Abstract
Early mathematical models varied in their predictions of the impact of HIV/AIDS on population growth from minimal impact to reductions in growth, in pessimistic scenarios, from positive to negative values over a period of 25 years. Models predicting negative rates of natural increase forecast little effect on the dependency ratio. Twenty years later, HIV prevalence in small towns, estates, and rural villages in eastern Zimbabwe, has peaked within the intermediate range predicted by the early models, but the demographic impact has been more acute than was predicted. Despite concurrent declines in fertility, fueled in part by HIV infections (total fertility is now 8% lower than expected without an epidemic), and a doubling of the crude death rate because of HIV/AIDS, the rate of natural population increase between 1998 and 2005 remained positive in each socioeconomic stratum. In the worst-affected areas (towns with HIV prevalence of 33%), HIV/AIDS reduced growth by two-thirds from 2.9% to 1.0%. The dependency ratio fell from 1.21 at the onset of the HIV epidemic to 0.78, the impact of HIV-associated adult mortality being outweighed by fertility decline. With the benefit of hindsight, the more pessimistic early models overestimated the demographic impact of HIV epidemics by overextrapolating initial HIV growth rates or not allowing for heterogeneity in key parameters such as transmissibility and sexual risk behavior. Data collected since the late 1980s show that there was a mismatch between the observed growth in the HIV epidemic and assumptions made about viral transmission.
Keywords: dependency ratio, population growth
The initial observation of a growing AIDS epidemic among heterosexual populations in the Americas and Africa generated concern over the eventual scale and impact of the pandemic. A range of models was developed to explore the future spread of infection and its impact on mortality based on the available pattern of growth in HIV prevalence, patterns of sexual behavior, transmissibility of the virus, and progression from infection to AIDS and death. Initial predictions, based on the early growth of the epidemic and a long duration of infectiousness, predicted that, over a period of decades, HIV could well turn population growth rates negative (1, 2). However, others disagreed (3), and the consensus at a United Nations and World Heath Organization workshop held in December 1989 was that the epidemic could have an important effect on population growth but that negative rates of natural increase were unlikely (4). A maximum reduction of 30% in the growth rate over a period of <50 years was predicted. The relationship between mortality in young adults and decreased births meant that, even in models with an extreme impact, dependency ratios were predicted to remain unchanged (1, 2).
Over time, our understanding of the spread of HIV and its impact on fertility and mortality has improved and has been included in more recent models (5–7). Model predictions can be compared with empirical estimates of demographic impact obtained in surveys, census, and vital registration (8). Discrepancies can arise, leading to debate over the validity of both model and empirical estimates (9). With the benefit of hindsight, we can explore the validity of the assumptions made in the different models of the heterosexual spread of HIV and reevaluate the approaches taken in predicting the demographic impact of AIDS.
HIV prevalence has now reached high levels in several countries in southern and eastern Africa, with rates generally being highest in cities and other centers of high labor migration (10).
In the Rakai district of Uganda, 1990–1991, Sewankambo et al. (11) observed substantial reductions in the rate of natural increase but continued population growth in three geographic strata in which HIV prevalence ranged from 13% to 35%. Using 1991 national census data for the same country, Low-Beer et al. (12) found instances of negative population growth at parish level but not at district or national levels. Population pyramids for severely affected areas exhibited deficits of adults and young children consistent with the distribution of reported AIDS cases. Despite much interest in the demographic impact of AIDS, these seem to be the only direct empirical measurements published to date on the impact of large-scale HIV epidemics on population growth and structure. Furthermore, we are aware of no previous reports of this kind in the countries in southern Africa that have experienced the most widely disseminated epidemics (13).
In Zimbabwe, nationally, the HIV/AIDS epidemic took hold in the mid-1980s, and HIV prevalence in adults aged 15–49 years is estimated to have peaked at 29% in 1997 and to have fallen to 20% by 2005 (14). Over a similar period, census reports estimated that the annual average inter-censal population growth rate declined from 3.1% between 1982 and 1992 to 1.1% between 1992 and 2002 (15, 16). This decline resulted from a combination of rising mortality [the crude death rate (CDR) almost doubled from 9.5 to 17.2 between 1992 and 2002], falling fertility [the total fertility rate [TFR] fell by a third from 5.5 live births per women in 1984–1988 to 3.8 in 2001–2005] (17, 18), and increasing international out-migration (United Nations Office for the Coordination of Humanitarian Affairs, “Zimbabwe government taps into remittances to ease forex shortage,” Integrated Regional Information Networks News, June 9, 2004).
Against this background, we present data on the impact of HIV/AIDS on mortality, fertility, population age structure, and population growth in small towns, estates, and rural villages in Manicaland, Zimbabwe's eastern province. To inform future modeling of the impact of infectious disease, we compare these data with early model predictions of the demographic impact of HIV/AIDS in sub-Saharan Africa and discuss likely explanations for observed discrepancies.
Results
Demographic Impact of HIV/AIDS in Manicaland, Eastern Zimbabwe.
Adult HIV prevalence and mortality.
Table 1 shows HIV prevalence and mortality rates per 1,000 person years (PY) by sex, socioeconomic stratum, and HIV-infection status for 1998–2005 [see expanded version in supporting information (SI) Table 5]. HIV prevalence in adults fell from 23% to 18% over an average 5-year inter-survey interval (19). During the same period, adult mortality seems to have stabilized in both sexes (unpublished data). Adult mortality was much higher among HIV-infected individuals, 81.6 per 1,000 PY, than in uninfected individuals, 7.2 per 1,000 PY [Cox proportional hazard ratio adjusted for sex, age, and location (AHR) 12.1; 95% confidence interval (C.I.), 9.9–14.9]. In HIV-infected individuals, death rates were highest in males (AHR, 1.3; 95% C.I., 1.0–1.5) and in village locations (AHR, 1.3; 95% C.I., 1.0–1.6).
Table 1.
Adult mortality (ages 15–54 years) per thousand PY by HIV status and location, Manicaland, Zimbabwe, 1998–2005
| Sex | Stratum | HIV prevalence,* % | HIV-positive |
HIV-negative |
Proportion of deaths associated with HIV, % | AR of HIV-associated mortality | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | Deaths | PY | Mortality | Deaths | PY | |||||
| Males | Towns | 26.9 | 72.8 | 31 | 426 | 7.0 | 8 | 1,137 | 79 | 72 |
| Estates | 20.3 | 68.0 | 45 | 662 | 3.1 | 9 | 2,884 | 83 | 80 | |
| Villages | 14.8 | 109.9 | 119 | 1,083 | 6.2 | 39 | 6,255 | 75 | 71 | |
| Females | Towns | 38.5 | 65.6 | 41 | 625 | 5.7 | 6 | 1,045 | 87 | 80 |
| Estates | 24.6 | 52.0 | 29 | 558 | 3.0 | 6 | 1,995 | 83 | 78 | |
| Villages | 19.2 | 80.5 | 173 | 2,149 | 4.9 | 56 | 11,498 | 76 | 71 | |
AR, attributable risk = proportion of all deaths in the age interval and location that could be averted in the absence of HIV.
*Weighted average of HIV prevalence at baseline and two rounds of follow-up.
The proportion of deaths associated with HIV infection was similar in both sexes and across socioeconomic locations (range 75–87%). The proportion of all deaths that would have been averted in the absence of HIV (attributable risk) was 73% in both sexes, the higher HIV prevalence in females balancing the greater mortality in infected males. Female HIV-associated deaths typically occurred at younger ages with 70% of deaths in females versus 56% of deaths in males occurring before the age of 40.
Fertility.
Fertility in the Manicaland study sites declined between 1998 and 2005 (20) and an average TFR of 3.46 was recorded for the period as whole. Based on the fertility experience of uninfected women, the TFR would have been 3.73 in the absence of the HIV epidemic. Thus, the population-attributable reduction in fertility associated with HIV/AIDS in 1998–2005 was 8%. Table 2 shows similar comparisons for the town, estate, and village populations.
Table 2.
Total fertility rate by HIV status and location, Manicaland, Zimbabwe, 1998–2005
| Stratum | All women | HIV-negative women | PAC |
|---|---|---|---|
| Towns | 3.01 | 3.51 | −0.14 |
| Estates | 3.37 | 3.58 | −0.06 |
| Villages | 3.56 | 3.79 | −0.06 |
Total fertility rates for 15- to 49-year-olds based on births in the last 5 years and HIV infection status at follow-up. PAC, population-attributable change in fertility.
Population structure and dependency ratios.
The sex and age structures of the town, estate, and village populations for 2003–2005 are shown in SI Fig. 2. The town and estate populations had a more even sex ratio than the village populations (0.98 and 1.02 versus 0.81 males per female) and greater numbers of males aged 25–54 years of age and females aged 25–34 years of age. The dependency ratio (defined here as the number of children below age 15 years and elderly people >65 years of age, divided by the number of adults aged 15–64 years) before and after including adult AIDS cases as dependents, is higher in the villages (0.85 and 0.91, respectively) than in the towns (0.55 and 0.61) or estates (0.55 and 0.61).
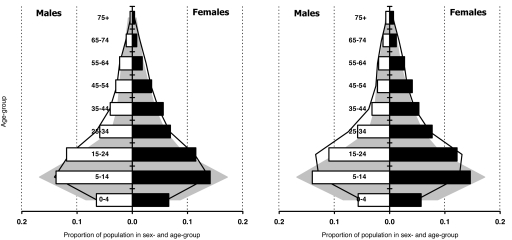
The dependency ratios for the combined study population were similar at the beginning (1998–2000: 0.78 and 0.81) and end of the study period (2003–2005: 0.78 and 0.84). In the absence of data for these areas before the spread of HIV, the dependency ratios and age-structures were compared with those observed in rural populations nationally in the mid-1980s (21). This comparison suggests a considerable reduction in the dependency ratio from 1.21 in the mid-1980s and that individuals in the age range 15–44 years have increased as a proportion of the total population whereas the proportion accounted for by children up to the age of 14 has reduced (Fig. 1).
Fig. 1.
Sex and age structure of combined study population in Manicaland, Zimbabwe from 1998–2000 (Left) and 2003–2005 (Right). Histograms show empirical data for proportions of population in each sex and age group. Also shown, for comparison, are the distribution of rural populations in Zimbabwe, as a whole, in 1987 (21) (shaded area) and mathematical model projections for the national rural population in 1999 (Left) and 2004 (Right), in which HIV prevalence peaks at 25% in the late 1990s and the TFR declines from 5.5 live births per woman in 1986 to 4.4 in 1992 and 3.5 in 1997 (solid line).
Population growth.
Table 3 shows the observed crude birth and death rates and rate of natural population increase and estimates of these rates in the absence of the HIV epidemic. In the absence of HIV/AIDS, the rate of natural increase is greater in the towns and estates because of the concentration of young adults with high fertility and low mortality. The presence of HIV/AIDS more than doubles the CDR and reduces the crude birth rate (CBR) in each population stratum. In the towns, where HIV prevalence is highest, the rate of natural increase is estimated to have been cut by two thirds from 2.9% per annum to 1.0% per annum. Smaller reductions were recorded in the estates and villages. If the HIV-infected adults living in the towns are taken to have experienced the higher death rates recorded in the villages (to allow for migration during terminal illness), the rate of natural increase reduces further to 0.7%, but still remains positive.
Table 3.
CBR, CDR, and population growth rate by socioeconomic stratum in the presence and absence of HIV, Manicaland, Zimbabwe, 1998–2005
| Stratum | HIV prevalence* |
Observed |
In the absence of HIV† |
|||||
|---|---|---|---|---|---|---|---|---|
| All ages, % | 15–54 yr, % | CBR | CDR | Rate of natural increase, % | CBR | CDR | Rate of natural increase, % | |
| Towns | 21.5 | 32.8 | 32.2 | 22.2 | 1.0 | 36.7 | 7.4 | 2.9 |
| Estates | 15.3 | 22.2 | 36.1 | 14.1 | 2.2 | 37.8 | 4.5 | 3.3 |
| Villages | 10.3 | 17.3 | 29.5 | 17.0 | 1.3 | 30.9 | 8.2 | 2.3 |
*Weighted average of HIV prevalence at baseline and two rounds of follow-up. HIV prevalence in children estimated from prevalence in pregnant women in the study communities, data on vertical transmission in Zimbabwe (37), and data on postinfection childhood survival (38).
†Based on fertility and mortality among uninfected individuals.
Comparison of Empirical Estimates with Early Model Predictions.
At the United Nations and World Health Organization workshop held in December 1989, mathematical modelers were given a standard set of inputs for demographic trends in the absence of HIV, patterns of marriage and extramarital relationships and parameters describing the natural history and transmissibility of the virus. They were requested to provide estimates for the spread and impact of HIV after 25 years. i.e., by 2010 from a starting point based on HIV prevalence levels in east Africa around 1985 (4). The intermediate and worst case predictions made at the workshop are presented in Table 4. It is important to note that the HIV prevalence estimates presented were for all age groups not prevalence within the most sexually active age range (typically 15–49 years of age) which is commonly used today. Based on best estimates of the biological, behavioral and demographic parameters, it was found that the HIV epidemic would have a moderate demographic effect “at most 30%” (4) rather than the profound impact predicted by Anderson et al. (1). Many other simulations did generate negative population growth rates but with parameter values that “may prove exaggerated when more complete and accurate evidence is gathered” (4). These parameter values included high transmission probabilities of HIV which, in turn, generated a high prevalence of infection and a large demographic impact.
Table 4.
Intermediate and worst-case estimates of HIV prevalence and rate of natural increase projected in mathematical models at a United Nations and World Health Organization workshop held in December 1989
| Model | Intermediate |
Worst |
||
|---|---|---|---|---|
| HIV prevalence* | Rate of natural increase,† % | HIV prevalence* | Rate of natural increase,† % | |
| Auvert | 31.0 | 0.5 | 55.0 | −2.0 |
| Brouard | 15.0 | 2.4 | 55.0 | −0.2 |
| Bulatao | 39.5 | 2.5 | 57.5 | 2.4 |
| Dietz | 21.2 | 1.5 | 43.9 | −0.7 |
| IWG‡ | 3.5 | 2.6 | 42.4 | −2.5 |
| Palloni | 2.8 | 2.8 | 30.3 | 0.0 |
*HIV prevalence is defined across the whole population.
†The initial growth rate based on demographic inputs was 3.5% per annum.
‡Interagency Working Group (4).
In this exercise, Anderson et al. (1) did not use the standard inputs but generated results based on doubling times of the epidemic, where a doubling time of 4.5, 3.5, 2.5, and 1.5 years generated a prevalence of ≈10%, 20%, 40%, and 48%, respectively, after 25 years. Only with a doubling time of 4.5 did population growth remain positive; however, it took >25 years to become negative with a doubling time of 3.5 years. In the mid-1990s, the same research group applied a model that additionally included heterogeneity in sexual behavior with parameter settings based on data from Zimbabwe. The model projected that HIV prevalence in adults would peak at ≈25% and, with concurrent fertility decline stabilizing at a TFR of 3.5 live births per woman, would cause the rate of natural increase to fall slightly below zero (22). A similar prediction was made by the United States Bureau of the Census (23).
The empirical estimates for HIV prevalence in Zimbabwe fall near the center of the range of the intermediate projections at the workshop (Table 4). In populations where the underlying rate of natural increase was slightly lower than the 3.5% per annum used at the workshop, the Dietz (4) and Brouard (24) models provided seemingly accurate predictions of the impact on population growth. However, these impacts were predicted to be later than observed and were predicted to continue increasing. The later projections for Zimbabwe (22) correctly predicted the peak in HIV prevalence but overestimated the impact on population growth.
Anderson's initial model had also predicted that HIV epidemics would have only a modest impact on the dependency ratio. The group's later model for Zimbabwe indicated that any impact would be outweighed by the effects of rapid fertility decline. These predictions are consistent with the empirical data, although recent high out-migration has led to deficits in the 15–34 year age groups, particularly among males (Fig. 1).
Discussion
Empirical Evidence: Summary and Consistency with Other Empirical Studies.
HIV has spread extensively within rural Manicaland, but our data indicate that, 20 years into the epidemic, the rate of natural increase remains positive, even in the areas of highest HIV prevalence. Demographic and epidemiological trends in Manicaland broadly reflect those for Zimbabwe as a whole. In both cases, antiretroviral treatment for HIV/AIDS began to become available only late in the study period.
Our findings are consistent with those obtained by Sewankambo and colleagues (11) between 1990 and 1991 in three rural socioeconomic strata in the Rakai District of Uganda. The HIV epidemic took hold in Rakai District more than a decade earlier than in Manicaland and HIV prevalence levels and the underlying rate of population increase were similar to those seen in the current study areas. HIV-associated subfertility was not accounted for in the Rakai study but mortality in HIV-infected individuals was lower in Manicaland, possibly because the longer periods of follow-up result in greater underreporting (25).
Perhaps the most surprising finding is the continued excess of fertility over mortality in the small towns. As in the trading centers in Rakai, Uganda (11), an adult HIV prevalence of >30% proved insufficient to reduce the annual rate of natural increase below 1%. There seem to be two main reasons for this finding. First, a strong concentration of women of peak childbearing age results in a high CBR despite lower age-specific fertility rates than in the other socioeconomic strata. Second, age-specific mortality rates among HIV-positive adults in the towns (and also in the estates) are lower than in the villages. Migration could have contributed to this result because Zimbabweans living in centers of employment frequently retain strong links with their “rural homes” (26). We minimized the effect of terminally ill individuals returning to rural villages once they become too ill to work by accounting for deaths according to place of residence when last interviewed, and, in earlier research, we found no evidence for selective out-migration of HIV-infected individuals from our study populations (27). A larger proportion of the deaths of HIV-infected adults in the towns and estates could have gone unreported in the survey given the greater social isolation in these locations, but the rate of natural increase remained above zero even when HIV-infected adults in the towns were taken to experience the higher death rates observed in villages. A further possibility is that circular migration could have led to underestimates in the true number of PY of residence in towns and estates in the age groups with highest HIV-associated mortality and, thereby, to underestimates of the CDR in these areas. Urban and estate residents frequently visit their rural homes during holidays and near the end of the month shortly after being paid. However, our surveys were conducted on a de jure basis, which should have minimized this bias.
Other sources of bias could have affected our estimates of the impact of HIV/AIDS on population growth in eastern Zimbabwe. We assumed that no AIDS deaths occurred within the age groups not covered by the individual cohort survey (i.e., 5–14 years of age and 55 years of age and above), which tends to make our findings conservative. We assumed that the levels of mortality and fertility seen in uninfected individuals represented the levels that would have pertained in the absence of the HIV epidemic. If infected individuals would have had higher mortality or lower fertility than other individuals even in the absence of infection, our estimates would tend to overstate the true impact of HIV/AIDS.
The data from eastern Zimbabwe indicate a reduction rather than an increase in the dependency ratio, and, unlike in south-west Uganda in the early 1990s (12), we found no evidence for deficits (relative to the pre-AIDS period) in the adult age groups with greatest HIV-associated mortality. This difference is probably because, unlike Uganda before 1990, Zimbabwe experienced a substantial fall in fertility during the 1980s and 1990s (20) which typically causes population aging. However, fertility declines have yet to commence or have been less pronounced in a number of other countries with large national epidemics (e.g., Zambia and Malawi). In these countries, deficits of (particularly, older middle-aged) adults could occur (28). We did find deficits in the early-childhood age groups. Low-Beer et al. (12) noted that reduced numbers of births because of smaller proportions of women being in the primary child-bearing age groups, as well as HIV-associated subfertility and pediatric mortality, could contribute to such deficits.
The recent collapse of the economy in Zimbabwe has led to international migration of young adults to South Africa and other countries in the region as well as to western countries since around 2000. This phenomenon is unlikely to have affected our findings that the rate of natural increase of the population has remained positive and that the combined effect of HIV/AIDS and fertility decline has reduced the dependency ratio. However, when migration is taken into account, the net growth rate since 2000 may have been negative in some parts of Zimbabwe. Migration has probably also increased the dependency ratio during this period although the economic effects of this increase have been offset by a substantial increase in remittances received from the newly expanded diaspora.
Post Hoc Evaluation of Early Models of the Demographic Impact of HIV/AIDS.
Empirical measurements are subject to bias of the forms just discussed. However, HIV prevalence has not reached the levels feared in the early model worst case scenarios. Whereas the Zimbabwe data are consistent with two of the intermediate scenarios, with the benefit of hindsight, one can question the wide range of predicted demographic impact, particularly when the anticipated prevalence of infection was similar.
At the time, early models were classified according to whether they adopted a standard demographic cohort projection approach (for example, the models described in the chapters by Bulatao and Palloni in ref. 4) or built from epidemiological foundations. However, the major differences in their predictions came from elsewhere. The predicted impact of HIV on population growth depends upon (i) the expected birth and death rates in the absence of HIV and the patterns of migration; (ii) the incidence of HIV infections over time as a function of sex and age; and (iii) the impact that infection has on fertility and mortality in the individual. The major differences between the models arise from both the HIV prevalence level achieved and how quickly it is attained and all of the models failed to capture what has subsequently been observed. Whereas the intermediate predictions for Auvert (4), Brouard (24), and Dietz (4) generate high prevalence, these rates increase more gradually than observed to the recorded levels. Projections from Anderson's models, based on initial HIV epidemic growth rates and without heterogeneity in risk behavior within age groups, go on increasing until most of the population is infected. The Palloni et al. models (and the Bongaarts and John model applied before the United Nations and World Health Organization workshop) did not reach the high HIV prevalence levels observed with the parameters provided for the workshop (4). The model of Bulatao, from the World Bank (4), is an outlier, in that, even at high prevalence, population growth is little affected. For some unexplained reason, the crude fertility rate (CFR) in this model increases along with increased HIV prevalence, and, despite a continued high prevalence of HIV, the projected life expectancy bounces back after an initial decline.
The subsequent HIV epidemics in eastern and southern Africa can broadly be described as less widespread than those predicted by models with a rapid rise in prevalence, but more widespread than the other predictions. These discrepancies came mainly from an attempt to fit the models to HIV prevalence levels observed in 1985 while using the observed per act transmission probabilities of 0.001 from female to male and 0.003 from male to female (4). In those models that started from the 1985 HIV prevalence levels, these transmission probabilities led to slow growth thereafter whereas the models that generated the 1985 HIV prevalence levels could do so only with extreme risk behaviors leading to the predicted epidemic eventually spreading too far.
The low per act transmission probabilities assumed that transmission likelihood was independent of partnership. At the time, there was concern over changing transmissibility, but this concern was largely ignored. The question asked of experts was whether HIV was transmissible at all in the long asymptomatic incubation period, rather than whether it was less transmissible (4). Further in, those models that did allow variation in transmissibility over the course of the infection mostly just assumed an increase toward the end of the incubation period. It is now clear that the high viral load associated with primary HIV infection leads to a high transmission probability per act early in the infection (29), which generates a rapidly growing and saturating epidemic (30). Early models that assumed a per partnership transmission probability, like that of Anderson and colleagues (4), would have better captured the heterogeneity in transmission probabilities per partnership. However, this model did not include behavioral heterogeneity which is needed to generate saturation effects. Dietz's model (4) is interesting in that it explicitly includes sexual partnerships as state variables. However, in that formulation, partnerships were protective, which slowed the spread of infection. Subsequent models describing the network of sexual partnerships show how overlapping partnerships combined with the high transmissibility early in infection can generate rapid epidemic spread (31).
Translating a given HIV prevalence into a demographic impact depends upon the rate at which infection leads to AIDS and then death. A median period of 10 years from infection to AIDS was assumed for the workshop. More rapid progression requires greater HIV incidence to maintain the observed prevalence, so observed urban prevalence levels could have led to a negative population growth rate if progression had been faster than in western countries (32). However, subsequent data suggest that the incubation period assumed initially was reasonable (33). None of the models took account of reduced fertility in those infected with HIV. At the time, the evidence was slim, but subsequent work has shown a decline of ≈25% in births to women infected (34, 40). Nonetheless, this effect may be offset by the lower than assumed mother-to-child transmission rate and the countervailing reduction in fallopian tube occlusion associated with declines in bacterial sexually transmitted diseases (35).
Models for Zimbabwe developed in the mid-1990s (22, 23) provided accurate forecasts of the timing and level of peak HIV prevalence within the sexually active age range but predicted a greater impact on population growth than is indicated by the data. Mortality may not have been fully captured in the survey for the reasons discussed above. However, errors in model assumptions (e.g., an underestimation of the median survival time from seroconversion) also contributed to this discrepancy.
Conclusion.
In Zimbabwe and some other parts of sub-Saharan Africa, HIV prevalence has reached the levels predicted in some intermediate case scenarios in the late 1980s. Whereas HIV/AIDS has not turned positive growth rates negative, the impact has exceeded the maximum 30% reduction envisaged at the time. The more pessimistic projections overestimated the demographic impact by overextrapolating initial epidemic growth rates or by not allowing for heterogeneity in transmissibility. It was not realized at the time that heightened transmissibility during the early stages of infection was combining with overlapping sexual partnerships to cause a rapid initial spread of epidemics. The more optimistic projections underestimated the potential for future growth in HIV epidemics, and the more pessimistic projections overestimated this potential.
Whereas the demographic impact of large-scale HIV epidemics may be less dramatic than was first feared, the effects are nonetheless very substantial and still unfolding. In the predominantly rural populations studied here, life expectancy has been reduced by a median of 19 years for males and 22 years for females (25), total fertility has been reduced by ≈0.3 live births per woman, and the rate of natural increase has been reduced by up to two-thirds. The proportion of children under the age of 15 years who were orphans doubled from 12% to 23% between 1998–2000 and 2003–2005 (36).
Methods
Study Setting.
The study was conducted in 12 locations (two small towns, two tea and coffee estates, two forestry plantations, two roadside trading centers, and four subsistence farming areas) in Manicaland province, eastern Zimbabwe between 1998 and 2005.
Data Collection and Laboratory Procedures.
The detailed procedures followed in the study have been published (19). In brief, we conducted a baseline census of all households in each location in a phased manner (one site at a time) between July 1998 and February 2000. Household residence was defined on a de jure basis. A random sample of adults aged 15–54 years resident within the study households was recruited into a longitudinal open cohort. First and second follow-up censuses and surveys were conducted in each of the same sites 3 years (July 2001 to February 2003) and 5 years (July 2003 to August 2005) after baseline, respectively. All baseline respondents and individuals who had previously been too young to participate but who now met the age criteria were considered eligible at each round of follow-up. Because of funding constraints, at the first follow-up, persons who had migrated into the study areas in the 3-year inter-survey period were only eligible for individual interview in the sites visited 5th to 12th in the phased enumeration. HIV serological testing was done on dried blood spot specimens by using a highly sensitive and specific antibody dipstick assay. Written informed consent was sought as a condition of enrollment and continuation in the study. Prior ethical approval for the study was obtained from the Research Council of Zimbabwe (Number 02187) and the Applied and Qualitative Research Ethics Committee in Oxford, United Kingdom (N97.039).
Following these procedures, 98%, 94%, and 96% of the households identified in the survey areas at baseline and at first and second follow-up, respectively, were enumerated. Individual participation rates were 79%, 79%, and 83% at baseline, first follow-up, and second follow-up, respectively. Sixty-one percent and 63% of those interviewed at baseline and first follow-up (and not known to have died subsequently) were reinterviewed at first and second follow-up, respectively. Out-migration was the principal reason for loss-to-follow-up; for example, at first follow-up, this reason was given directly by village guides or household respondents in 56% of cases and the individuals or their households could not be located in a further 42% of cases. Only 1% of baseline respondents and 2% of first follow-up respondents declined to participate in the next round of the survey.
Data Analysis.
For the purposes of this study, households and their residents were recoded according to their location within the three principal socioeconomic strata found in eastern Zimbabwe: small towns, large-scale tea, coffee and forestry estates, and rural villages. Rural villages were defined as those characterized by subsistence farming and, for practical reasons, were taken to be those located >10 min walking distance from an urban center or an estate compound.
Mortality.
Sex- and age-specific mortality rates during each of two inter-survey periods, stratified by HIV infection status and location of residence at the start of the period, were calculated for the age intervals 15–24 years, 25–39 years, and 40–54 years, by using data from the cohort of individuals enrolled at the start of the period. Weighted averages of the age-specific mortality rates for the two inter-survey periods were calculated to give estimates for the full study period (1998–2005), by using the lengths of the inter-survey periods as weights.
Crude death rates for the full study period were calculated for each socioeconomic stratum as weighted averages of the age-specific mortality rates by using the proportion of all PY exposed in each age group as weights. PY of exposure were periods of residence within households located within the socioeconomic stratum during the study period. These periods were calculated for each inter-survey period from data on dates of initial and follow-up household interview and dates of migration into/from household and/or death, where appropriate, and were then aggregated. For infant and early childhood mortality rates, we used indirect estimates derived from data on HIV prevalence in pregnant women (pregnant in the inter-survey periods) collected in the socioeconomic stratum, an estimate of 30.7% for the probability of vertical transmission of HIV infection in Zimbabwe (37), and estimates for age-specific mortality from 0–1 years (1m0) and 1–4 years (4m1) of 0.429 and 0.132 for HIV-infected infants (38) and 0.056 and 0.007 for uninfected infants in sub-Saharan African populations. We assumed that no AIDS deaths occurred in the other age intervals not covered in the individual cohort and estimated non-AIDS mortality for these ages using data from the longitudinal household census adjusted for underreporting (25). Estimates for CDR in the absence of the HIV epidemic were based on the mortality experience of uninfected individuals.
Fertility.
Standard procedures (39) were applied to calculate 5-year age-specific and TFR for women aged 15–49 years in each socioeconomic stratum by using data on live births in the inter-survey periods. Separate estimates were derived and compared for women found to be infected with HIV and uninfected at follow-up. The estimates for uninfected women were taken to be indicative of levels in the absence of the HIV epidemic and population-attributable change (PAC) in fertility was estimated by using the formula PAC = [(TFR in total population) − (TFR in HIV-negative population)]/(TFR in HIV-negative population) (40). Crude birth rates were estimated from age-specific fertility rates and PY of exposure within households in the same way as for CDR. Women who died were treated as lost to follow-up in the calculation of age-specific fertility rates by HIV-infection status.
Population structure and dependency ratios.
Population pyramids were constructed to identify the differences in sex and age structure found between the different socioeconomic strata by using data from the third household census (2003–2005). The overall sex and age distributions for the 12 study sites combined at baseline and second follow-up were compared with that for rural areas of Zimbabwe in 1987 (21) (i.e., before the impact of the HIV epidemic) and a mathematical model projection made in the mid-1990s (22). Dependency ratios were calculated for the combined study population and for each of the socioeconomic strata by using the standard age groups for dependents and economically active individuals: (number of individuals aged 0–14 years and 65 years and above)/(number of individuals aged 15–64 years); this ratio was compared with the equivalent ratio for 1987. We also calculated and compared alternative dependency ratios in which estimates for the numbers of HIV-infected adults in the economically active age groups incapacitated by AIDS (based on an average incubation period of 8 years and an average survival period with AIDS of 1 year) were added to the numerator and subtracted from the denominator.
Rate of natural population increase.
Estimates for the rate of natural increase for each socioeconomic strata, in the presence and absence of the HIV epidemic, were obtained by taking the difference between the estimates of the CBR and the CDR in each case.
Data analysis was done in Stata Version 9 (Stata Corporation, College Station, TX).
Supplementary Material
Acknowledgments
We thank L. Chisvo, E. Dauka, M. Kakowa, J. Magwere, P. R. Mason, M. Mlilo, C. Mundandi, J. Mutsvangwa, Z. Mupambireyi, M. Wambe, and C. Zvidzai for assistance with data collection, data processing, and laboratory diagnostics. This work was supported by the Wellcome Trust and the Joint United Nations Program on HIV/AIDS (UNAIDS).
Abbreviations
- TFR
total fertility rate
- PY
person year
- CDR
crude death rate
- CBR
crude birth rate.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611540104/DC1.
References
- 1.Anderson RM, May RM, McLean AR. Nature. 1988;332:228–234. doi: 10.1038/332228a0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RM, May RM, Boily M-C, Garnett GP, Rowley JT. Nature. 1991;352:581–589. doi: 10.1038/352581a0. [DOI] [PubMed] [Google Scholar]
- 3.Bongaarts J. Stat Med. 1989;9:103–120. doi: 10.1002/sim.4780080111. [DOI] [PubMed] [Google Scholar]
- 4.United Nations and World Health Organization. The AIDS Epidemic and its Demographic Consequences: Proceedings of the United Nations/World Health Organization Workshop on Modelling the Demographic Impact of the AIDS Epidemic in Pattern II Countries–Progress to Date and Policies for the Future; United Nations, New York. 1991. pp. 1–140. [Google Scholar]
- 5.Stover J. Sex Transm Infect. 2004;80:i14–i18. doi: 10.1136/sti.2004.010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson LF, Dorrington RE. Demogr Res. 2006;14:541–574. [Google Scholar]
- 7.Reference Group on Estimates, Modelling and Projections. [(last accessed May 23, 2007)];Joint United Nations Programme on HIV/AIDS (UNAIDS) 2005 www.epidem.org/publications.htm.
- 8.Stover J, Ghys PD, Walker N. AIDS. 2004;18:S67–S73. doi: 10.1097/00002030-200406002-00008. [DOI] [PubMed] [Google Scholar]
- 9.Grassly NC, Lewis JJC, Mahy M, Walker N, Timaeus IM. Popul Stud. 2004;58:207–217. doi: 10.1080/0032472042000224431. [DOI] [PubMed] [Google Scholar]
- 10.Carael M. In: Sexual Cultures and Migration in the Era of AIDS: Anthropological and Demographic Perspectives. Herdt G, editor. Oxford: Oxford Univ Press; 1997. pp. 107–126. [Google Scholar]
- 11.Sewankambo NK, Wawer MJ, Gray RH, Serwadda D, Li C, Stallings RY, Musgrave SD, Konde-Lule J. AIDS. 1994;8:1707–1713. doi: 10.1097/00002030-199412000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Low-Beer D, Stoneburner RL, Mukulu A. Nat Med. 1997;3:553–557. doi: 10.1038/nm0597-553. [DOI] [PubMed] [Google Scholar]
- 13.UNAIDS. Report on the Global HIV/AIDS Epidemic. Geneva: UNAIDS; 2006. pp. 1–689. [Google Scholar]
- 14.Zimbabwe Ministry of Health and Child Welfare. Antenatal Clinic HIV Surveillance Report 2004. Harare, Zimbabwe: Zimbabwe Ministry of Health and Child Welfare; 2005. pp. 1–28. [Google Scholar]
- 15.Zimbabwe Central Statistical Office. Census 1992: National Report. Harare, Zimbabwe: Zimbabwe Central Statistical Office; 1994. pp. 1–226. [Google Scholar]
- 16.Zimbabwe Central Statistical Office. Census 2002: National Report. Harare, Zimbabwe: Zimbabwe Central Statistical Office; 2004. pp. 1–261. [Google Scholar]
- 17.Zimbabwe Central Statistical Office. Zimbabwe Demographic and Health Survey, 1999. Harare, Zimbabwe: Zimbabwe Central Statistical Office and Macro International; 2000. pp. 1–289. [Google Scholar]
- 18.Zimbabwe Central Statistical Office; MEASURE Evaluation DHS. Zimbabwe Demographic and Health Survey, 2005–2006: Preliminary Report. Harare, Zimbabwe: Zimbabwe Central Statistical Office; 2006. pp. 1–31. [Google Scholar]
- 19.Gregson S, Garnett GP, Nyamukapa CA, Hallett TB, Lewis JJC, Mason PR, Chandiwana SK, Anderson RM. Science. 2006;311:664–666. doi: 10.1126/science.1121054. [DOI] [PubMed] [Google Scholar]
- 20.Terceira N, Gregson S, Zaba B, Mason PR. Popul Stud. 2003;57:149–164. doi: 10.1080/0032472032000097074. [DOI] [PubMed] [Google Scholar]
- 21.Zimbabwe Central Statistical Office. Zimbabwe Intercensal Demographic Survey, 1987. Harare, Zimbabwe: Zimbabwe Central Statistical Office; 1991. pp. 1–166. [Google Scholar]
- 22.Blair Research Institute; Oxford University. The Early Socio-Demographic Impact of the HIV-1 Epidemic in Rural Zimbabwe. Harare, Zimbabwe: Blair Research Institute; 1996. pp. 1–557. [Google Scholar]
- 23.U.S. Bureau of the Census. The Demographic Impact of HIV/AIDS: Perspectives from the World Population Profile, 1996. Washington, DC: U.S. Bureau of the Census; 1997. [Google Scholar]
- 24.Brouard N. Population. 1987;42:797–818. [Google Scholar]
- 25.Lopman BA, Barnabas R, Hallett TB, Nyamukapa CA, Mundandi C, Mushati P, Garnett GP, Gregson S. Bull WHO. 2005;84:189–197. doi: 10.2471/blt.05.025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potts DH, Mutambirwa CC. J S Afr Stud. 1990;16:177–198. [Google Scholar]
- 27.Mundandi C, Vissers D, Voeten HACM, Habbema JDF, Gregson S. Trop Med Int Health. 2006;11:705–711. doi: 10.1111/j.1365-3156.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 28.UNAIDS. Report on the Global HIV/AIDS Epidemic. Geneva: UNAIDS; 2002. [Google Scholar]
- 29.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Layendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, et al. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 30.Jacquez JA, Koopman JS, Simon CP, Longini IMJ. J Acquired Immune Defic Syndr. 1994;7:1169–1184. [PubMed] [Google Scholar]
- 31.Morris M, Kretzschmar M. AIDS. 1997;11:641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Gregson S, Garnett GP, Anderson RM. J Acquired Immune Defic Syndr. 1994;7:839–852. [PubMed] [Google Scholar]
- 33.Morgan D, Mahe C, Mayanja B, Okongo JM, Lubega R, Whitworth JAG. AIDS. 2002;16:597–603. doi: 10.1097/00002030-200203080-00011. [DOI] [PubMed] [Google Scholar]
- 34.Gray R, Wawer M, Serwadda D, Sewankambo N, Li C, Wabwire-Mangen F, Paxton L, Kiwanuka N, Kigozi G, Konde-Lule J, et al. Lancet. 1998;351:98–103. doi: 10.1016/S0140-6736(97)09381-1. [DOI] [PubMed] [Google Scholar]
- 35.Garnett GP, Gregson S. Math Popul Stud. 2000;8:251–277. [Google Scholar]
- 36.Watts H, Lopman B, Nyamukapa CA, Gregson S. AIDS. 2005;19:717–725. doi: 10.1097/01.aids.0000166095.62187.df. [DOI] [PubMed] [Google Scholar]
- 37.Zijenah LS, Moulton LH, Iliff P, Nathoo K, Munjoma MW, Mutasa K, Malaba L, Zvandasara P, Ward BJ, Humphrey J. AIDS. 2004;18:273–280. doi: 10.1097/00002030-200401230-00017. [DOI] [PubMed] [Google Scholar]
- 38.Marston M, Zaba B, Salomon J, Brahmbhatt H, Bagenda D. J Acquired Immune Defic Syndr. 2005;38:219–227. doi: 10.1097/00126334-200502010-00015. [DOI] [PubMed] [Google Scholar]
- 39.Preston SH, Heuveline P, Guillot M. Demography: Measuring and Modelling Population Processes. Oxford: Blackwell; 2001. [Google Scholar]
- 40.Zaba B, Gregson S. AIDS. 1998;12:S41–S50. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.