Abstract
G protein-gated, inwardly rectifying K+ channels (GIRK) are effectors of G protein-coupled receptors for neurotransmitters and hormones and may play an important role in the regulation of neuronal excitability. GIRK channels may be important in neurodevelopment, as suggested by the recent finding that a point mutation in the pore region of GIRK2 (G156S) is responsible for the weaver (wv) phenotype. The GIRK2 G156S gene gives rise to channels that exhibit a loss of K+ selectivity and may also exert dominant-negative effects on Gβγ-activated K+ currents. To investigate the physiological role of GIRK2, we generated mutant mice lacking GIRK2. Unlike wv/wv mutant mice, GIRK2 −/− mice are morphologically indistinguishable from wild-type mice, suggesting that the wv phenotype is likely due to abnormal GIRK2 function. Like wv/wv mice, GIRK2 −/− mice have much reduced GIRK1 expression in the brain. They also develop spontaneous seizures and are more susceptible to pharmacologically induced seizures using a γ-aminobutyric acid antagonist. Moreover, wv/− mice exhibit much milder cerebellar abnormalities than wv/wv mice, indicating a dosage effect of the GIRK2 G156S mutation. Our results indicate that the weaver phenotypes arise from a gain-of-function mutation of GIRK2 and that GIRK1 and GIRK2 are important mediators of neuronal excitability in vivo.
Keywords: GIRK1, embryonic stem cells, genetics, weaver, cerebellum
G protein-gated, inwardly rectifying K+ channels (GIRK) are regulated by neurotransmitters and hormones through G protein-coupled receptors (1–3). GIRK channels are believed to determine neuronal membrane excitability by selectively permitting the flux of K+ ions near the resting membrane potential (4–7). The weaver mouse, a neurological mutant characterized by extensive cerebellar granule cell death during development (8–10), age-dependent dopaminergic neuronal loss in the substantia nigra (11, 12), male infertility (13), and spontaneous seizures (14), carries a G156S point mutation in the pore-forming region H5 of GIRK2 (15). This mutation leads to a loss of K+ selectivity of homomeric GIRK2 channels and strongly reduces heteromeric GIRK1/GIRK2 channel function (16–18). Electrophysiological recordings from weaver and wild-type cerebellar granular cells have yielded conflicting reports, supporting either a loss of K+ selectivity (16) or a loss of channel function (19). To study the physiological effects of GIRK2 in vivo and to address the question whether the phenotypic defects in the weaver mouse are due to gain-of-function effects such as the loss of K+ selectivity or due to loss-of-function or dominant-negative effects on GIRK1/GIRK2 heteromultimeric channels, we have generated GIRK2-deficient mice and compared them to mice carrying one or two copies of the wv allele but no wild-type GIRK2 gene.
MATERIALS AND METHODS
Genomic Cloning and Construction of a Targeting Vector.
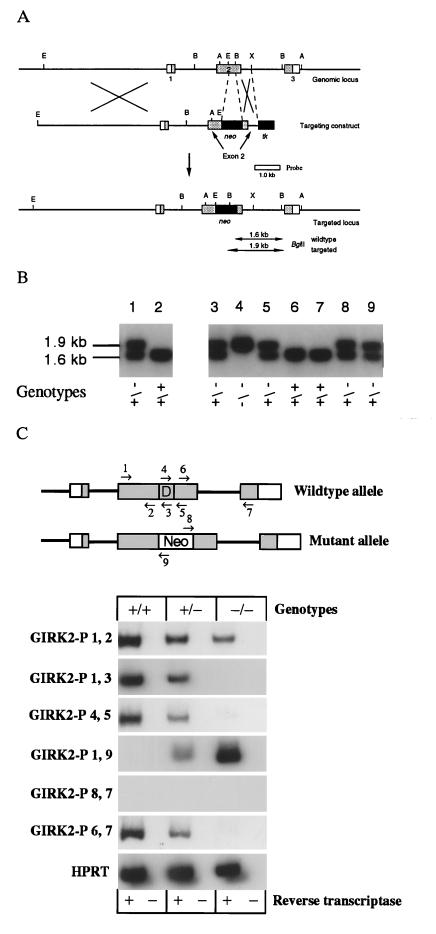
Genomic clones containing the murine GIRK2 gene were isolated from a λFIX II murine 129/Sv genomic library (Stratagene) by screening the library using the full-length hamster GIRK2 cDNA as a probe (20). Two identical phage clones containing the entire murine GIRK2 gene were identified, and three exons containing the entire open reading frame were mapped. To generate the GIRK2 targeting vector pPNT-76, an ≈8-kb EcoRI fragment containing exon 1 and part of exon 2 was inserted into the targeting vector pPNT (21) such that its 3′ end was adjacent to the PGK promotor upstream of the neomycin gene. The 3′ end of the targeting construct was generated from the same GIRK2 genomic clone and contained a 0.47-kb BglII–XbaI fragment that was inserted into the exon 2 EcoRI/BglII deletion and included sequences from exon and intron 2 (Fig. 1A). The targeting vector was linearized by NotI and electroporated into R1 embryonic stem (ES) cells at 200 V and 800 mF. Stable colonies were grown under double selection in 350 μg/ml G418 and 0.2 mM gancyclovin in ES cell medium (22). By Southern blotting, 150 colonies were analyzed for homologous recombination. One clone (G2) was identified by the presence of a 1.9-kb BglII band and was microinjected into blastocysts to generate GIRK2-deficient mice (Fig. 1B).
Figure 1.
(A) Targeted disruption of the mouse GIRK2 gene in ES cells and mice. The genomic structure and restriction map of the mouse GIRK2 gene locus and targeting vectors pPNT-76 used to disrupt the GIRK2 gene are shown. Shaded boxes represent coding sequences of the exons (boxes). The probe 3′ of the deletion is used for Southern blot analysis and shown as an open bar. B, BglII; A, AccI; E, EcoRI; X, XbaI. (B) Southern analysis of transfected ES cells. Lanes: 1, targeted clone containing the targeted allele, as assessed by the presence of an additional 1.9-kb BglII fragment; 2: parental clone containing the normal 1.6-kb fragment; 3–9, genotypes from tail biopsies of GIRK2 +/+, +/−, and −/− mice. (C) Reverse transcriptase (RT)-PCR analysis from brain mRNA of GIRK2 +/+, +/−, and −/− mice. (Upper) GIRK2 wild-type and mutant alleles are shown with the position of oligonucleotides used as PCR primers. D, deleted region into which the pgk-neomycin resistance cassette was inserted. No primer-pair amplified products in the absence of reverse transcriptase. Each sample started with an equal amount of cDNAs. A 5′-truncated mRNA terminating in pgk-neomycin can be detected by PCR at reduced levels in +/− and −/− animals. The sequences of the oligonucleotide used are available upon request.
Western Blot Analysis.
Mouse brain membrane (50 μg), prepared as described (23, 24), was solubilized in 2% SDS/sample buffer (125 mM Tris, pH 6.8/20% glycerol/5% 2-mercaptoethanol) and loaded onto each lane. Western blots were probed with 1 μg/ml affinity-purified rabbit polyclonal antibodies against the N terminus of GIRK2 or GIRK1 or against the C terminus of IRK1 (23). Donkey anti-rabbit-horseradish peroxidase was used as secondary antibody at 1:5000 dilution. The blots were developed with enhanced chemiluminescence reagents (Amersham) and exposed to Hyperfilm-ECL (Amersham).
Immunohistochemistry.
Mice were perfused intracardially with 4% formaldehyde and 0.1% glutaraldehyde in PBS (pH 7.4). Brains were dissected and postfixed overnight at 4°C. Fifty-micrometer vibratome sections were collected in 0.1 M Tris (pH 7.6); blocked with 2% H2O2; washed in 50 mM Tris, pH 7.5/100 mM NaCl/0.1% Triton X-100 (TBST); and then blocked in 4% normal goat serum and 3% BSA in TBST. Rabbit antibodies were affinity-purified, and sections were incubated in 1–2 μg/ml primary antibody overnight (23, 24). Monoclonal antibodies against tyrosine hydroxylase (TH; Pel-Freez Biologicals) were used at 1:1000. Biotinylated donkey anti-rabbit or anti-mouse IgG Fab (The Jackson Laboratory) were used at 1:200, and sections were developed with the ABC kit (Vector Laboratories) and diaminobenzidine.
Induction of Seizures Using Pentylenetetrazole (PTZ).
PTZ (Sigma) was dissolved in PBS and injected i.p. at a dose of 50 mg/kg in ≈0.1 ml. Animals were housed in a room with controlled light/dark cycle (12 hr light/12 hr dark) and temperature (23°C). All experiments were performed between 11 a.m. and 1 p.m. Animals were injected and observed without prior knowledge of their genotype. Each mouse was placed in a transparent cage and observed for 30 min after injection. All mice were littermates between 10 and 14 weeks of age and weighed ≥20 g.
RESULTS AND DISCUSSION
Generation of GIRK2 Null Mice.
The GIRK2 gene was disrupted in ES cells by homologous recombination using a targeting vector in which exon 2 was disrupted and partially deleted by a pgk-neomycin resistance cassette (Fig. 1A). One ES cell clone that carried the targeted allele was used to generate chimeric male animals that passed the mutant allele to their offspring. GIRK2 +/− mice were indistinguishable from wild-type mice and were inbred to produce GIRK2 −/− mice (Fig. 1B). No normal GIRK2 mRNA could be detected in brains of adult GIRK2 −/− mice by RT-PCR analysis, but a truncated GIRK2 mRNA was present (Fig. 1C). No GIRK2 immunoreactivity was detectable using antibodies against either the N terminus or C terminus of GIRK2 (Figs. 2A and 3 A and B; data not shown). We conclude, therefore, that we have generated GIRK2 null mice.
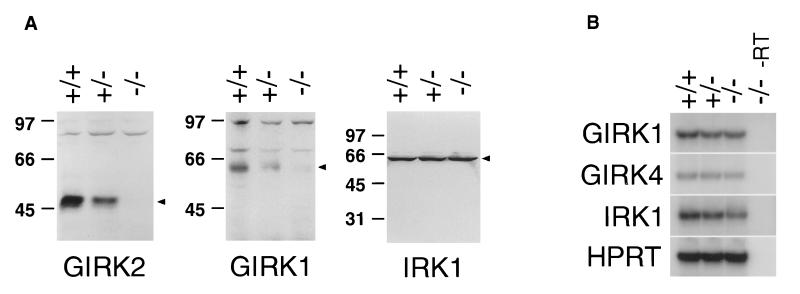
Figure 2.
(A) Western blots of membrane prepared from GIRK2 +/+, +/−, and −/− mouse brains show that both GIRK2 and GIRK1 protein levels are reduced in the GIRK2 knockout mice. (B) RT-PCR showing equal mRNA expression of GIRK1 in +/+, +/−, and −/− mouse brain. No product is amplified by any of the four pairs of primers in the absence of reverse transcriptase (−/− −RT; lane 4), confirming that all products were amplified from cDNA rather than contaminating genomic DNA. Hypoxanthine phosphoribosyltransferase (HPRT) primers amplify a comparable level of product in all samples, indicating that the same amount of template is present. Amplified products by GIRK1, GIRK4, IRK1, and HPRT primers are of expected sizes.
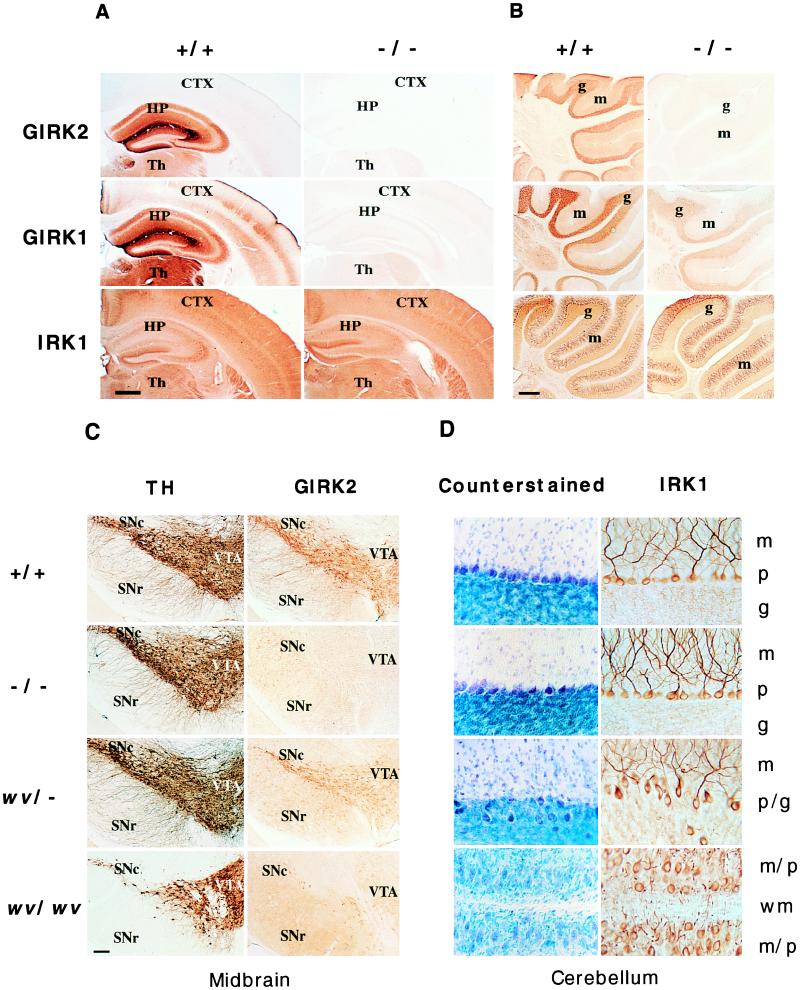
Figure 3.
(A) Coronal sections of brain from GIRK2 +/+ and −/− mouse brains stained with antibodies against the N terminus of GIRK2, the N terminus of GIRK1, and the C terminus of IRK1 show that there is no detectable GIRK2 and dramatically reduced GIRK1 immunoreactivity in the GIRK2 −/− mice. The IRK1 staining patterns are the same for these mice. CTX, cerebral cortex; HP, hippocampus; Th, thalamus. (Bar = 10 mm.) (B) Sagittal views of cerebella from GIRK2 +/+ and −/− mice stained with the antibodies described in A. Although there is no detectable GIRK2 protein in the −/− mice, there is still significant GIRK1 staining, whereas the level of IRK1 expression appears to be the same. g, granule cell layer; m, molecular layer. (Bar = 20 mm.) (C) Coronal sections of ventral midbrain from +/+, −/−, wv/−, and wv/wv mice are stained with antibodies against TH and the N terminus of GIRK2. The TH staining of +/+, −/−, and wv/− midbrain appears similar, whereas there are fewer TH-positive neurons in the substantia nigra pars compacta (SNc) of the wv/wv midbrain. GIRK2 immunoreactivity is absent in the −/− midbrain but can still be found in the wv/− and wv/wv mice, although the dendritic staining is dramatically reduced. SNr, substantia nigra pars reticulata; VTA, ventral tegmental area. (Bar = 1 mm.) (D) High magnification views of parasagittal cerebellar sections from +/+, −/−, wv/−, and wv/wv mice. The sections are counterstained with toluidine blue or stained with antibody against the C terminus of IRK1, which stains the cell body and dendrites of the Purkinje cells as well as the dendrites in the granule cell layer. The wv/− cerebellum is mostly wild-type in appearance except for regions with a Purkinje cell layer more disorganized and broader than that in GIRK2 +/+ and −/− mice. Some Purkinje cells and their dendrites can be found in the granule cell layer of wv/− mice. In the wv/wv cerebellum, there is no granule cell layer, and the Purkinje cells with disorganized dendrites are scattered throughout the cerebellum. m, molecular layer; p, Purkinje cell layer; g, granule cell layer; p/g, cell layer where both Purkinje cells and granule cells are found; m/p, cell layer where molecular layer and Purkinje cell layers collapse into one in the absence of granule cell layer; wm, white matter. (Bar = 0.2 mm.)
Down-Regulation of GIRK1 Protein in GIRK2 Null Mice.
GIRK2 −/− mice are born at the expected frequency and are viable. Given that GIRK2 and GIRK1 have partly overlapping temporal and spatial expression patterns and are known to form functional heteromultimers in vitro (16–18, 25–28) and in vivo (24), we examined the expression of GIRK1 and other related inward rectifier channels by using affinity-purified polyclonal antibodies against GIRK1, IRK1, and GIRK4 in Western blot and immunohistochemical studies of GIRK2 +/+, +/−, and −/− mice. Immunoblot analysis showed that GIRK1 levels were reduced in brain membranes of GIRK2 +/− mice and nearly undetectable in −/− mice, whereas IRK1 protein levels remained constant in mice of all three genotypes (Fig. 2A). RT-PCR analysis showed that GIRK1, GIRK4, and IRK1 mRNA were similar in all animals, suggesting that the down-regulation of GIRK1 in GIRK2 −/− mice occurred posttranscriptionally (Fig. 2B). Immunohistochemical analysis showed dramatic reduction of GIRK1 immunoreactivity in many brain regions in GIRK2 −/− mice, whereas IRK1 and GIRK4 immunoreactivities were normal in these GIRK2 mutants (Fig. 3 A and B; data not shown). The extent of reduction in GIRK1 varied with the brain regions; expression of GIRK1 in the cerebral cortex and hippocampus was virtually undetectable, whereas in the cerebellum, significant amounts of GIRK1 remained in the granule cell layer (Fig. 3 A and B). The reduction of GIRK1 protein levels throughout the brain suggests that a majority of GIRK1 proteins in the brain interact with GIRK2, and in the absence of GIRK2, there is a concurrent loss of GIRK1 subunits that normally form heteromultimers with GIRK2.
Differences Between the GIRK2 Null Phenotypes and the Weaver Phenotypes.
The GIRK2 −/− and wv/wv mice showed striking differences. Visual inspection and histological examination of the brain and other organs of GIRK2 −/− animals revealed no anomalies. GIRK2 −/− mice exhibited normal cerebellar morphology except for the reduced GIRK1 and GIRK2 protein expression (Fig. 3 B and D). Midbrain dopaminergic neurons and their dendrites also appeared normal despite the absence of GIRK2 protein (Fig. 3C). While male wv/wv mice are infertile, male GIRK2 −/− mice are fertile; superovulated CD-1 mice mated with either GIRK2 −/− males or their wild-type littermates produced a comparable number of fertilized eggs. The apparent normal phenotype in GIRK2 −/− mice provides strong evidence that loss of homomeric GIRK2 channel and/or heteromeric GIRK1/GIRK2 channel function is not the primary cause of the weaver phenotype.
The Weaver Gene Dosage Effect.
When GIRK2 −/− mice were compared with mice carrying one or two copies of the wv allele (GIRK2 wv/− and/or wv/wv), we found that both −/− and wv/− mice exhibit normal locomotive behavior, unlike the wv/wv mice. In +/+, −/−, and wv/− animals, there was no obvious loss of TH-positive neurons or dendrites in the substantia nigra pars compacta or in the ventral tegmental area, whereas substantial cell loss was evident in the substantia nigra pars compacta of the wv/wv midbrain (Fig. 3C). In the substantia nigra pars compacta and ventral tegmental area of wv/− mice, GIRK2 immunoreactivity was present but of lower intensity. In contrast to the heterozygous GIRK2 +/− mice, most of the GIRK2 immunoreactivity in the wv/− mice was found in the cell bodies of the dopaminergic neurons; the GIRK2 immunoreactivity in the dendrites was much reduced (Fig. 3C). The size and gross morphology of the cerebellum of wv/− animals are not significantly different from that of the wild-type animals. Histologically, the wv/− cerebellum appeared more similar to that of the wv/+ (8, 9) than the cerebellum of +/+ or wv/wv mice. The wv/− granule cell layer often appeared thinner. The Purkinje cell layer was disorganized in various locations, and some of the Purkinje cells were found deep in the granule cell layer (Fig. 3D). The similarity between wv/− and wv/+ cerebella and the difference between wv/− and wv/wv cerebella suggest that cerebellar development is sensitive to the dosage of the GIRK2 G156S mutant gene.
Seizure Activities of GIRK2 Null Mice.
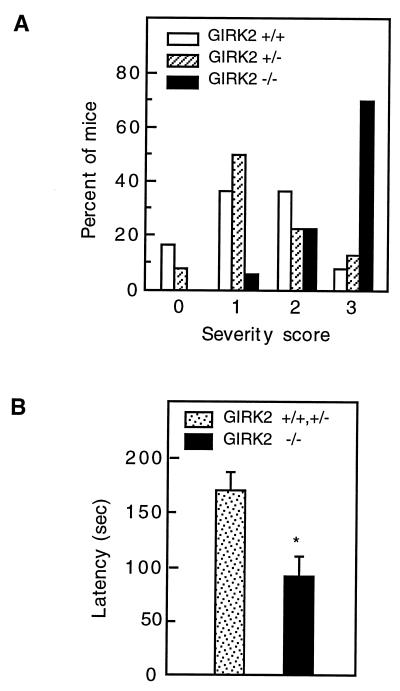
The GIRK2 −/− mice exhibited sporadic seizures characterized by jerking of head and body, vocalization, and infrequently progression to a tonic-clonic seizure. Typically, the episodes lasted for 30 sec and were followed by complete physical inactivity. All witnessed seizures occurred when some kind of stress was exerted on the animal (changing cages, setting up matings), and the behavior of mice returned to normal after the seizure. Seizures were never observed before weaning and seemed to occur at equal frequencies in young and old mutant mice. Pharmacological challenge with the convulsant agent PTZ (29), a γ-aminobutyric acid antagonist, revealed that GIRK2 −/− mice were hyperexcitable when challenged with a single injection of PTZ (50 mg/kg). At this dose, 70% of GIRK2 −/− mice but only 25% of heterozygous or wild-type littermates developed severe stage 3 tonic-clonic seizures frequently associated with death (P < 0.004, Mann–Wilcoxon rank sum test). The severity of seizure, in the range from 0 to 3, was shifted toward increased severity in GIRK2 −/− mice as compared with +/− and +/+ controls. No statistically significant difference was seen between heterozygous and wild-type mice (Fig. 4A). The time taken to develop seizure activities was significantly shorter in GIRK2 −/− mice compared with +/− and +/+ animals (P < 0.002, unpaired t-test; Fig. 4B). Seizure activity has previously been noted in weaver mice and might be due to altered or reduced G protein-activated K+ channel function (14). Our observation that GIRK1/GIRK2-deficient mice are susceptible to spontaneous and pharmacologically induced seizures was consistent with numerous studies demonstrating that agonists of G protein-coupled receptors, such as receptors for opioid peptides, somatostatin, and dopamine, can have significant effects on seizure thresholds in several different experimental seizure model systems (30).
Figure 4.
Susceptibility of GIRK2-deficient mice to PTZ-induced seizures. (A) Response of mice receiving one injection of 50 mg/kg PTZ i.p. (0, no response; 1, isolated twitches; 2, tonic-clonic convulsions; 3, tonic extension and/or death). GIRK2 −/− mice (n = 16) tend to progress to more severe stages than +/+ or +/− mice (n = 13 and 12, respectively; P < 0.004, Mann–Whitney U–Wilcoxon rank sum test). No statistically significant difference was observed between +/+ and +/− animals. (B) Seizure latency. The PTZ seizure latency was defined as the time elapsed from PTZ injection to the first obvious sign of tonic-clonic convulsion or tonic extension. The latency to seizures was shorter for the −/− mice (∗, P < 0.002, unpaired test).
In conclusion, we show that GIRK2-deficient mice have greatly reduced GIRK1 protein levels in the brain, suggesting that the majority of GIRK1 proteins in the brain associate with GIRK2. Phenotypic characteristics of GIRK2 −/−, wv/−, wv/+, and wv/wv mice suggest that gain-of-function and gene dosage mechanisms are responsible for the developmental defects in weaver mutants. Moreover, loss of GIRK2 function results in sporadic seizures and increased susceptibility to a convulsant agent, implicating GIRK1 and GIRK2 in the control of neural excitability in vivo.
Acknowledgments
We thank Paul Slesinger for his suggestions and discussions throughout the course of this work. We also thank Jan L. Breslow, James E. Darnell, Jr., Paolo Mocarelli, Annemarie Walsh, and the Rockefeller transgenic facility for their support. The research was supported by the National Parkinson Foundation (M.S.), a National Institute of Mental Health grant to the Silvio Conte Neuroscience Center at the University of California at San Francisco, and the University of California at San Francisco Neuroscience Graduate Program. L.Y.J. is a Howard Hughes Medical Institute investigator.
Footnotes
Abbreviations: PTZ, pentylenetetrazol; ES cells, embryonic stem cells; RT, reverse transcriptase; TH, tyrosine hydroxylase.
References
- 1.Sakman B, Noma A, Trautwein W. Nature (London) 1983;303:250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- 2.Breitwieser G E, Szabo G. Nature (London) 1985;317:538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- 3.Pfaffinger P J, Martin J M, Hunter D D, Nathanson N H, Hille B. Nature (London) 1985;217:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- 4.Hille B. Ion Channels of Excitable Membranes. 2nd Ed. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 5.Jan L Y, Jan Y N. Nature (London) 1994;371:119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- 6.Kubo Y. Neurosci Res (NY) 1994;21:109–117. doi: 10.1016/0168-0102(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 7.Wickman K, Clapham D E. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- 8.Rakic P, Sidman R L. J Comp Neurol. 1973;152:103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- 9.Rakic P, Sidman R L. J Comp Neurol. 1973;152:133–162. doi: 10.1002/cne.901520203. [DOI] [PubMed] [Google Scholar]
- 10.Hatten M E, Liem R K, Mason C A. J Neurosci. 1984;4:1163–1172. doi: 10.1523/JNEUROSCI.04-04-01163.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt M J, Sawyer B D, Perry K W, Fuller R W, Foreman M M, Ghetti B. J Neurosci. 1982;2:376–380. doi: 10.1523/JNEUROSCI.02-03-00376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roffler-Tarlov S, Graybiel A M. Nature (London) 1984;307:62–66. doi: 10.1038/307062a0. [DOI] [PubMed] [Google Scholar]
- 13.Harrison S M W, Roffler-Tarlov S. Dev Dyn. 1994;200:26–38. doi: 10.1002/aja.1002000104. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg B, Messer A. Neurosci Lett. 1989;96:168–172. doi: 10.1016/0304-3940(89)90052-9. [DOI] [PubMed] [Google Scholar]
- 15.Patil N, Cox D R, Bhay D, Faham M, Myers R M, Peterson A S. Nat Genet. 1996;11:126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- 16.Slesinger P A, Patil N, Liao Y J, Jan Y N, Jan L Y, Cox D R. Neuron. 1996;16:321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- 17.Kofuji P, Hofer M, Millen K J, Millonig J H, Davidson N, Lester H A, Hatten M E. Neuron. 1996;16:941–952. doi: 10.1016/s0896-6273(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 18.Navarro B, Kennedy M E, Velimirovic B, Bhat B, Peterson A S, Clapham D E. Science. 1996;272:1950–1953. doi: 10.1126/science.272.5270.1950. [DOI] [PubMed] [Google Scholar]
- 19.Surmeier D J, Mermelstein P G, Goldowitz D. Proc Natl Acad Sci USA. 1996;93:11191–11195. doi: 10.1073/pnas.93.20.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsaur M-E, Menzel S, Lai F P, Espinosa R, III, Concannon P, Spielman R S, Hanis C L, Cox N J, Le Beau M M, German M S, Jan L Y, Bell G I, Stoffel M. Diabetes. 1995;44:592–595. doi: 10.2337/diab.44.5.592. [DOI] [PubMed] [Google Scholar]
- 21.Tybulewicz V L J, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 22.Robertson E J. In: Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. Robertson E J, editor. Oxford: IRL Press; 1987. pp. 71–112. [Google Scholar]
- 23.Sheng M, Tsaur M-L, Jan Y N, Jan L Y. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- 24.Liao, Y. J., Jan, Y. N. & Jan, L. Y. (1996) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 25.Kobayashi T, Ikeda K, Ichikawa T, Abe S, Togashi S, Kumanishi T. Biochem Biophys Res Commun. 1995;208:1166–1173. doi: 10.1006/bbrc.1995.1456. [DOI] [PubMed] [Google Scholar]
- 26.Duprat F, Lesage K, Guillemare E, Fink M, Hugnot J P, Bigay J, Lazdunski M, Romey G, Barhanin J. Biochem Biophys Res Commun. 1995;212:657–663. doi: 10.1006/bbrc.1995.2019. [DOI] [PubMed] [Google Scholar]
- 27.Kofuji P, Davidson N, Lester H A. Proc Natl Acad Sci USA. 1995;92:6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, Romey G, Barhanin J, Lazdunski M. J Biol Chem. 1995;270:28660–28667. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- 29.Orloff M J, Williams H L, Pfeiffer C C. Proc Soc Exp Biol Med. 1949;70:254–325. doi: 10.3181/00379727-70-16891. [DOI] [PubMed] [Google Scholar]
- 30.Schwartzkroin P A. Epilepsy: Models, Mechanisms and Concepts. 1st Ed. Cambridge, U.K.: Cambridge Univ. Press; 1993. [Google Scholar]