movements. As convergent extension begins, cells extend lamellipodia in random directions. The cells are then polarized and become narrow along the mediolateral direction, followed by the intercalation of these cells. Xdsh function is required for this regulation of cell polarity (Wallingford et al. 2000). We clearly showed that PKCδ MO-injected cells were not polarized, nor did they participate in the intercalation, indicating that PKCδ is essential for controlling cell polarity and the change in cell shape during convergent extension movements.
Interestingly, PKCδ and Xdsh tagged with Venus were localized around the tips of the elongated cells. In addition, Rac and Arp3 are also localized in the same regions. It is known that these regions have a lamellipodial protrusive activity. Arp3 is one of Arp2/3 complex components, which is a key regulator of the actin polymerization in lamellipodial protrusion of membranes (Suetsugu et al. 2002; Weaver et al. 2003). The Arp2/3 complex is also known to be regulated by Rac (Suetsugu et al. 2002). It is strongly suggested that the proper localization of Rac, Arp3, and other cytoskeletal regulators may be important for cell elongation and intercalative movements. Without PKCδ function, cells were round-shaped, and Rac and Arp3 did not localize in the specific region. This suggests that PKCδ, Xdsh and the downstream PCP signaling may be required for the localization of such machinery, including the Arp2/3 complex.
Materials and methods
Plasmids, RNA synthesis, and morpholino oligos
Our Xenopus EST database (NIBB XDB, http://Xenopus.nibb.ac.jp) was searched with the cDNA sequences of mammalian nPKC family members using BLAST. Full-length cDNA clones, XL011f02 and XL066d07, were identified. These clones were sequenced and designated PKCδ1 and PKCδ2, respectively. GenBank accession numbers are AB109739 and AB109740, respectively. Plasmids for the expression in Xenopus embryos were constructed with PCR products inserted into the expression vector pCS2+. Capped mRNAs were synthesized using the mMESSAGE mMACHINE kit (Ambion).
PKCδΔC contained the N-terminal regulatory domain (1–347 amino acids) of PKCδ1. A plasmid bearing the gene for constitutively active MKK7 (MKK7 DED) was a kind gift from Dr. E. Nishida (Kyoto University, Japan). The plasmid bearing the gene for GAL4(DBD)-tagged c-Jun was a kind gift from Dr. M. Tada (National Institute for Medical Research, UK). The myc-Xdsh was a kind gift from Dr. R. Harland (University of California, Berkeley). For the Venus-tagged constructs, the indicated fragments were amplified with PCR, fused to Venus gene (Nagai et al. 2002), and sequenced. For mb-Venus, a cDNA fragment of the C-terminal region (158–188 amino acids) of Xenopus K-ras (Baum and Bebernitz 1990) was cloned by PCR using the plasmid XL213p09 (NIBB XDB) as a template. XdshΔDEP contained the N-terminal region (1–426 amino acids). Xenopus Rac (GenBank accession no. AF174644) was cloned by PCR using neurula cDNA. Arp3 was cloned by PCR using the plasmid XL019o05 (NIBB XDB). PKCδ and Arp3 were fused to the N terminus of Venus, and Xdsh and XdshΔDEP were fused to the C terminus.
Antisense morpholinos were obtained from Gene Tools. The morpholino oligo sequences were as follows: PKCδ1 MO, 5′-AGGATATGCGTAGGAAGGAGACATG-3′; PKCδ2 MO, 5′-AGGATAAGCGTAGGAAAGGAGCCAT-3′; Control MO, 5′-CCTCTTACCTCAGTTACAATTTATA-3′.
In situ hybridization and RT–PCR analysis
In situ hybridization in Xenopus was carried out as described in Harland (1991). The detection of β-galactosidase activity for tracing cell lineage was carried out as described by Kurata and Ueno (2003). For RT–PCR analyses, RNA from Xenopus embryos was prepared with Trizol (Life Technologies). cDNA was synthesized with Reverse Transcriptase (#TRT-101, Toyobo). Sequences of the primers for Xbra, Xnr3, and Xotx2 were as described in Yamamoto et al. (2001) and those for chordin, goosecoid, siamois, and ODC were as described in Dr. De Robertis' home page (http://www.hhmi.ucla.edu/derobertis/index.html). Primers for Xmyf5 were 5′-CAGAATGGA GATGG TAGATAGC-3′ and 5′-AGCCTGGTTCACTTTCTT TAGC-3′; those for Xwnt11 were 5′-AAGT-GCCACGGAGT GTCT GG-3′ and 5′-CTCAGACTCTCTCACTGGCC-3′; and those for PKCδ were 5′-TTTATTAACCCCAAGATGGAGCG-3′ and 5′-AACTACATTCAAGTAACCAG-3′.
Whole-mount immunostaining and immunocytochemistry of Xenopus embryos
The procedure for whole-mount immunostaining was as described in Kurata et al. (2001). The antibodies were MZ15 for notochord (a kind gift from Dr. F. Watt; Smith and Watt 1985) and 12/101 for somites (Development Studies Hybridoma Bank; Kintner and Brockes 1984). As secondary antibodies, horseradish peroxidase-conjugated and alkaline phosphatase-conjugated antibodies were used for MZ15 and 12/101, respectively.
For immunocytochemistry, each epitope-tagged mRNA was injected into the animal pole of two-cell embryos. The animal caps were dissected from stage 9–10 embryos and fixed with MEMFA, followed by immunostaining by a standard method using a fluorescence-labeled secondary antibody. The localizations were determined by laser-scanning confocal microscopy, using a Carl Zeiss LSM510 microscope. The antibodies for immunocytochemistry were anti-myc 9E1 (Boehringer Mannheim) and rabbit polyclonal anti-Flag (Sigma) antibodies. For PMA treatment, phorbol 12–myristate 13–acetate (#P1585, Sigma) was used.
Elongation assay in Xenopus animal cap and DMZ explants
For the animal cap explants, mRNAs or a morpholino oligonucleotide were coinjected with 0.5 pg activin mRNA into the animal pole of two-cell embryos. The animal cap was dissected manually from stage 9 embryos. For DMZ explants, mRNA or a morpholino oligonucleotide were injected into the two dorsal blastomeres of four-cell embryos. Explants were isolated at stage 10+. These explants were cultured in 0.1% BSA/1× Steinberg's solution until sibling embryos reached stage 17. The procedure for observing cells during convergent extension movements was basically according to Wallingford et al. (2000) with some modifications. Explants were isolated at stage 10+ and cultured in 1× Steinberg's solution on a cover glass coated with fibronectin. The explants were observed by laser-scanning confocal microscopy.
Immunoprecipitation and Western blotting
HEK293T cells were transiently transfected with the indicated constructs using Lipofectamine Plus (Invitrogen). Cell lysates were prepared in PBS containing 0.1% Triton-X100, 20 mM NaF, 0.5 mM PMSF, and a 1/200 volume of protease inhibitor cocktail (#P8340, Sigma), and spun at 15,000g for 10 min. The indicated antibodies were added to the supernatants, and incubated at 4°C overnight. Protein A/G agarose (#SC-2003, Santa Cruz Biotechnology) was added, and the mixture was incubated for 1 h in a tumbling mixer. The agarose beads were washed five times with the lysis buffer. The antibodies used for immunoprecipitation and Western blotting were anti-myc 9E1 (Boehringer Mannheim), anti-Flag monoclonal M2 (Sigma), and anti-GFP (#598, Molecular Biological Laboratories) antibodies.
JNK assay
mRNA encoding GAL4 (DBD)-tagged c-Jun (100 pg) was injected into two-cell embryos. The animal caps were isolated at stage 10 and smashed by pipetting in sample buffer for SDS-PAGE. These samples were boiled and fractionated by SDS-PAGE. Western blotting was performed using anti-GAL4 (DBD; #SC-510, Santa Cruz Biotechnology) and anti-phospho-c-Jun (#9261S, Cell Signaling) antibodies.
Acknowledgments
We thank Dr. Eisuke Nishida for the MKK7 plasmid and helpful advice, Dr. Atsushi Miyawaki for the Venus plasmid, Dr. Masazumi Tada for the c-Jun plasmid and protocol for the JNK assay, Dr. Richard Harland for the Xdsh plasmids, Dr. Fiona Watt for the MZ15 antibody, and Dr. Junko Miura for comments on the manuscript. We are grateful to all the members of Dr. Ueno's laboratory for their kind support. This work was supported by grants from the Ministry of Education, Science and Culture of Japan to N.U. and N.K.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1101303.
Corresponding author.
References
- Adler P.N. 2002. Planar signaling and morphogenesis in Drosophila. Dev. Cell 2: 525–535. [DOI] [PubMed] [Google Scholar]
- Axelrod J.D. 2001. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes & Dev. 15: 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J.D., Miller, J.R., Shulman, J.M., Moon, R.T., and Perrimon, N. 1998. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes & Dev. 12: 2610–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum E.Z. and Bebernitz, G.A. 1990. K-ras oncogene expression in Xenopus laevis. Oncogene 5: 763–767. [PubMed] [Google Scholar]
- Bell R.M. and Burns, D.J. 1991. Lipid activation of protein kinase C. J. Biol. Chem. 266: 4661–4664. [PubMed] [Google Scholar]
- Boutros M. and Mlodzik, M. 1999. Dishevelled: At the crossroads of divergent intracellular signaling pathways. Mech. Dev. 83: 27–37. [DOI] [PubMed] [Google Scholar]
- Boutros M., Paricio, N., Strutt, D.I., and Mlodzik, M. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94: 109–118. [DOI] [PubMed] [Google Scholar]
- Brannon M. and Kimelman, D. 1996. Activation of Siamois by the Wnt pathway. Dev. Biol. 180: 344–347. [DOI] [PubMed] [Google Scholar]
- Cadigan K.M. and Nusse, R. 1997. Wnt signaling: A common theme in animal development. Genes & Dev. 11: 3286–3305. [DOI] [PubMed] [Google Scholar]
- Carnac G., Kodjabachian, L., Gurdon, J.B., and Lemaire, P. 1996. The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development 122: 3055–3065. [DOI] [PubMed] [Google Scholar]
- Chen K.H., Peng, Z.G., Lavu, S., and Kung, H.F. 1988. Molecular cloning and sequence analysis of two distinct types of Xenopus laevis protein kinase C. Second Messengers Phosphoproteins 12: 251–260. [PubMed] [Google Scholar]
- Cohen E.D., Mariol, M.C., Wallace, R.M., Weyers, J., Kamberov, Y.G., Pradel, J., and Wilder, E.L. 2002. DWnt4 regulates cell movement and focal adhesion kinase during Drosophila ovarian morphogenesis. Dev. Cell 2: 437–448. [DOI] [PubMed] [Google Scholar]
- Djiane A., Riou, J., Umbhauer, M., Boucaut, J., and Shi, D. 2000. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development 127: 3091–3100. [DOI] [PubMed] [Google Scholar]
- Habas R., Dawid, I.B., and He, X. 2003. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes & Dev. 17: 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R.M. 1991. In situ hybridization: An improved whole-mount method for Xenopus embryos. Methods Cell Biol. 36: 685–695. [DOI] [PubMed] [Google Scholar]
- Heisenberg C.P., Tada, M., Rauch, G.J., Saude, L., Concha, M.L., Geisler, R., Stemple, D.L., Smith, J.C., and Wilson, S.W. 2000. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405: 76–81. [DOI] [PubMed] [Google Scholar]
- Kemp B.E., Parker, M.W., Hu, S., Tiganis, T., and House, C. 1994. Substrate and pseudosubstrate interactions with protein kinases: Determinants of specificity. Trends Biochem. Sci. 19: 440–444. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Kishimoto, A., and Nishizuka, Y. 1989. The protein kinase C family: Heterogeneity and its implications. Annu. Rev. Biochem. 58: 31–44. [DOI] [PubMed] [Google Scholar]
- Kintner C. and Brockes, J. P. 1984. Monoclonal antibodies identify blastemal cells derived from differentiating muscle in newt limb regeneration. Nature 308: 67–69. [DOI] [PubMed] [Google Scholar]
- Kuhl M. 2002. Noncanonical Wnt signaling in Xenopus: Regulation of axis formation and gastrulation. Semin. Cell Dev. Biol. 13: 243–249. [DOI] [PubMed] [Google Scholar]
- Kuhl M., Sheldahl, L.C., Park, M., Miller, J.R., and Moon, R.T. 2000. The Wnt/Ca2+ pathway: A new vertebrate Wnt signaling pathway takes shape. Trends Genet. 16: 279–283. [DOI] [PubMed] [Google Scholar]
- Kuhl M., Geis, K., Sheldahl, L.C., Pukrop, T., Moon, R.T., and Wedlich, D. 2001. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/β-catenin and Wnt/Ca2+ signaling. Mech. Dev. 106: 61–76. [DOI] [PubMed] [Google Scholar]
- Kurata T. and Ueno, N. 2003. Xenopus Nbx, a novel NK-1 related gene essential for neural crest formation. Dev. Biol. 257: 30–40. [DOI] [PubMed] [Google Scholar]
- Kurata T., Nakabayashi, J., Yamamoto, T.S., Mochii, M., and Ueno, N. 2001. Visualization of endogenous BMP signaling during Xenopus development. Differentiation 67: 33–40. [DOI] [PubMed] [Google Scholar]
- Li W., Michieli, P., Alimandi, M., Lorenzi, M.V., Wu, Y., Wang, L.H., Heidaran, M.A., and Pierce, J.H. 1996. Expression of an ATP binding mutant of PKC-δ inhibits Sis-induced transformation of NIH3T3 cells. Oncogene 13: 731–737. [PubMed] [Google Scholar]
- Liu T., DeCostanzo, A.J., Liu, X., Wang, H., Hallagan, S., Moon, R.T., and Malbon, C.C. 2001. G protein signaling from activated rat frizzled-1 to the β-catenin–Lef–Tcf pathway. Science 292: 1718–1722. [DOI] [PubMed] [Google Scholar]
- Liu X., Liu, T., Slusarski, D.C., Yang-Snyder, J., Malbon, C.C., Moon, R.T., and Wang, H. 1999. Activation of a frizzled-2/β-adrenergic receptor chimera promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via Galphao and Galphat. Proc. Natl. Acad. Sci. 96: 14383–14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z.G., Wang, Q., Zhou, J.Z., Wang, J., Luo, Z., Liu, M., He, X., Wynshaw-Boris, A., Xiong, W.C., Lu, B., et al. 2002. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron 35: 489–505. [DOI] [PubMed] [Google Scholar]
- McKendry R., Hsu, S.C., Harland, R.M., and Grosschedl, R. 1997. LEF-1/TCF proteins mediate wnt-inducible transcription from the Xenopus nodal-related 3 promoter. Dev. Biol. 192: 420–431. [DOI] [PubMed] [Google Scholar]
- Medina A. and Steinbeisser, H. 2000. Interaction of Frizzled 7 and Dishevelled in Xenopus. Dev. Dyn. 218: 671–680. [DOI] [PubMed] [Google Scholar]
- Medina A., Reintsch, W., and Steinbeisser, H. 2000. Xenopus frizzled 7 can act in canonical and noncanonical Wnt signaling pathways: Implications on early patterning and morphogenesis. Mech. Dev. 92: 227–237. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Kawachi, K., Kamakura, S., Masuyama, N., Yamanaka, H., Matsumoto, K., Kikuchi, A., and Nishida, E. 1999. Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J. Biol. Chem. 274: 30957–30962. [DOI] [PubMed] [Google Scholar]
- Nagai T., Ibata, K., Park, E.S., Kubota, M., Mikoshiba, K., and Miyawaki, A. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20: 87–90. [DOI] [PubMed] [Google Scholar]
- Newton A.C. 1997. Regulation of protein kinase C. Curr. Opin. Cell Biol. 9: 161–167. [DOI] [PubMed] [Google Scholar]
- Nieukoop P.D. and Faber, J. 1994. Normal Table of Xenopus laevis (Daudin), pp. 163–188. Garland Publishing, Inc., New York.
- Nishizuka Y. 1995. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 9: 484–496. [PubMed] [Google Scholar]
- Orr J.W. and Newton, A.C. 1994. Intrapeptide regulation of protein kinase C. J. Biol. Chem. 269: 8383–8387. [PubMed] [Google Scholar]
- Otte A.P. and Moon, R.T. 1992. Protein kinase C isozymes have distinct roles in neural induction and competence in Xenopus. Cell 68: 1021–1029. [DOI] [PubMed] [Google Scholar]
- Otte A.P., Koster, C.H., Snoek, G.T., and Durston, A.J. 1988. Protein kinase C mediates neural induction in Xenopus laevis. Nature 334: 618–620. [DOI] [PubMed] [Google Scholar]
- Pandur P., Lasche, M., Eisenberg, L.M., and Kuhl, M. 2002. Wnt-11 activation of a noncanonical Wnt signalling pathway is required for cardiogenesis. Nature 418: 636–641. [DOI] [PubMed] [Google Scholar]
- Rauch G.J., Hammerschmidt, M., Blader, P., Schauerte, H.E., Strahle, U., Ingham, P.W., McMahon, A.P., and Haffter, P. 1997. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb. Symp. Quant. Biol. 62: 227–234. [PubMed] [Google Scholar]
- Rothbacher U., Laurent, M.N., Deardorff, M.A., Klein, P.S., Cho, K.W., and Fraser, S.E. 2000. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 19: 1010–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl L.C., Park, M., Malbon, C.C., and Moon, R.T. 1999. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr. Biol. 9: 695–698. [DOI] [PubMed] [Google Scholar]
- Shih J. and Keller, R. 1992. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development 116: 901–914. [DOI] [PubMed] [Google Scholar]
- Slusarski D.C., Corces, V.G., and Moon, R.T. 1997a. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390: 410–413. [DOI] [PubMed] [Google Scholar]
- Slusarski D.C., Yang-Snyder, J., Busa, W.B., and Moon, R.T. 1997b. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 182: 114–120. [DOI] [PubMed] [Google Scholar]
- Smith J.C. and Watt, F.M. 1985. Biochemical specificity of Xenopus notochord. Differentiation 29: 109–115. [DOI] [PubMed] [Google Scholar]
- Smith W.C. and Harland, R.M. 1991. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 67: 753–765. [DOI] [PubMed] [Google Scholar]
- Suetsugu S., Miki, H., and Takenawa, T. 2002. Spatial and temporal regulation of actin polymerization for cytoskeleton formation through Arp2/3 complex and WASP/WAVE proteins. Cell Motil. Cytoskeleton 51: 113–122. [DOI] [PubMed] [Google Scholar]
- Sun T.Q., Lu, B., Feng, J.J., Reinhard, C., Jan, Y.N., Fantl, W.J., and Williams, L.T. 2001. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat. Cell Biol. 3: 628–636. [DOI] [PubMed] [Google Scholar]
- Tada M. and Smith, J.C. 2000. Xwnt11 is a target of Xenopus Brachyury: Regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127: 2227–2238. [DOI] [PubMed] [Google Scholar]
- Tada M., Concha, M.L., and Heisenberg, C.P. 2002. Noncanonical Wnt signalling and regulation of gastrulation movements. Semin. Cell Dev. Biol. 13: 251–260. [DOI] [PubMed] [Google Scholar]
- Vielhaber E. and Virshup, D.M. 2001. Casein kinase I: From obscurity to center stage. IUBMB Life 51: 73–78. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B., Rowning, B.A., Vogeli, K.M., Rothbacher, U., Fraser, S.E., and Harland, R.M. 2000. Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405: 81–85. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B., Fraser, S.E., and Harland, R.M. 2002. Convergent extension: The molecular control of polarized cell movement during embryonic development. Dev. Cell 2: 695–706. [DOI] [PubMed] [Google Scholar]
- Weaver A.M., Young, M.E., Lee, W.-L., and Cooper, J.A. 2003. Integration of signals to the Arp2/3 complex. Curr. Op. Cell Biol. 15: 23–30. [DOI] [PubMed] [Google Scholar]
- Wharton K.A. 2003. Runnin' with the Dvl: Proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 253: 1–17. [DOI] [PubMed] [Google Scholar]
- Willert K., Brink, M., Wodarz, A., Varmus, H., and Nusse, R. 1997. Casein kinase 2 associates with and phosphorylates dishevelled. EMBO J. 16: 3089–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. and Keller, R. 1991. Cell rearrangement during gastrulation of Xenopus: Direct observation of cultured explants. Development 112: 289–300. [DOI] [PubMed] [Google Scholar]
- Winklbauer R., Medina, A., Swain, R.K., and Steinbeisser, H. 2001. Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature 413: 856–860. [DOI] [PubMed] [Google Scholar]
- Yamamoto T.S., Takagi, C., Hyodo, A.C., and Ueno, N. 2001. Suppression of head formation by Xmsx-1 through the inhibition of intracellular nodal signaling. Development 128: 2769–2779. [DOI] [PubMed] [Google Scholar]
- Yamanaka H., Moriguchi, T., Masuyama, N., Kusakabe, M., Hanafusa, H., Takada, R., Takada, S., and Nishida, E. 2002. JNK functions in the noncanonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 3: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S., van Leeuwen, F., Wodarz, A., Klingensmith, J., and Nusse, R. 1995. The dishevelled protein is modified by wingless signaling in Drosophila. Genes & Dev. 9: 1087–1097. [DOI] [PubMed] [Google Scholar]
- Yang-Snyder J., Miller, J.R., Brown, J.D., Lai, C.J., and Moon, R.T. 1996. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr. Biol. 6: 1302–1306. [DOI] [PubMed] [Google Scholar]
- Zhang G., Kazanietz, M.G., Blumberg, P.M., and Hurley, J.H. 1995. Crystal structure of the cys2 activator-binding domain of protein kinase C δ in complex with phorbol ester. Cell 81: 917–924. [DOI] [PubMed] [Google Scholar]