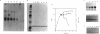Abstract
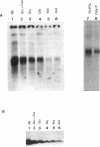
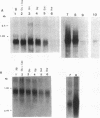
Acetyl coenzyme A (CoA) carboxylase catalyzes the synthesis of malonyl-CoA, the first intermediate of fatty acid synthesis. The Escherichia coli enzyme is encoded by four subunits located at three different positions on the E. coli chromosome. The accBC genes lie in a small operon at min 72, whereas accA and accD are located at min 4.3 and 50, respectively. We examined the expression of the genes that encode the E. coli acetyl-CoA carboxylase subunits (accA, accBC, and accD) under a variety of growth conditions by quantitative Northern (RNA) blot analysis. We found a direct correlation between the levels of transcription of the acc genes and the rate of cellular growth. Consistent results were also obtained upon nutritional upshift and downshift experiments and upon dilution of stationary-phase cultures into fresh media. We also determined the 5' end of the accA and accD mRNAs by primer extension and did transcriptional fusion analysis of the previously reported accBC promoter. Several interesting features were found in the promoter regions of these genes, including a bent DNA sequence and an open reading frame within the unusually long leader mRNA of the accBC operon, potential stem-loop structures in the accA and accD mRNA leader regions, and a stretch of GC-rich sequences followed by AT-rich sequences common to all three promoters. In addition, both accA and accD are located in complex gene clusters. For example, the accA promoter was localized within the upstream polC gene (which encodes the DNA polymerase III catalytic subunit), suggesting that additional regulatory mechanisms exist.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arps P. J., Winkler M. E. Structural analysis of the Escherichia coli K-12 hisT operon by using a kanamycin resistance cassette. J Bacteriol. 1987 Mar;169(3):1061–1070. doi: 10.1128/jb.169.3.1061-1070.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. F., Campbell A. M. The birA gene of Escherichia coli encodes a biotin holoenzyme synthetase. J Mol Biol. 1981 Mar 15;146(4):451–467. doi: 10.1016/0022-2836(81)90042-5. [DOI] [PubMed] [Google Scholar]
- Bognar A. L., Osborne C., Shane B. Primary structure of the Escherichia coli folC gene and its folylpolyglutamate synthetase-dihydrofolate synthetase product and regulation of expression by an upstream gene. J Biol Chem. 1987 Sep 5;262(25):12337–12343. [PubMed] [Google Scholar]
- Bognar A., Pyne C., Yu M., Basi G. Transcription of the folC gene encoding folylpolyglutamate synthetase-dihydrofolate synthetase in Escherichia coli. J Bacteriol. 1989 Apr;171(4):1854–1861. doi: 10.1128/jb.171.4.1854-1861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton Z. F., Gross C. A., Watanabe K. K., Burgess R. R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell. 1983 Feb;32(2):335–349. doi: 10.1016/0092-8674(83)90453-1. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Magasanik B. Transcription of the lac operon of Escherichia coli. J Biol Chem. 1974 Oct 25;249(20):6556–6561. [PubMed] [Google Scholar]
- Cooper T. G., Whitney P., Magasanik B. Reaction of lac-specific ribonucleic acid from Escherichia coli with lac deoxyribonucleic acid. J Biol Chem. 1974 Oct 25;249(20):6548–6555. [PubMed] [Google Scholar]
- Cronan J. E., Jr The E. coli bio operon: transcriptional repression by an essential protein modification enzyme. Cell. 1989 Aug 11;58(3):427–429. doi: 10.1016/0092-8674(89)90421-2. [DOI] [PubMed] [Google Scholar]
- Crowell D. N., Reznikoff W. S., Raetz C. R. Nucleotide sequence of the Escherichia coli gene for lipid A disaccharide synthase. J Bacteriol. 1987 Dec;169(12):5727–5734. doi: 10.1128/jb.169.12.5727-5734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall R. R., Vagelos P. R. Acetyl coenzyme A carboxylase. Molecular forms and subunit composition of biotin carboxyl carrier protein. J Biol Chem. 1972 Dec 25;247(24):8005–8015. [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Guchhait R. B., Polakis S. E., Dimroth P., Stoll E., Moss J., Lane M. D. Acetyl coenzyme A carboxylase system of Escherichia coli. Purification and properties of the biotin carboxylase, carboxyltransferase, and carboxyl carrier protein components. J Biol Chem. 1974 Oct 25;249(20):6633–6645. [PubMed] [Google Scholar]
- Henry M. F., Cronan J. E., Jr A facile and reversible method to decrease the copy number of the ColE1-related cloning vectors commonly used in Escherichia coli. J Bacteriol. 1989 Oct;171(10):5254–5261. doi: 10.1128/jb.171.10.5254-5261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H. Regulation of acetyl-CoA carboxylase. Curr Top Cell Regul. 1983;22:143–176. doi: 10.1016/b978-0-12-152822-5.50009-9. [DOI] [PubMed] [Google Scholar]
- Kornblum J. S., Projan S. J., Moghazeh S. L., Novick R. P. A rapid method to quantitate non-labeled RNA species in bacterial cells. Gene. 1988;63(1):75–85. doi: 10.1016/0378-1119(88)90547-1. [DOI] [PubMed] [Google Scholar]
- Li S. J., Cronan J. E., Jr The gene encoding the biotin carboxylase subunit of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992 Jan 15;267(2):855–863. [PubMed] [Google Scholar]
- Li S. J., Cronan J. E., Jr The genes encoding the two carboxyltransferase subunits of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992 Aug 25;267(24):16841–16847. [PubMed] [Google Scholar]
- Li S. J., Rock C. O., Cronan J. E., Jr The dedB (usg) open reading frame of Escherichia coli encodes a subunit of acetyl-coenzyme A carboxylase. J Bacteriol. 1992 Sep;174(17):5755–5757. doi: 10.1128/jb.174.17.5755-5757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. C., Kim K. H. An enhancer element in the house-keeping promoter for acetyl-CoA carboxylase gene. Nucleic Acids Res. 1990 Jun 11;18(11):3249–3254. doi: 10.1093/nar/18.11.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Casillas F., Pape M. E., Bai D. H., Kuhn D. N., Dixon J. E., Kim K. H. Preparation of functional acetyl-CoA carboxylase mRNA from rat mammary gland. Arch Biochem Biophys. 1987 Aug 15;257(1):63–68. doi: 10.1016/0003-9861(87)90543-1. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Muramatsu S., Mizuno T. Nucleotide sequence of the fabE gene and flanking regions containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 1989 May 25;17(10):3982–3982. doi: 10.1093/nar/17.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa J., Tanabe T., Ogiwara H., Shiba T., Numa S. Inhibitory effects of long-chain acyl coenzyme A analogues on rat liver acetyl coenzyme A carboxylase. FEBS Lett. 1979 Jun 15;102(2):223–226. doi: 10.1016/0014-5793(79)80005-8. [DOI] [PubMed] [Google Scholar]
- Nonet M. L., Marvel C. C., Tolan D. R. The hisT-purF region of the Escherichia coli K-12 chromosome. Identification of additional genes of the hisT and purF operons. J Biol Chem. 1987 Sep 5;262(25):12209–12217. [PubMed] [Google Scholar]
- Polakis S. E., Guchhait R. B., Zwergel E. E., Lane M. D., Cooper T. G. Acetyl coenzyme A carboxylase system of Escherichia coli. Studies on the mechanisms of the biotin carboxylase- and carboxyltransferase-catalyzed reactions. J Biol Chem. 1974 Oct 25;249(20):6657–6667. [PubMed] [Google Scholar]
- Rao N. N., Torriani A. Utilization by Escherichia coli of a high-molecular-weight, linear polyphosphate: roles of phosphatases and pore proteins. J Bacteriol. 1988 Nov;170(11):5216–5223. doi: 10.1128/jb.170.11.5216-5223.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Muramatsu S., Yamada H., Mizuno T. Systematic characterization of curved DNA segments randomly cloned from Escherichia coli and their functional significance. Mol Gen Genet. 1991 May;226(3):367–376. doi: 10.1007/BF00260648. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz H. G., McHenry C. S. Sequence analysis of the Escherichia coli dnaE gene. J Bacteriol. 1987 Dec;169(12):5735–5744. doi: 10.1128/jb.169.12.5735-5744.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Mechanisms and regulation of biosynthesis of saturated fatty acids. Physiol Rev. 1976 Apr;56(2):339–417. doi: 10.1152/physrev.1976.56.2.339. [DOI] [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K., Joshi V. C. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Physiological regulation of a decontrolled lac operon. J Bacteriol. 1977 Apr;130(1):212–222. doi: 10.1128/jb.130.1.212-222.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]