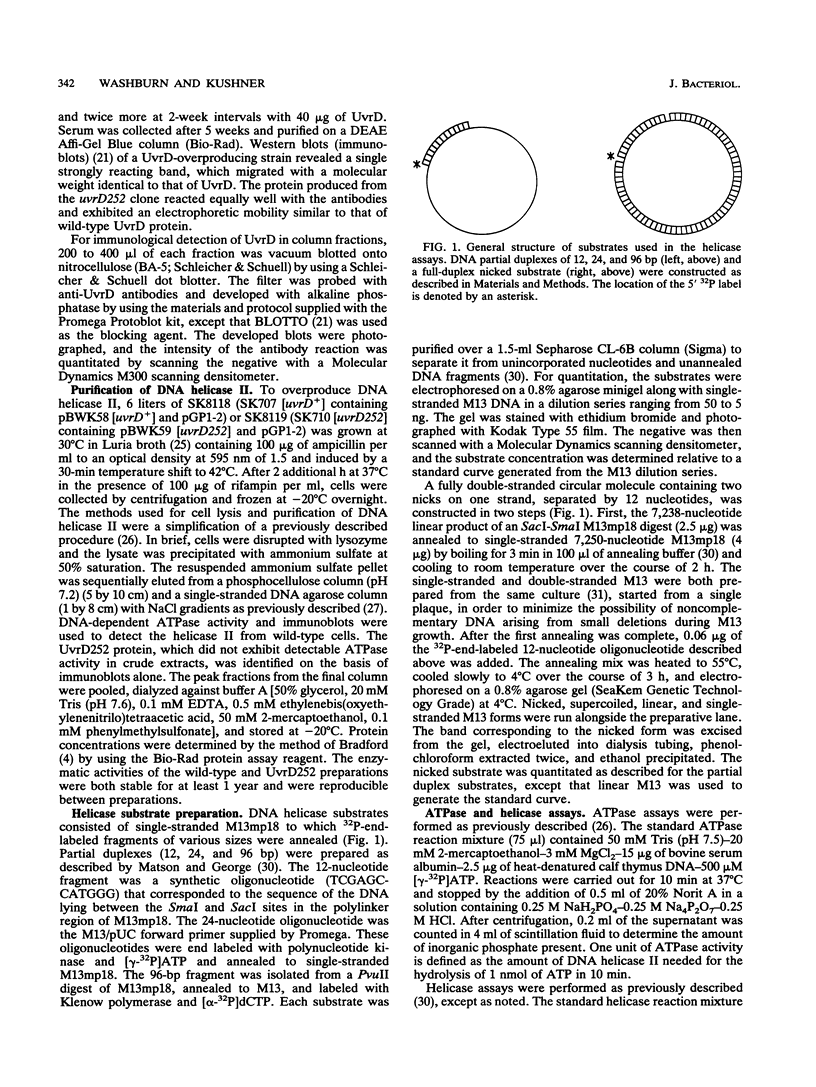
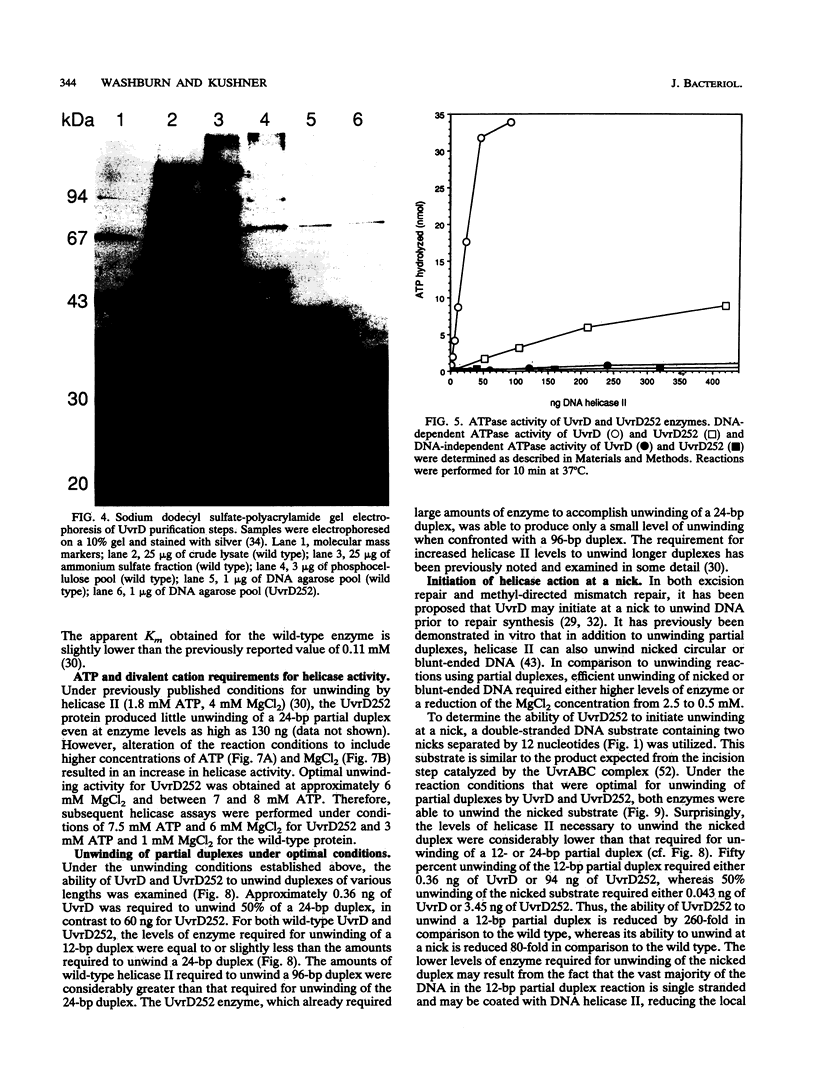
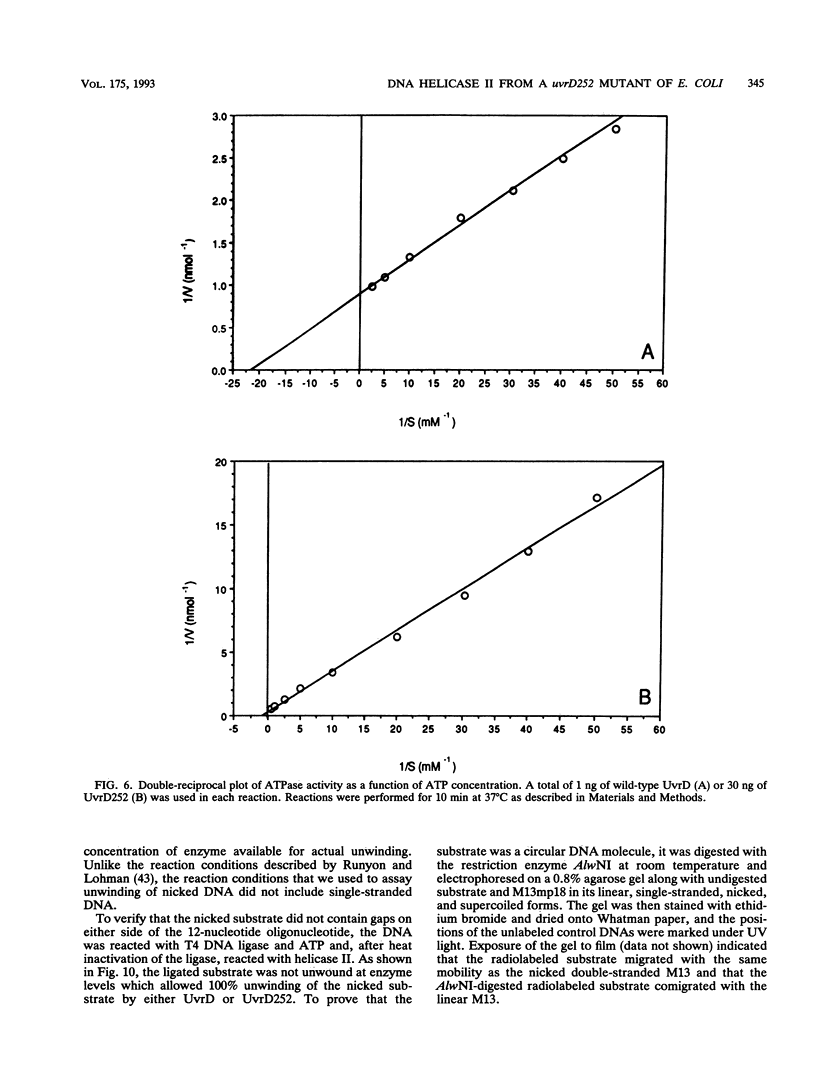
Abstract
The loss of DNA helicase II (UvrD) in Escherichia coli results in sensitivity to UV light and increased levels of spontaneous mutagenesis. While the effects of various uvrD alleles have been analyzed in vivo, the proteins produced by these alleles have not been examined in any detail. We have cloned one of these alleles, uvrD252, and determined the site of the mutation conferring the phenotype. In addition, the protein it encodes has been purified to homogeneity and characterized in vitro. The mutation responsible for the phenotype was identified as a glycine-to-aspartic-acid change in the putative ATP-binding domain. In comparison to wild-type DNA helicase II, the UvrD252 enzyme exhibited reduced levels of ATPase activity and a large increase in the Km for ATP. The ability of UvrD252 to unwind DNA containing single-stranded regions, as well as DNA containing only nicks, was reduced in comparison to that of the wild-type enzyme. Possible interpretations of these results in relation to the phenotypes of the uvrD252 mutant are discussed. This represents the first detailed analysis of the biochemical properties of a mutant DNA helicase II protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M., Dürwald H., Hoffmann-Berling H. DNA unwinding enzyme II of Escherichia coli. 2. Characterization of the DNA unwinding activity. Eur J Biochem. 1977 Sep 15;79(1):39–45. doi: 10.1111/j.1432-1033.1977.tb11781.x. [DOI] [PubMed] [Google Scholar]
- Arthur H. M., Lloyd R. G. Hyper-recombination in uvrD mutants of Escherichia coli K-12. Mol Gen Genet. 1980;180(1):185–191. doi: 10.1007/BF00267368. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brennan R. G., Matthews B. W. The helix-turn-helix DNA binding motif. J Biol Chem. 1989 Feb 5;264(4):1903–1906. [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron P. R., Kushner S. R., Grossman L. Involvement of helicase II (uvrD gene product) and DNA polymerase I in excision mediated by the uvrABC protein complex. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4925–4929. doi: 10.1073/pnas.82.15.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A., Eisenthal R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot andother methods. Biochem J. 1974 Jun;139(3):721–730. doi: 10.1042/bj1390721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein S. I., Low K. B. Hyper-recombining recipient strains in bacterial conjugation. Genetics. 1986 May;113(1):13–33. doi: 10.1093/genetics/113.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Emmerson P. T. The nucleotide sequence of the uvrD gene of E. coli. Nucleic Acids Res. 1984 Jul 25;12(14):5789–5799. doi: 10.1093/nar/12.14.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi-Geisberger P., Hoffmann-Berling H. Direction of the DNA-unwinding reaction catalysed by Escherichia coli DNA helicase II. Eur J Biochem. 1990 Sep 24;192(3):689–693. doi: 10.1111/j.1432-1033.1990.tb19277.x. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V. Superfamily of UvrA-related NTP-binding proteins. Implications for rational classification of recombination/repair systems. J Mol Biol. 1990 Jun 20;213(4):583–591. doi: 10.1016/S0022-2836(05)80243-8. [DOI] [PubMed] [Google Scholar]
- Hamilton C. M., Aldea M., Washburn B. K., Babitzke P., Kushner S. R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989 Sep;171(9):4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgman T. C. A new superfamily of replicative proteins. Nature. 1988 May 5;333(6168):22–23. doi: 10.1038/333022b0. [DOI] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Abdel-Monem M., Sancar A. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6774–6778. doi: 10.1073/pnas.82.20.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Klinkert M. Q., Klein A., Abdel-Monem M. Studies on the functions of DNA helicase I and DNA helicase II of Escherichia coli. J Biol Chem. 1980 Oct 25;255(20):9746–9752. [PubMed] [Google Scholar]
- Kuemmerle N. B., Masker W. E. Effect of the uvrD mutation on excision repair. J Bacteriol. 1980 May;142(2):535–546. doi: 10.1128/jb.142.2.535-546.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B., Abdel-Monem M. DNA synthesis at a fork in the presence of DNA helicases. Eur J Biochem. 1982 Jun 15;125(1):63–68. doi: 10.1111/j.1432-1033.1982.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Kushner S. R. In vivo studies of temperature-sensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1213–1218. doi: 10.1128/jb.120.3.1213-1218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson S. W. Escherichia coli helicase II (urvD gene product) translocates unidirectionally in a 3' to 5' direction. J Biol Chem. 1986 Aug 5;261(22):10169–10175. [PubMed] [Google Scholar]
- Matson S. W., George J. W. DNA helicase II of Escherichia coli. Characterization of the single-stranded DNA-dependent NTPase and helicase activities. J Biol Chem. 1987 Feb 15;262(5):2066–2076. [PubMed] [Google Scholar]
- Modrich P. Mechanisms and biological effects of mismatch repair. Annu Rev Genet. 1991;25:229–253. doi: 10.1146/annurev.ge.25.120191.001305. [DOI] [PubMed] [Google Scholar]
- Morrison J. F. Approaches to kinetic studies on metal-activated enzymes. Methods Enzymol. 1979;63:257–294. doi: 10.1016/0076-6879(79)63013-6. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nevers P., Spatz H. C. Escherichia coli mutants uvr D and uvr E deficient in gene conversion of lambda-heteroduplexes. Mol Gen Genet. 1975 Aug 27;139(3):233–243. doi: 10.1007/BF00268974. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Richet E., Nishimura Y., Hirota Y., Kohiyama M. Escherichia coli uvrD mutants with thermosensitive DNA-dependent adenosine triphosphatase I (helicase II). Mol Gen Genet. 1983;192(3):378–385. doi: 10.1007/BF00392178. [DOI] [PubMed] [Google Scholar]
- Rothman R. H. Dimer excision and repair replication patch size in recL152 mutant of Escherichia coli K-12. J Bacteriol. 1978 Oct;136(1):444–448. doi: 10.1128/jb.136.1.444-448.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman R. H., Fried B. Long repair replication patches are produced by the short-patch pathway in a uvrD252 (recL152) mutant of Escherichia coli K-12. J Bacteriol. 1984 May;158(2):749–753. doi: 10.1128/jb.158.2.749-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon G. T., Bear D. G., Lohman T. M. Escherichia coli helicase II (UvrD) protein initiates DNA unwinding at nicks and blunt ends. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6383–6387. doi: 10.1073/pnas.87.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon G. T., Lohman T. M. Escherichia coli helicase II (uvrD) protein can completely unwind fully duplex linear and nicked circular DNA. J Biol Chem. 1989 Oct 15;264(29):17502–17512. [PubMed] [Google Scholar]
- Seetharam S., Dicker I. B. A rapid and complete 4-step protocol for obtaining nucleotide sequence from E. coli genomic DNA from overnight cultures. Biotechniques. 1991 Jul;11(1):32–34. [PubMed] [Google Scholar]
- Siegel E. C., Race H. M. Phenotypes of UV-sensitive uvrD3, recL152, and uvrE15 mutants of Escherichia coli. Mutat Res. 1981 Aug;83(1):49–59. doi: 10.1016/0027-5107(81)90070-1. [DOI] [PubMed] [Google Scholar]
- Siegel E. C. The Escherichia coli uvrD gene is inducible by DNA damage. Mol Gen Genet. 1983;191(3):397–400. doi: 10.1007/BF00425753. [DOI] [PubMed] [Google Scholar]
- Siegel E. C., Wain S. L., Meltzer S. F., Binion M. L., Steinberg J. L. Mutator mutations in Escherichia coli induced by the insertion of phage mu and the transposable resistance elements Tn5 and Tn10. Mutat Res. 1982 Mar;93(1):25–33. doi: 10.1016/0027-5107(82)90122-1. [DOI] [PubMed] [Google Scholar]
- Sinzinis B. I., Smirnov G. B., Saenko A. A. Repair deficiency in Escherichia coli UV-sensitive mutator strain uvr502. Biochem Biophys Res Commun. 1973 Jul 2;53(1):309–316. doi: 10.1016/0006-291x(73)91435-6. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taucher-Scholz G., Hoffmann-Berling H. Identification of the gene for DNA helicase II of Escherichia coli. Eur J Biochem. 1983 Dec 15;137(3):573–580. doi: 10.1111/j.1432-1033.1983.tb07864.x. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Analysis of sequential steps of nucleotide excision repair in Escherichia coli using synthetic substrates containing single psoralen adducts. J Biol Chem. 1988 Nov 15;263(32):16553–16560. [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sluis C. A., Mattern I. E., Paterson M. C. Properties of uvrE mutants of Escherichia coli K12. I. Effects of UV irradiation on DNA metabolism. Mutat Res. 1974 Dec;25(3):273–279. doi: 10.1016/0027-5107(74)90055-4. [DOI] [PubMed] [Google Scholar]
- Wang R. F., Kushner S. R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991 Apr;100:195–199. [PubMed] [Google Scholar]
- Washburn B. K., Kushner S. R. Construction and analysis of deletions in the structural gene (uvrD) for DNA helicase II of Escherichia coli. J Bacteriol. 1991 Apr;173(8):2569–2575. doi: 10.1128/jb.173.8.2569-2575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Ogawa T., Shinagawa H., Nakayama T., Matsuo H., Ogawa H. Determination of the initiation sites of transcription and translation of the uvrD gene of Escherichia coli. J Biochem. 1986 Jun;99(6):1579–1590. doi: 10.1093/oxfordjournals.jbchem.a135631. [DOI] [PubMed] [Google Scholar]
- Yancey J. E., Matson S. W. The DNA unwinding reaction catalyzed by Rep protein is facilitated by an RHSP-DNA interaction. Nucleic Acids Res. 1991 Jul 25;19(14):3943–3951. doi: 10.1093/nar/19.14.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarranton G. T., Gefter M. L. Enzyme-catalyzed DNA unwinding: studies on Escherichia coli rep protein. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1658–1662. doi: 10.1073/pnas.76.4.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]