Abstract
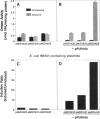
Proteus mirabilis urease catalyzes the hydrolysis of urea, initiating the formation of urinary stones. The enzyme is critical for kidney colonization and the development of acute pyelonephritis. Urease is induced by urea and is not controlled by the nitrogen regulatory system (ntr) or catabolite repression. Purified whole-cell RNA from induced and uninduced cultures of P. mirabilis and Escherichia coli harboring cloned urease sequences was probed with a 4.2-kb BglI fragment from within the urease operon. Autoradiographs of slot blots demonstrated 4.2- and 5.8-fold increases, respectively, in urease-specific RNA upon induction with urea. Structural and accessory genes necessary for urease activity, ureD, A, B, C, E, and F, were previously cloned and sequenced (B. D. Jones and H. L. T. Mobley, J. Bacteriol. 171:6414-6422, 1989). A 1.2-kb EcoRV-BamHI restriction fragment upstream of these sequences confers inducibility upon the operon in trans. Nucleotide sequencing of this fragment revealed a single open reading frame of 882 nucleotides, designated ureR, which is transcribed in the direction opposite that of the urease structural and accessory genes and encodes a 293-amino-acid polypeptide predicted to be 33,415 Da in size. Autoradiographs of sodium dodecyl sulfate-polyacrylamide gels of [35S]methionine-labeled polypeptides obtained by in vitro transcription-translation of the PCR fragments carrying only ureR yielded a single band with an apparent molecular size of 32 kDa. Fragments carrying an in-frame deletion within ureR synthesized a truncated product. The predicted UreR amino acid sequence contains a potential helix-turn-helix motif and an associated AraC family signature and is similar to that predicted for a number of DNA-binding proteins, including E. coli proteins that regulate acid phosphatase synthesis (AppY), porin synthesis (EnvY), and rhamnose utilization (RhaR). These data suggest that UreR governs the inducibility of P. mirabilis urease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison C., Lai H. C., Hughes C. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol Microbiol. 1992 Jun;6(12):1583–1591. doi: 10.1111/j.1365-2958.1992.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Atlung T., Nielsen A., Hansen F. G. Isolation, characterization, and nucleotide sequence of appY, a regulatory gene for growth-phase-dependent gene expression in Escherichia coli. J Bacteriol. 1989 Mar;171(3):1683–1691. doi: 10.1128/jb.171.3.1683-1691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel F. M. Regulation of nitrogen fixation genes. Cell. 1984 May;37(1):5–6. doi: 10.1016/0092-8674(84)90294-0. [DOI] [PubMed] [Google Scholar]
- Belas R., Erskine D., Flaherty D. Transposon mutagenesis in Proteus mirabilis. J Bacteriol. 1991 Oct;173(19):6289–6293. doi: 10.1128/jb.173.19.6289-6293.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenbach J. M., Hausinger R. P. Proteus mirabilis urease. Partial purification and inhibition by boric acid and boronic acids. Biochem J. 1988 Mar 15;250(3):917–920. doi: 10.1042/bj2500917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990 Sep 11;18(17):5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Magasanik B. Urease of Klebsiella aerogenes: control of its synthesis by glutamine synthetase. J Bacteriol. 1977 Aug;131(2):446–452. doi: 10.1128/jb.131.2.446-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardini F. C., Hobbs M. M., Stamm L. V., Bassford P. J., Jr Complementation of an Escherichia coli proC mutation by a gene cloned from Treponema pallidum. J Bacteriol. 1990 Jun;172(6):2996–3002. doi: 10.1128/jb.172.6.2996-3002.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gicquelais K. G., Baldini M. M., Martinez J., Maggi L., Martin W. C., Prado V., Kaper J. B., Levine M. M. Practical and economical method for using biotinylated DNA probes with bacterial colony blots to identify diarrhea-causing Escherichia coli. J Clin Microbiol. 1990 Nov;28(11):2485–2490. doi: 10.1128/jcm.28.11.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Gargan R. A. Rapid screening for urease inhibitors. Invest Urol. 1979 Mar;16(5):327–328. [PubMed] [Google Scholar]
- Harrison S. C., Aggarwal A. K. DNA recognition by proteins with the helix-turn-helix motif. Annu Rev Biochem. 1990;59:933–969. doi: 10.1146/annurev.bi.59.070190.004441. [DOI] [PubMed] [Google Scholar]
- Hu L. T., Nicholson E. B., Jones B. D., Lynch M. J., Mobley H. L. Morganella morganii urease: purification, characterization, and isolation of gene sequences. J Bacteriol. 1990 Jun;172(6):3073–3080. doi: 10.1128/jb.172.6.3073-3080.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Jones B. D., Lockatell C. V., Johnson D. E., Warren J. W., Mobley H. L. Construction of a urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1990 Apr;58(4):1120–1123. doi: 10.1128/iai.58.4.1120-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Genetic and biochemical diversity of ureases of Proteus, Providencia, and Morganella species isolated from urinary tract infection. Infect Immun. 1987 Sep;55(9):2198–2203. doi: 10.1128/iai.55.9.2198-2203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J Bacteriol. 1988 Aug;170(8):3342–3349. doi: 10.1128/jb.170.8.3342-3349.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Proteus mirabilis urease: nucleotide sequence determination and comparison with jack bean urease. J Bacteriol. 1989 Dec;171(12):6414–6422. doi: 10.1128/jb.171.12.6414-6422.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leucine-zipper motif update. Nature. 1989 Jul 13;340(6229):103–104. doi: 10.1038/340103a0. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Friedrich M. J., Kadner R. J. Nucleotide sequence of the Escherichia coli porin thermoregulatory gene envY. Nucleic Acids Res. 1989 Jan 25;17(2):800–800. doi: 10.1093/nar/17.2.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland N., McCarter L., Artz S., Kustu S. Nitrogen regulatory locus "glnR" of enteric bacteria is composed of cistrons ntrB and ntrC: identification of their protein products. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2135–2139. doi: 10.1073/pnas.78.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Cortesia M. J., Rosenthal L. E., Jones B. D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988 May;26(5):831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Jones B. D., Jerse A. E. Cloning of urease gene sequences from Providencia stuartii. Infect Immun. 1986 Oct;54(1):161–169. doi: 10.1128/iai.54.1.161-169.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Jones B. D., Penner J. L. Urease activity of Proteus penneri. J Clin Microbiol. 1987 Dec;25(12):2302–2305. doi: 10.1128/jcm.25.12.2302-2305.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Warren J. W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987 Nov;25(11):2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney S. B., Lynch M. J., Mobley H. L., Hausinger R. P. Purification, characterization, and genetic organization of recombinant Providencia stuartii urease expressed by Escherichia coli. J Bacteriol. 1988 May;170(5):2202–2207. doi: 10.1128/jb.170.5.2202-2207.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney S. B., Pankratz H. S., Hausinger R. P. Regulation of gene expression and cellular localization of cloned Klebsiella aerogenes (K. pneumoniae) urease. J Gen Microbiol. 1989 Jun;135(6):1769–1776. doi: 10.1099/00221287-135-6-1769. [DOI] [PubMed] [Google Scholar]
- Mörsdorf G., Kaltwasser H. Cloning of the genes encoding urease from Proteus vulgaris and sequencing of the structural genes. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):67–73. doi: 10.1016/0378-1097(90)90260-w. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of computer programs for DNA and protein sequence management, analysis and homology determination. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):643–655. doi: 10.1093/nar/12.1part2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein I. J., Hamilton-Miller J. M., Brumfitt W. Role of urease in the formation of infection stones: comparison of ureases from different sources. Infect Immun. 1981 Apr;32(1):32–37. doi: 10.1128/iai.32.1.32-37.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Takayama H., Konishi T., Tomoyoshi T. Scanning electron microscopy detects bacteria within infection stones. J Urol. 1984 Jul;132(1):67–69. doi: 10.1016/s0022-5347(17)49466-3. [DOI] [PubMed] [Google Scholar]
- Tobin J. F., Schleif R. F. Positive regulation of the Escherichia coli L-rhamnose operon is mediated by the products of tandemly repeated regulatory genes. J Mol Biol. 1987 Aug 20;196(4):789–799. doi: 10.1016/0022-2836(87)90405-0. [DOI] [PubMed] [Google Scholar]
- Walz S. E., Wray S. K., Hull S. I., Hull R. A. Multiple proteins encoded within the urease gene complex of Proteus mirabilis. J Bacteriol. 1988 Mar;170(3):1027–1033. doi: 10.1128/jb.170.3.1027-1033.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982 Dec;146(6):719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]