Abstract
Objectives
To determine, in a rural and urban population in Cameroon, the prevalence of the metabolic syndrome (MS) using three definitions and to assess the association between components of the MS and central obesity and HOMA insulin resistance (HOMA-IR) index.
Methods
A representative sample of 1573 adults (638 rural, 935 urban) were interviewed on their personal medical history. Blood pressure and anthropometric measures used standardised methods. After an overnight fast, blood samples were collected before and 2 hours after an OGTT and plasma glucose, plasma insulin and blood lipids determined. Modified WHO, NCEP-ATP III, and IDF definitions of the MS were used.
Results
Central obesity was the most prevalent component of the syndrome, but prevalence varied widely according to the definition used. Hypertriglyceridemia was almost non-existent. The highest prevalences of the MS were with the WHO definition and the lowest with the NCEP-ATP III definition. Central obesity was more tightly associated with components of the MS than was HOMA-IR.
Conclusions
The prevalence of the MS varied greatly by rural/urban residence with the various definitions used. Central obesity appears to be the key determinant of the prevalence of the MS in sub Saharan Africa. Many MS definitions may not be appropriate for African population.
Keywords: Metabolic syndrome, central obesity, Sub Saharan Africa, urbanisation
Keywords: Adiposity, Adult, Age Factors, Aged, Cameroon, epidemiology, Cluster Analysis, Female, Humans, Insulin Resistance, Male, Metabolic Syndrome X, epidemiology, etiology, metabolism, pathology, Middle Aged, Obesity, complications, pathology, Risk Factors, Sex Factors
The metabolic syndrome (MS) is a constellation of interrelated risk factors of metabolic origin that are associated with the development of atherosclerotic cardiovascular disease [1]. Several expert groups and institutions have proposed different criteria to diagnose the MS in clinical practice [2,3]. They include various combinations of central obesity, high blood pressure, high plasma glucose, dyslipidemia and insulin resistance. One of the most recently published definitions takes into account ethnic specific cut points for central obesity [2]. However, European cut-points are still recommended in African populations where specific data are not yet available.
The underlying risk factors for this syndrome appear to be abdominal obesity [4] and insulin resistance [3]; other associated conditions are physical inactivity [5], ageing [6], and polycystic ovarian syndrome [7]. The contribution of obesity and insulin resistance to the criteria defining the MS varies between the different definitions. The WHO and EGIR definitions gave a central role to insulin resistance, whereas in the IDF definition a pivotal role is assigned to central obesity, which is a mandatory criteria. This conflicting interpretation and variation in the components of the MS provide an insight into the scientific limitations of our understanding of the MS.
Africa is currently experiencing one of the most rapid demographic and epidemiological transitions in world history. The future impact of this on the prevalence of the MS unknown, but is a matter of concern. Although the prevalences of individual components of the MS have been reported from Sub-Saharan Africa [8–11], we are not aware of any population-based study of the prevalence of the MS in this part of the world. This study aims to assess the prevalence of the components of the MS and of the MS in Sub-Saharan Africa using three existing definitions, and to compare the association between the main components of the MS and central obesity and Homeostasis Assessment model for insulin resistance (HOMA-IR).
RESEARCH DESIGN AND METHODS
Subjects
The study population, sampling method and recruitment procedures have been described previously. [8,9] A total of 1986 Cameroonians aged 24–74 years from the States Housing District of Cité Verte in the capital city Yaoundé (urban area), and three villages of Evodoula (rural area) were included in this study.
Methods
Interviews were conducted by trained and fortnightly re-certified survey workers on personal medical history of hypertension, diabetes and dyslipidemia. Clinical examination included measurements of height and weight with subjects in light clothes. The means of three standard measurements of waist and hip circumferences were recorded, and the waist-hip ratio (WHR) calculated. Three consecutive diastolic and systolic blood pressures (SBP and DBF) were recorded on the right arm using a standard mercury sphygmomanometer with appropriate cuff sizes. The average of the second and third readings was used in the present analyses. A standard 75 g OGTT was performed in all subjects between 07.00 and 10.00 a.m., after an overnight fast of at least 12 hours. In each subject, 12.5 ml of whole blood was drawn before and 120 min after the glucose load for the determination of plasma glucose, insulin, total cholesterol and triglycerides. Blood for insulin determination was collected on ice, centrifuged immediately, separated and stored at −70° C until assayed. Plasma glucose was determined by the glucose-oxidase method using a spectrophotometer with external quality control on every 4th sample by a Cobas bio hexokinase fluorometric method. Insulin level was assayed in the Welcome Laboratories, Department of Medicine, University of Newcastle Upon Tyne, England, by ELISA method using DAKO kits (Hersteller, UK). The inter-assay coefficients of variation were low, 8.7%, medium, 2.7% and high 2.6% for the insulin assay. Serum cholesterol and triglycerides were measured by enzymatic colorimetric methods in the same laboratory in Newcastle.
Overall 1986 subjects (786 in the rural area and 1160 in the urban area) were invited to participate in the study. The response rate was 95 % and 91 % respectively in rural and urban areas. At least one clinical variable was not available for 104 subjects. Fasting triglycerides, total cholesterol and insulin were not available for 147 subjects. Analyses were on 1573 subjects.
Definitions
Homeostasis assessment model for insulin resistance (HOMA-IR) was calculated as: Fasting plasma glucose (mmol/l) × Fasting plasma insulin (μU/ml)/22.5. High cholesterol was defined as fasting total cholesterol ≥ 5.2 mmol/l[12].
Three definitions of the MS were used after some modification to account for the fact that HDL-cholesterol and microalbuminuria were not available:
-
WHO 1999 definition[13], Impaired Glucose Tolerance, Impaired Fasting Glycaemia, diabetes or highest quartile of HOMA plus two or more of any of the following:
WHR > 0.85 (women) 0.90 (men) and/or BMI > 30 kg/m2.
Fasting triglycerides ≥ 1.70 mmol/l.
SBP ≥ 140 mmHg and/or DBF ≥ 90 mmHg.
Fasting total cholesterol ≥ 5.2 mmol/l
-
NCEP-ATP III definition[14], three or more of any of the following:
Waist circumference > 88 cm (women) 102 cm (men).
Fasting triglycerides ≥ 1.70 mmol/l.
SBP ≥ 130 mmHg and/or DBF ≥ 85 mmHg.
Fasting glucose ≥ 6.1 mmol/l.
Fasting total cholesterol ≥ 5.2 mmol/l.
-
IDF 2005 definition[2], waist circumference ≥ 80 cm (women) ≥ 94 cm (men) plus two or more of any of the following:
Fasting triglycerides > 1.70 mmol/l or triglyceride lowering drugs.
SBP ≥ 130 mmHg and/or DBF ≥ 85 mmHg, or blood pressure lowering treatment.
Fasting plasma glucose ≥ 5.6 mmol/l, or anti-diabetic treatment.
Fasting total cholesterol ≥ 5.2 mmol/l.
Statistical analysis
Analyses used STATA® 8.2 Software. All analyses were stratified by sex. Results are presented as means (standard error), percentages (95 % CI) and adjusted odds ratios (95 % CI). Fasting triglyceride, fasting and 2 hour post load insulin and HOMA-IR were non-normally distributed and were logarithmically transformed before comparisons. Means and percentages between groups were compared using the Student-t, and Chi-square or two-sided Fisher Exact tests. Since the mean age of study participants was higher in the rural than the urban area, age-adjusted linear and logistic regression models were used to compare means and proportions of different components of the MS within sites of residence, after stratification on gender. The trend in the prevalence of the MS across age classes was tested by the Armitage test. The association between waist circumference, HOMA-IR and other components of the MS (as dependent variables) used linear regression models with adjustments for 1) age, place of residence and sex; 2) age, place of residence, sex and BMI. The relations were expressed as standardised beta coefficients and the percent increase in the adjusted R2 when central obesity or HOMA-IR was added to the model in comparison to the basic models 1) and 2)
Ethical considerations
The study protocol was approved by the National Ethics Committee of the Ministry of Public Health, Cameroon. All subjects gave informed consent to participate and the authors followed the Declaration of Helsinki on biomedical research involving human subjects.
RESULTS
General characteristics of the study population
After adjustment for age, all anthropometric parameters (except waist circumference for women and WHR for both sexes), SBP and DBF, and all biological parameters (except plasma glucose and triglycerides for women) were significantly increased among subjects living in the urban area (Table 1).
Table 1.
Age adjusted, gender specific rural - urban characteristics of the study population
| Clinical and biological variables | WOMEN
|
MEN
|
||||
|---|---|---|---|---|---|---|
| Rural | Urban | P | Rural | Urban | P | |
| N
|
374 | 513 | 252 | 414 | ||
| Age (years)
|
46 (13) | 37 (9) | < 0.001 | 46 (14) | 38 (9) | < 0.001 |
| Anthropometric parameters
|
||||||
| Body mass index (kg/m2) | 22.1 (0.2) | 26.4 (0.2) | < 0.001 | 21.7 (0.2) | 25.1 (0.2) | < 0.001 |
| Waist circumference (cm) | 81.2 (0.5) | 81.3 (0.4) | 0.9 | 79.6 (0.5) | 84.4 (0.4) | < 0.001 |
| Hip circumference (cm) | 91.2 (0.5) | 103.8 (0.4) | < 0.001 | 89.2 (0.5) | 98.7 (0.4) | < 0.001 |
| Waist hip ratio | 0.90 (0.004) | 0.78 (0.004) | < 0.001 | 0.90 (0.004) | 0.85 (0.003) | < 0.001 |
| Blood Pressure (mmHg)
|
||||||
| Systolic | 112.4 (0.9) | 114.8 (0.7) | 0.007 | 118.1 (1.1) | 123.3 (0.9) | < 0.001 |
| Diastolic | 69.9 (0.6) | 74.7 (0.5) | < 0.001 | 72.6 (0.9) | 81.8 (0.7) | < 0.001 |
| Plasma glucose (mmol/l)
|
||||||
| Fasting | 4.1 (0.1) | 4.2 (0.1) | 0.5 | 4.1 (0.1) | 4.3 (0.1) | 0.09 |
| 2 hour post load | 5.1 (0.1) | 4.9 (0.1) | 0.2 | 5.2 (0.1) | 5.2 (0.1) | 0.8 |
| Insulin (μU/ml)
|
||||||
| Fasting* | 2.9 (1.0) | 5.4 (1.0) | < 0.001 | 1.9 (1.0) | 4.5 (1.0) | < 0.001 |
| 2 hour post load* | 10.5 (1.0) | 24.2 (1.0) | < 0.001 | 8.2 (1.0) | 21.8 (1.0) | < 0.001 |
| Blood lipids
|
||||||
| Total cholesterol (mmol/l) | 2.6 (0.04) | 3.6 (0.03) | < 0.001 | 2.5 (0.1) | 3.5 (0.04) | < 0.001 |
| Triglycerides (mmol/l)* | 0.48 (1.0) | 0.47 (1.0) | 0.2 | 0.48 (1.0) | 0.53 (1.0) | 0.001 |
| HOMA-IR* |
0.09 (1.0) | 0.20 (1.0) | <0.001 | 0.06 (1.0) | 0.10 (1.0) | <0.001 |
Variables logarithmically transformed before comparisons; non transformed values are expressed for easier interpretation. Data are means (standard errors of the mean).
Prevalence of the components of the metabolic syndrome
There was a wide difference in the prevalence of central obesity and obesity (Table 2). Central obesity defined by WHR was three to six times higher in the rural than the urban area. The prevalence of central obesity using the WHO definition (which is based on BMI and WHR) was higher in the rural region, but this difference was no longer significant for men after adjustment for age. Age adjusted prevalence of central obesity (NCEP-ATP III and IDF definitions, based on waist circumference) were lower in the rural than the urban area (Table 2), and higher in women compared to men.
Table 2.
Gender specific rural - urban characteristics (unadjusted prevalences and age adjusted odds ratios) for components of the metabolic syndrome:
| Components of the metabolic syndrome | WOMEN
|
MEN
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted prevalences
|
Age adjusted ORs
|
Unadjusted prevalences
|
Age adjusted ORs
|
|||||||
| Rural | Urban | P | Rural | Urban | Rural | Urban | P | Rural | Urban | |
| Central obesity or obesity
|
||||||||||
| WHR (M>0.90/W>0.85) | 73.5 | 13.5 | <0.001 | 1 | 0.07 (0.05–0.1)* | 35.7 | 13.3 | < 0.001 | 1 | 0.4 (0.3 – 0.7)* |
| BMI ≥ 30 kg/m2 | 2.4 | 20.5 | <0.001 | 1 | 17 (8 – 37)* | 1.2 | 10.9 | < 0.001 | 1 | 21 (6 – 74)* |
| WHO definition | 74.1 | 29.6 | <0.001 | 1 | 0.2 (0.15 – 0.3)* | 35.7 | 20.3 | < 0.001 | 1 | 0.8 (0.5 – 1.1) |
| IDF definition | 58.6 | 49.5 | 0.008 | 1 | 1.0 (0.8 – 1.4) | 7.5 | 12.8 | 0.03 | 1 | 3.2(1.7–6.2)‡ |
| NCEP-ATP III Definition | 22.2 | 23.8 | 0.6 | 1 | 1.4 (0.97 – 2.0) | 2.4 | 4.4 | 0.2 | 1 | 4.0 (1.3– 11)† |
| High blood Pressure
|
||||||||||
| WHO definition | 9.4 | 8.7 | 0.7 | 1 | 2.0 (1.1– 3.4)† | 14.3 | 21.6 | 0.02 | 1 | 2.9(1.7–4.7)* |
| NCEP-ATP III definition | 18.3 | 15.7 | 0.3 | 1 | 1.6 (1.1– 2.5)† | 29.1 | 35.3 | 0.09 | 1 | 2.0 (1.4 – 3.0)* |
| IDF definition | 19.0 | 18.5 | 0.9 | 1 | 2.0 (1.3 – 3.0)* | 29.4 | 37.7 | 0.03 | 1 | 2.3 (1.5 – 3.3)* |
| High glucose
|
||||||||||
| WHO definition | 6.4 | 3.2 | 0.02 | 1 | 0.7 (0.6 – 1.5) | 11.5 | 6.0 | 0.02 | 1 | 0.8 (0.4 – 1.4) |
| NCEP-ATP III definition | 1.1 | 1.2 | 0.9 | 1 | 2.1 (0.5 – 8.8) | 1.2 | 1.2 | 1.0 | 1 | 1.6 (0.3 – 8) |
| IDF definition | 1.3 | 2.5 | 0.2 | 1 | 2.8 (0.9 – 8.7) | 1.6 | 2.4 | 0.6 | 1 | 2.2 (0.6 – 7.9) |
| High lipids
|
||||||||||
| High triglycerides | 0.3 | 0.4 | 1.0 | 1 | 1.1 (0.1 – 13) | 0.4 | 1.4 | 0.3 | 1 | 3.6 (0.4 – 32) |
| High total cholesterol | 0.3 | 2.7 | 0.005 | 1 | 13.0 (2– 110)† | 0.0 | 2.9 | 0.006 | --- | --- |
| ≥ 4th quartile HOMA-IR (%)
|
13.2 | 37.8 | <0.001 | 1 | 4.1 (2.8 – 5.9)* | 5.8 | 31.7 | <0.001 | 1 | 12 (5 – 23)* |
| IR and/or IGR (WHO)
|
19.7 | 38.9 | <0.001 | 1 | 2.7 (2.0 – 3.8)* | 17.1 | 33.4 | <0.001 | 1 | 3.7 (2.7 – 3.8)* |
| Prevalence of metabolic syndrome
|
||||||||||
| WHO definition | 1.8 | 5.9 | 0.002 | 1 | 6.4 (2.5 – 16)† | 1.9 | 7.3 | 0.001 | 1 | 13 (4– 39)† |
| NCEP-ATP III definition | 0.0 | 0.2 | 1.0 | 1 | --- | 0.0 | 0.5 | 0.5 | 1 | --- |
| IDF definition | 0.3 | 1.5 | 0.09 | 1 | 11(1.3 – 100)‡ | 0.0 | 1.2 | 0.08 | 1 | --- |
P for difference: < 0.05
< 0.01
<0.001.
IR: insulin resistance; IGR: impaired glucose regulation (impaired glucose tolerance + impaired fasting glycaemia + diabetes)
WHO and IDF definitions gave respectively the lowest and the highest prevalences of hypertension (Table 2). When adjusted for age, the prevalence of high blood pressure was two times higher in the urban compared to the rural area, irrespective of the definition used. Lowering the diagnostic criteria of hypertension from 140/90 mmHg to 130/85 mmHg doubled the prevalence of hypertension in the rural and the urban area.
The NCEP-ATP III definition gave the lowest prevalence of impaired glycaemia (women: rural, 1.1% and urban, 1.2%, p=0.9; men: rural, 1.2% and urban, 1.2%, p=1.0) while the WHO definition gave the highest prevalence (women: rural, 6.4% and urban, 3.2%, p<0.02; men: rural, 11.5% and urban, 6.0%, p<0.02). After adjusting for age, the differences were no longer significant between the places of residence.
None of the subjects was taking lipids-lowering drug. There was no difference between rural and urban area for the prevalence of high triglycerides, while high total cholesterol was more frequent in the urban area (women: 0.3% rural vs 2.7% urban, p<0.005 and men: 0.0 % rural vs 2.9 % urban, p < 0.006).
Clustering of the components of the metabolic syndrome
The clustering of the components was assessed using the NCEP-ATP III definition. For two components the most frequent combinations were central obesity and high blood pressure (81% in women and 52% in men), high blood pressure and hypercholesterolemia (6% in women and 24% in men), high blood pressure and hyperglycaemia (6% in women and 12% in men). Two combinations of three components were found: central obesity, high blood pressure, high cholesterol and high blood pressure (100% in women), high blood glucose, high triglycerides. No subject had four components.
Prevalence of the metabolic syndrome
The prevalence of the MS, using the WHO definition, increased with age classes in men in rural area and in both genders in urban area (Figure 1). The highest prevalences of the MS were obtained with the WHO criteria (women: rural, 1.8 (0.6–3.5) % and urban, 5.9 (3.3–7.3) %, p=0.002; men: rural, 1.9 (0.6–4.6) % and urban, 7.9 (5.6–11.3) %, p = 0.001). These differences were still significant after age adjustments, even if the urban subjects were younger than the rural. No subject in the rural area in either gender (NCEP-ATP III definition) or men (IDF definition) had the MS while its prevalence in the urban area was under 2.0 % (Table 2).
Figure 1.
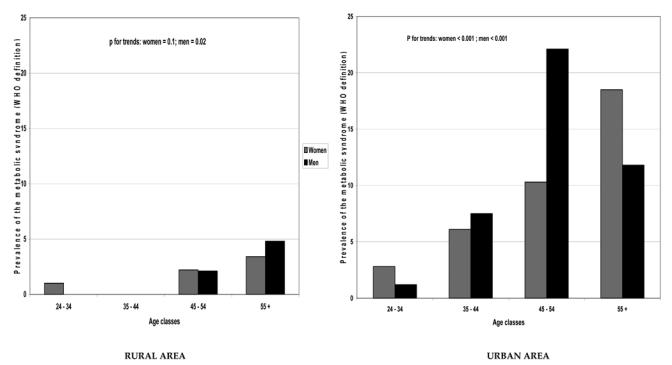
Relationship between age classes and the prevalence of metabolic syndrome, stratified on gender and place of residence.
Central obesity, HOMA-IR and the other components of the MS
After adjusting for age, place of residence and sex, HOMA-IR was positively associated with all the components of the metabolic syndrome. Waist circumference was also associated with components of the MS, except the associations with plasma glucose were not significant (Table 3). However, apart from fasting and 2 hour glucose, the standardised beta coefficients and the increases in the adjusted R2 were higher for all the components for waist circumference than for the HOMA-IR. This suggested the association with these components of the MS was stronger in this population for waist circumference than it was for HOMA-IR. After adjusting for BMI, the association between total cholesterol and waist circumference was no longer significant, while for HOMA-IR, its association with systolic and diastolic blood pressure, and total cholesterol were no longer significant (Table 3).
Table 3.
Standardised beta coefficients of the relation between each component of the metabolic syndrome and 1) waist circumference 2) HOMA-IR after adjustments, and adjusted R2 associated with the introduction of these terms in the basic models
| Waist circumference
|
HOMA-IR* |
|||||
|---|---|---|---|---|---|---|
| Model I† | Standardised β | P | Δ Adjusted R2§ | Standardised β | P | Δ Adjusted R2§ |
| Systolic blood pressure (mmHg) | 0.25 | < 0.001 | + 5.7 % | 0.10 | < 0.001 | + 0.72 % |
| Diastolic blood pressure (mmHg) | 0.26 | < 0.001 | + 6.3 % | 0.07 | 0.006 | +0.37 % |
| Fasting plasma glucose (mmol/l) | 0.02 | 0.4 | − 0.01 % | 0.32 | < 0.001 | + 8.00 % |
| 2 hours plasma glucose (mmol/l) | 0.045 | 0.08 | + 0.13 % | 0.25 | < 0.001 | + 4.78 % |
| Total cholesterol (mmol/l) | 0.12 | < 0.001 | + 1.4 % | 0.06 | 0.02 | + 0.23 % |
| Total triglycerides (mmol/l)* | 0.16 | < 0.001 | + 4.1 % | 0.12 | < 0.01 | + 2.04 % |
| Model II|| |
||||||
| Systolic blood pressure (mmHg) | 0.18 | < 0.001 | + 1.16 % | 0.04 | 0.2 | + 0.05 % |
| Diastolic blood pressure (mmHg) | 0.16 | < 0.001 | + 0.94 % | 0.001 | 1.0 | − 0.06 % |
| Fasting plasma glucose (mmol/l) | − 0.008 | 0.8 | − 0.06 % | 0.33 | < 0.001 | + 8.09 % |
| 2 hours plasma glucose (mmol/l) | − 0.013 | 0.7 | − 0.06 % | 0.23 | < 0.001 | + 4.30 % |
| Total cholesterol (mmol/l) | − 0.006 | 0.87 | − 0.05 % | 0.013 | 0.6 | − 0.04 % |
| Total triglycerides (mmol/l)* | 0.15 | < 0.001 | + 1.78 % | 0.09 | < 0.001 | + 1.13 % |
Variables logarithmically transformed before comparisons
Variation of adjusted R2 when waist circumference (‡) or HOMA-IR (§) is introduced in the model.
Model adjusted for age, place of residence, and sex
Model adjusted for age, place of residence, sex and BMI
CONCLUSION
This is the first report on the prevalence of the MS in Sub Saharan Africa. Although reported at low rates compared to other developing countries such as India and China, the prevalence of the MS in rural and urban Cameroon varied considerably across definitions and place of residence. The WHO definition which included a measure of insulin resistance, gave the highest prevalence because, HOMA-IR, by definition, affected 25 % of the population. Central obesity was more tightly associated with the other components of the MS than HOMA-IR.
Potential limitations which may affect the generalisability of the findings are that the urban population is ethnically heterogeneous, while the rural population is homogeneous. Although 14 % of the subjects were not included in the analyses because of missing data, this was not likely to have caused bias, as the main clinical variables available (age, BMI, wait and hip circumference, SBP, DBF) were not significantly different between subjects with missing and non-missing data. HDL cholesterol was replaced by total cholesterol as a diagnostic criterion. This had no major impact on the prevalence of the MS, as the prevalence of high cholesterol was very low.
This study provides the lowest reported population-based prevalence of the MS in the literature, compared to prevalences found in other developing and developed countries [15].
Many hypotheses could explain this low prevalence of MS. The first reason is the relatively young age our population. It is well known that the prevalence of the MS increase with age[6,16–18]. With the ageing of our population, the prevalence of MS will probably increase to levels similar to those described elsewhere, notably in African American populations. The prevalence MS components in Cameroon can provide another explanation. High prevalences of central obesity and hypertension were balanced by low prevalences of diabetes and dyslipidemia: hypertriglyceridaemia using the same threshold was low in Cameroon (less than 2 % in urban area), compared to 12.6 to 24.8 % in China and 28.6 % to 32.3 % in India[16–18]. These differences are probably an indicator of the temporal pathway from exposure to weight gain to development of the MS and subsequently diabetes and cardiovascular disease. The high prevalence of central obesity and hypertension should be considered as indicators of an imminent surge in the prevalence of diabetes. In fact, studied Cameroonians were probably not obese for long enough to develop metabolic complications of obesity. This is illustrated by the lack of association between waist circumference and glucose in this study. Cameroon is a developing country where undernutrition is common, and the prevalence of childhood obesity is very low (unpublished data). Subjects usually gain weight later in their adult life. Although the prevalence of central obesity and obesity being at the level of developed countries, the mean HOMA-IR was in this population 10 to 20 times lower than values found in developed countries[15]. Ethnicity can provide another clue. Blacks are known to have lower body fat for the same BMI than Caucasians[19]. This has implications on the interrelationship between obesity and the other components of the MS, but also raises the question on the use of diagnostic tools in Blacks based on criteria derived from Caucasian populations. Urbanization is the last possible explanation. Total body fat[20], as well as the prevalence of the components of the MS has been shown to be related to urbanisation[21,22]. This is substantiated by the high urban-rural difference in the prevalence of MS.
The higher prevalence of the MS in the urban area is in accordance with other studies on the components of the MS[8,9,23]. Urbanisation is associated with lifestyles that favour the development of obesity and the MS. Such changes are operating already in the rapidly urbanising Cameroonian population. As previously reported subjects in rural Cameroon exhibit higher occupational and leisure time physical activity[23] compared to urban dwellers. This can lead to different body composition and body shape[19,24], by influencing the percent body fat, and the body build. In contrast, in this study rural subjects had higher WHR but lower waist and hip circumference, and were less insulin resistant compared to the urban population.
The prevalence of obesity was ten times higher in women than in men, irrespective of the definition used. Apart from genetic and hormonal differences, this can partly be explained by the high number of deliveries and as well their lower level of physical activity [23]. Although more noted in the rural area, there was a great disparity in the prevalence of adiposity according to the definition used. WHR measures central fat deposition but is a poor measure of visceral fat mass, particularly in lean individuals[25], as demonstrated in this study. Therefore, WHR is probably not adapted in this population to assess central obesity. It appears that the cut-points used are not adapted to African populations, stressing the need for studies to identify criteria for obesity and central obesity suitable for African populations.
Differences in the prevalence of high plasma glucose between WHO/IDF-ATP III definitions are explained by the high prevalence of IGT in this population[9]. This study also confirms previous reports of lower triglyceride and total cholesterol level in African, compared to Caucasian populations[12,26–28]. Apart from dietary and physical activity patterns as highlighted by the rural-urban difference in this study, genetic predisposition may explain this variability, since even migrant Africans display lower blood lipid values than Caucasians[27,28]. Further the atherogenic lipid profile in our population may be different from the one described in Caucasians. This emerging issue has to be addressed in order to provide successful cardiovascular preventive measures in this setting.
Central obesity had stronger associations with components of the MS than HOMA-IR. Although this was a cross sectional study, these findings support the central role of abdominal adiposity in the MS in comparison to IR. This conclusion has to be taken as IR was artificially fixed at 25%. Also, the use of the WHO definition in daily practice in is not possible, as insulinemia is not measured routinely.
This study illustrates the complexity of the application of diagnostic tools developed in one population to a different population. The three MS definitions have generated a range of prevalences, not only as a consequence of the real burden of the MS in this population, but also because of the components in each definition and cutoff levels used. That the reported results are the translation of the burden of the MS in this population is questionable. The prevalence of individual risk factors and the rural-urban trends in these prevalences are indicators of the burgeoning epidemic of the MS and its subsequent consequences in Sub-Saharan Africa. The trends in this phenomenon have to be monitored in future studies, and specific cut-points, at least for central obesity, need to be defined for the African population. Preventive actions have to be implemented to reduce the impending impact of the MS in this population.
Acknowledgments
This study was supported by a grant from the European Union, contract NO TS3* CT92-0142. The insulin assays were undertaken in the Department of Medicine, University of Newcastle upon Tyne thanks to a generous support from Professor George Alberti. We wish to thank Pierre Ducimetierre, Marie-Aline Charles and Dominique Simon from INSERM Unité 780 for their input during the writing of this article. We also wish to thank Dr Richard Edwards for his help in the final correction of the article.
References
- 1.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 4.Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–94. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 5.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–36. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 7.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–35. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 8.Mbanya JC, Minkoulou EM, Salah JN, Balkau B. The prevalence of hypertension in rural and urban Cameroon. Int J Epidemiol. 1998;27:181–5. doi: 10.1093/ije/27.2.181. [DOI] [PubMed] [Google Scholar]
- 9.Mbanya JC, Ngogang J, Salah JN, et al. Prevalence of NIDDM and impaired glucose tolerance in a rural and an urban population in Cameroon. Diabetologia. 1997;40:824–9. doi: 10.1007/s001250050755. [DOI] [PubMed] [Google Scholar]
- 10.Puoane T, Steyn K, Bradshaw D, et al. Obesity in South Africa: the South African demographic and health survey. Obes Res. 2002;10:1038–48. doi: 10.1038/oby.2002.141. [DOI] [PubMed] [Google Scholar]
- 11.van der Sande MA, Milligan PJ, Nyan OA, et al. Blood pressure patterns and cardiovascular risk factors in rural and urban gambian communities. J Hum Hypertens. 2000;14:489–96. doi: 10.1038/sj.jhh.1001050. [DOI] [PubMed] [Google Scholar]
- 12.Fuentes R, Uusitalo T, Puska P, et al. Blood cholesterol level and prevalence of hypercholesterolaemia in developing countries: a review of population-based studies carried out from 1979 to 2002. Eur J Cardiovasc Prev Rehabil. 2003;10:411–9. doi: 10.1097/01.hjr.0000085247.65733.4f. [DOI] [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Balkau B, Charles MA, Drivsholm T, et al. Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28:364–76. [PubMed] [Google Scholar]
- 16.Gu D, Reynolds K, Wu X, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R, Deedwania PC, Gupta A, et al. Prevalence of metabolic syndrome in an Indian urban population. Int J Cardiol. 2004;97:257–61. doi: 10.1016/j.ijcard.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Ko GT, Cockram CS, Chow CC, et al. High prevalence of metabolic syndrome in Hong Kong Chinese--comparison of three diagnostic criteria. Diabetes Res Clin Pract. 2005;69:160–8. doi: 10.1016/j.diabres.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–71. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 20.Luke A, Durazo-Arvizu R, Rotimi C, et al. Relation between body mass index and body fat in black population samples from Nigeria, Jamaica, and the United States. Am J Epidemiol. 1997;145:620–8. doi: 10.1093/oxfordjournals.aje.a009159. [DOI] [PubMed] [Google Scholar]
- 21.Fezeu L, Minkoulou E, Balkau B, et al. Association between socioeconomic status and adiposity in urban Cameroon. Int J Epidemiol. 2006;35:105–11. doi: 10.1093/ije/dyi214. [DOI] [PubMed] [Google Scholar]
- 22.Kim MH, Kim MK, Choi BY, Shin YJ. Educational disparities in the metabolic syndrome in a rapidly changing society--the case of South Korea. Int J Epidemiol. 2005;34:1266–73. doi: 10.1093/ije/dyi175. [DOI] [PubMed] [Google Scholar]
- 23.Sobngwi E, Mbanya JC, Unwin NC, et al. Physical activity and its relationship with obesity, hypertension and diabetes in urban and rural Cameroon. Int J Obes Relat Metab Disord. 2002;26:1009–16. doi: 10.1038/sj.ijo.0802008. [DOI] [PubMed] [Google Scholar]
- 24.Deurenberg P, Deurenberg Yap M, Wang J, et al. The impact of body build on the relationship between body mass index and percent body fat. Int J Obes Relat Metab Disord. 1999;23:537–42. doi: 10.1038/sj.ijo.0800868. [DOI] [PubMed] [Google Scholar]
- 25.Han TS, van Leer EM, Seidell JC, Lean ME. Waist circumference as a screening tool for cardiovascular risk factors: evaluation of receiver operating characteristics (ROC) Obes Res. 1996;4:533–47. doi: 10.1002/j.1550-8528.1996.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 26.Berrios X, Koponen T, Huiguang T, et al. Distribution and prevalence of major risk factors of noncommunicable diseases in selected countries: the WHO Inter-Health Programme. Bull World Health Organ. 1997;75:99–108. [PMC free article] [PubMed] [Google Scholar]
- 27.Ford ES, Giles WH. A comparison of the prevalence of the metabolic syndrome using two proposed definitions. Diabetes Care. 2003;26:575–81. doi: 10.2337/diacare.26.3.575. [DOI] [PubMed] [Google Scholar]
- 28.Zoratti R. A review on ethnic differences in plasma triglycerides and high-density-lipoprotein cholesterol: is the lipid pattern the key factor for the low coronary heart disease rate in people of African origin? Eur J Epidemiol. 1998;14:9–21. doi: 10.1023/a:1007492202045. [DOI] [PubMed] [Google Scholar]


