Abstract
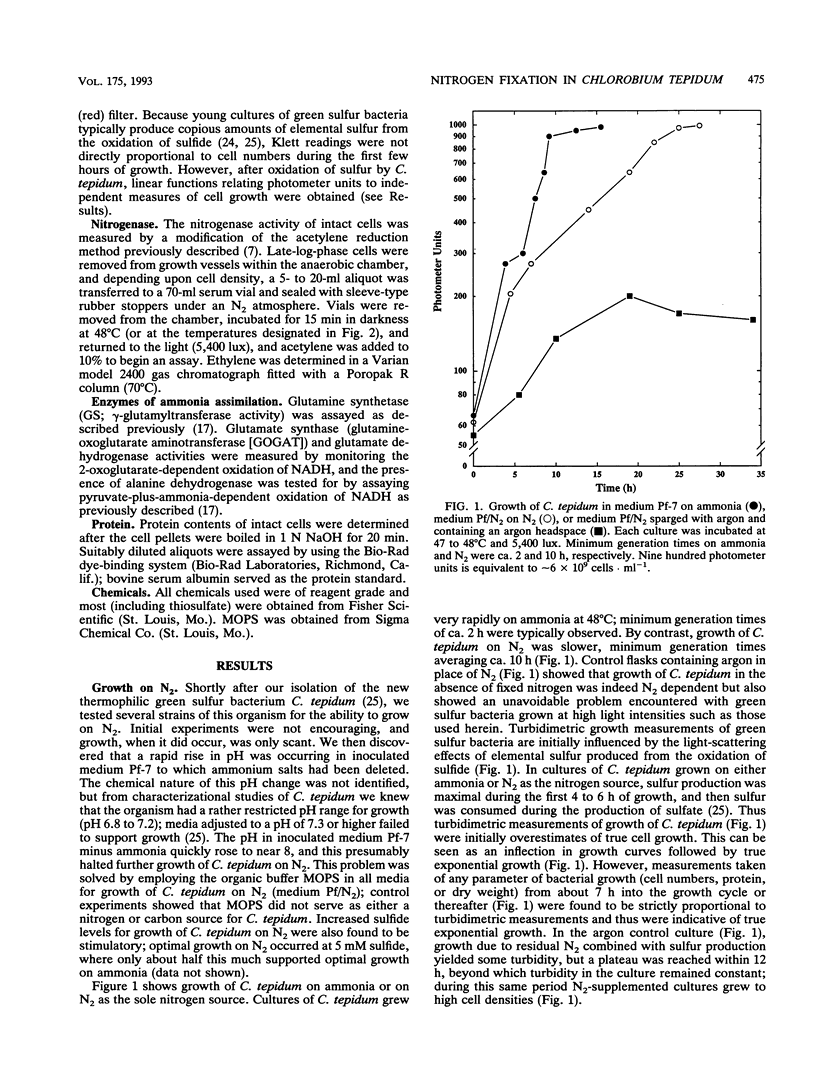
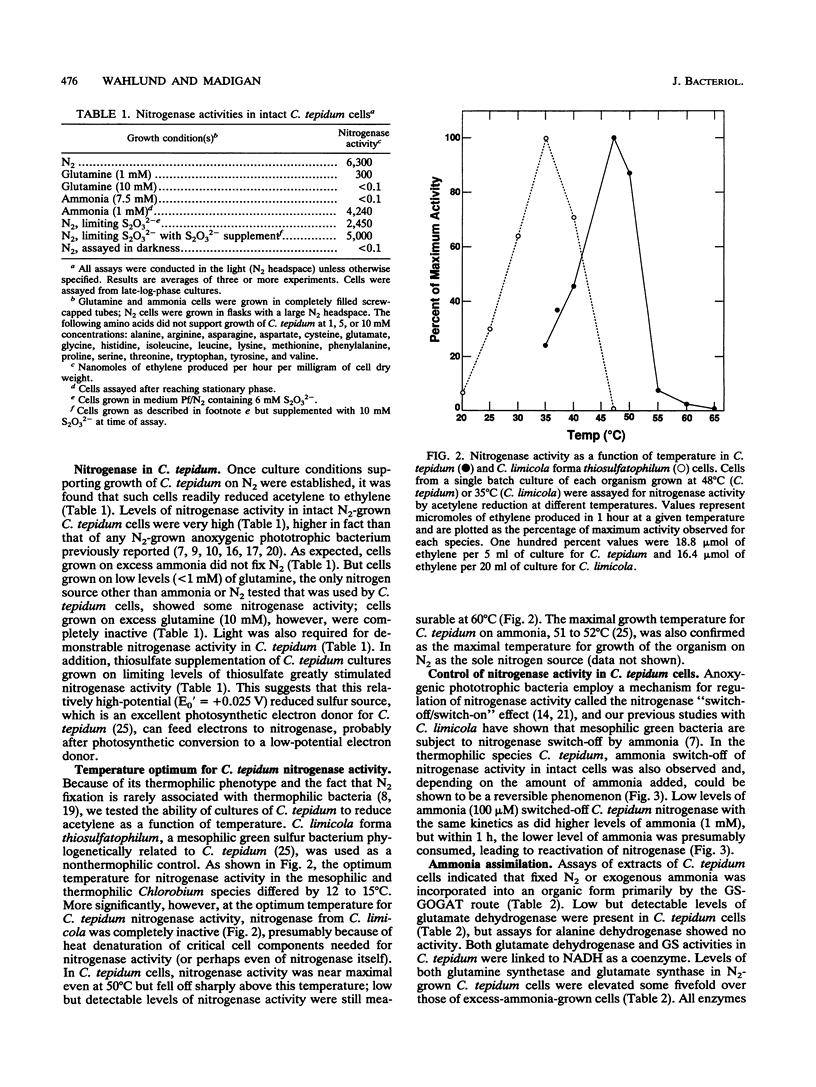
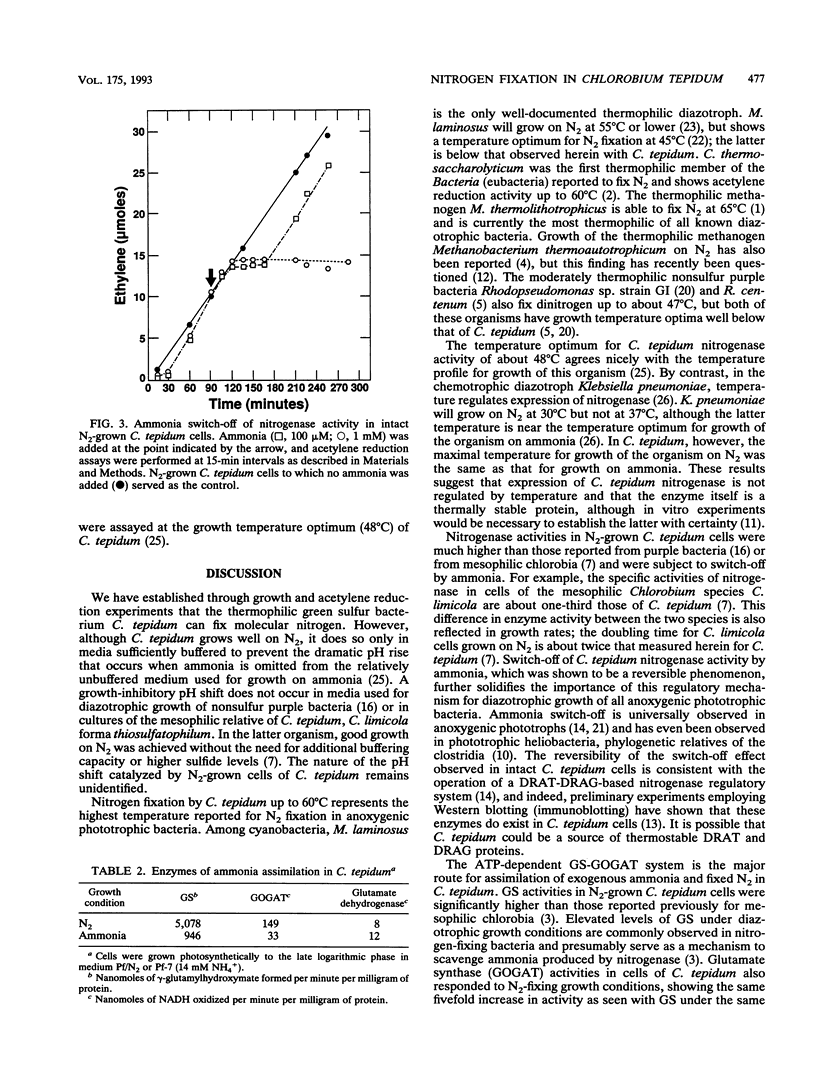
The thermophilic green sulfur bacterium Chlorobium tepidum grew with N2, NH4+, or glutamine as the sole nitrogen source under phototrophic (anaerobic-light) conditions. Growth on N2 required increased buffering capacity to stabilize uncharacterized pH changes that occurred during diazotrophic growth. Increased sulfide levels were stimulatory for growth on N2. Levels of nitrogenase activity (acetylene reduction) in N2-grown C. tepidum cells were very high, among the highest ever reported for anoxygenic phototrophic bacteria. Maximal acetylene reduction rates in C. tepidum cells were observed at 48 to 50 degrees C, which is about 15 degrees C higher than the optimum temperature for nitrogenase activity in mesophilic chlorobia, and nitrogenase activity in C. tepidum responded to addition of ammonia by a "switch-off/switch-on" mechanism like that in phototrophic purple bacteria. C. tepidum cells assimilated ammonia mainly via the glutamine synthetase-glutamate synthase pathway, elevated levels of both of these enzymes being present in cells grown on N2. These results show that N2 fixation can occur in green sulfur bacteria up to at least 60 degrees C and that regulatory mechanisms important in control of nitrogenase activity in mesophilic anoxygenic phototrophs also appear to regulate thermally active forms of the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belay N., Sparling R., Daniels L. Dinitrogen fixation by a thermophilic methanogenic bacterium. Nature. 1984 Nov 15;312(5991):286–288. doi: 10.1038/312286a0. [DOI] [PubMed] [Google Scholar]
- Bogdahn M., Kleiner D. N2 fixation and NH4+ assimilation in the thermophilic anaerobes Clostridium thermosaccharolyticum and Clostridium thermoautotrophicum. Arch Microbiol. 1986 Feb;144(1):102–104. doi: 10.1007/BF00454964. [DOI] [PubMed] [Google Scholar]
- Favinger J., Stadtwald R., Gest H. Rhodospirillum centenum, sp. nov., a thermotolerant cyst-forming anoxygenic photosynthetic bacterium. Antonie Van Leeuwenhoek. 1989 Mar;55(3):291–296. doi: 10.1007/BF00393857. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Roberts G. P. Regulation of nitrogenase activity by reversible ADP ribosylation. Curr Top Cell Regul. 1989;30:23–56. doi: 10.1016/b978-0-12-152830-0.50004-9. [DOI] [PubMed] [Google Scholar]
- Madigan M., Cox S. S., Stegeman R. A. Nitrogen fixation and nitrogenase activities in members of the family Rhodospirillaceae. J Bacteriol. 1984 Jan;157(1):73–78. doi: 10.1128/jb.157.1.73-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters R. A., Madigan M. Nitrogen metabolism in the phototrophic bacteria Rhodocyclus purpureus and Rhodospirillum tenue. J Bacteriol. 1983 Jul;155(1):222–227. doi: 10.1128/jb.155.1.222-227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Brill W. J. Temperature sensitivity of the regulation of nitrogenase synthesis by Klebsiella pneumoniae. J Bacteriol. 1981 Feb;145(2):1116–1118. doi: 10.1128/jb.145.2.1116-1118.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



