Summary
Primary rabbit kidney epithelial cell cultures can be obtained that express renal proximal tubule functions. Toward these ends, renal proximal tubules are purified from the rabbit kidney by the method of Brendel and Meezan. To summarize, each kidney is perfused with iron oxide, which becomes associated with glomeruli. The renal cortex is sliced and homogenized to liberate nephron segments. Renal proximal tubules and glomeruli are purified by sieving. The glomeruli, covered with iron oxide, are removed using a magnet. After a brief collagenase treatment (to disrupt basement membrane), the tubules are plated in hormonally defined serum-free medium supplemented with 5 μg/mL bovine insulin, 5 μg/mL human transferrin, and 5 × 10−8 M hydrocortisone. After 5–6 d of incubation, confluent monolayers are obtained that possess multicellular domes, indicative of their capacity for transepithelial solute transport.
Keywords: Primary culture, kidney, renal proximal tubule, serum-free medium, epithelial cell growth
1. Introduction
An important application of hormonally defined serum-free media is for preparing primary cultures of differentiated cells that lack fibroblast overgrowth. Of particular interest in this regard is the serum-free culture of primary kidney tubule epithelial cells (1–4). Investigations with primary kidney cell cultures are particularly advantageous for several reasons. First, kidney cells can be grown in vitro from the animal of choice. Thus, the results of tissue culture studies can be more closely correlated with animal studies. Second, new tissue culture systems can be developed that more closely resemble the kidney cells in vivo than presently available established kidney cell lines. Third, the use of serum-free medium permits a precise analysis of the mechanisms by which hormones and growth factors regulate growth and expression of differentiated function.
Of particular interest to this chapter is the use of serum-free medium to grow primary cultures of rabbit kidney epithelial cells that express renal proximal tubule functions. Rabbit kidney proximal tubules are first purified from the renal cortex by a modification (4–6) of the Brendel and Meezan method (7,8) and then placed into tissue culture dishes containing serum-free medium supplemented with three growth supplements: insulin, transferrin, and hydrocortisone. Within the first day of culture, the tubules attach to the culture dish. Subsequently, epithelial cells grow out from the tubule explants. After 1 wk, confluent monolayers are obtained, which possess multicellular domes (see Fig. 1), indicative of their capacity for transepithelial solute transport from the cells’ apical surface (facing the culture medium) through the basolateral surface (facing the culture dish). An examination of the primary kidney epithelial cells by transmission electron microscopy (TEM) (see Fig. 2) indicates that the cells do indeed possess such a polarized morphology and are interconnected by tight junctions. An examination of the apical surface by scanning electron microscopy (SEM) (see Fig. 3) also shows the presence of dense clusters of microvilli, typical of the renal proximal tubule. In addition to retaining a polarized morphology, the primary cultures express a number of renal proximal tubule functions, including γ-glutamyl transpeptidase, phosphoenolpyruvate carboxykinase, a sodium–glucose cotransport system, and a p-aminohippurate transport system, which are distinctive of renal proximal tubule cells (see Table 1).
Fig. 1.
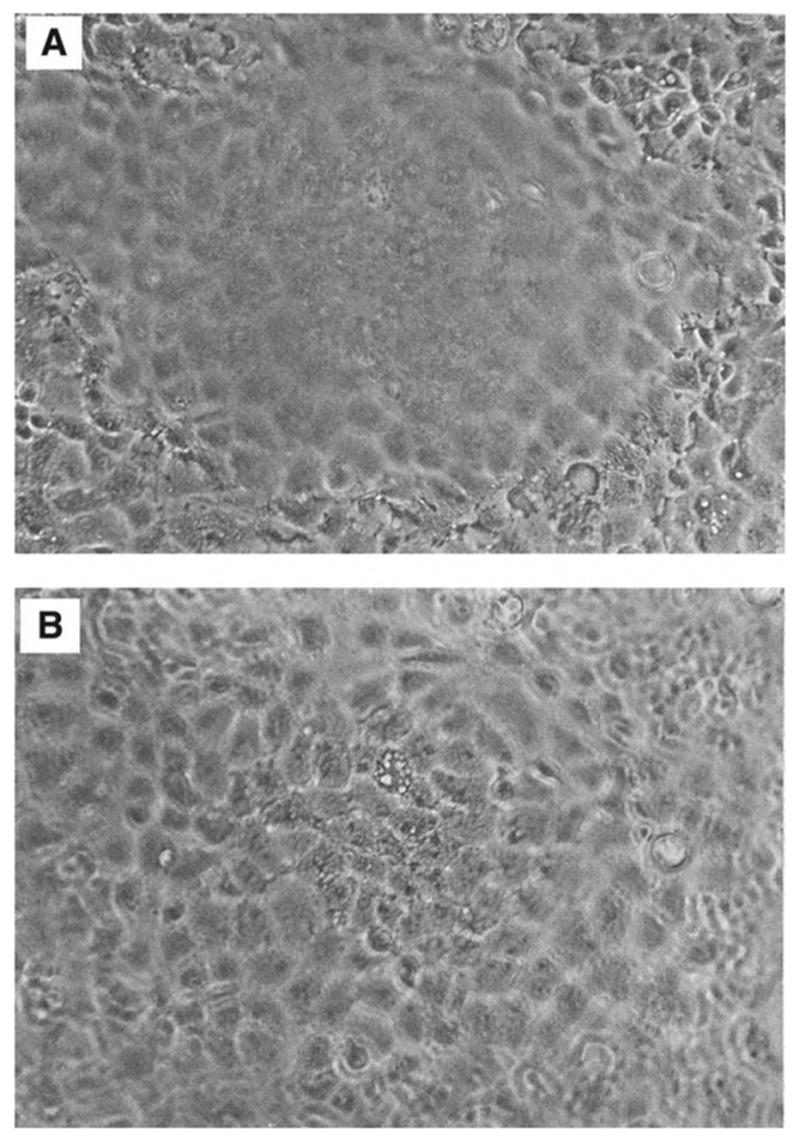
Dome formation by rabbit kidney proximal tubule cell cultures. A photomicrograph was taken of a confluent monolayer under an inverted microscope at ×100 magnification. Panel A focuses on the cells in the monolayer, whereas panel B focuses on the cells in the dome.
Fig. 2.
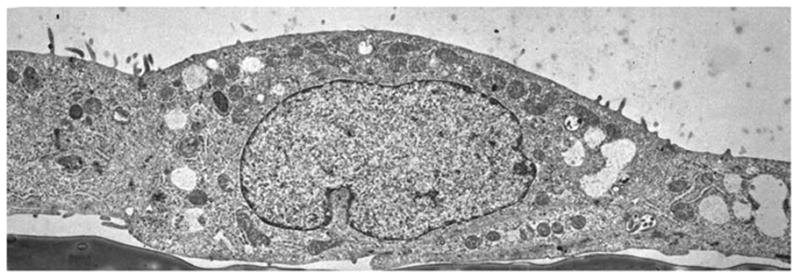
Transmission electron microscopy (TEM) of primary renal proximal tubule cells. Primary rabbit kidney proximal tubule cells were cultured on a plastic substratum in serum-free medium (as in Fig. 1), processed for TEM (9), and photographed at ×4000 magnification.
Fig. 3.
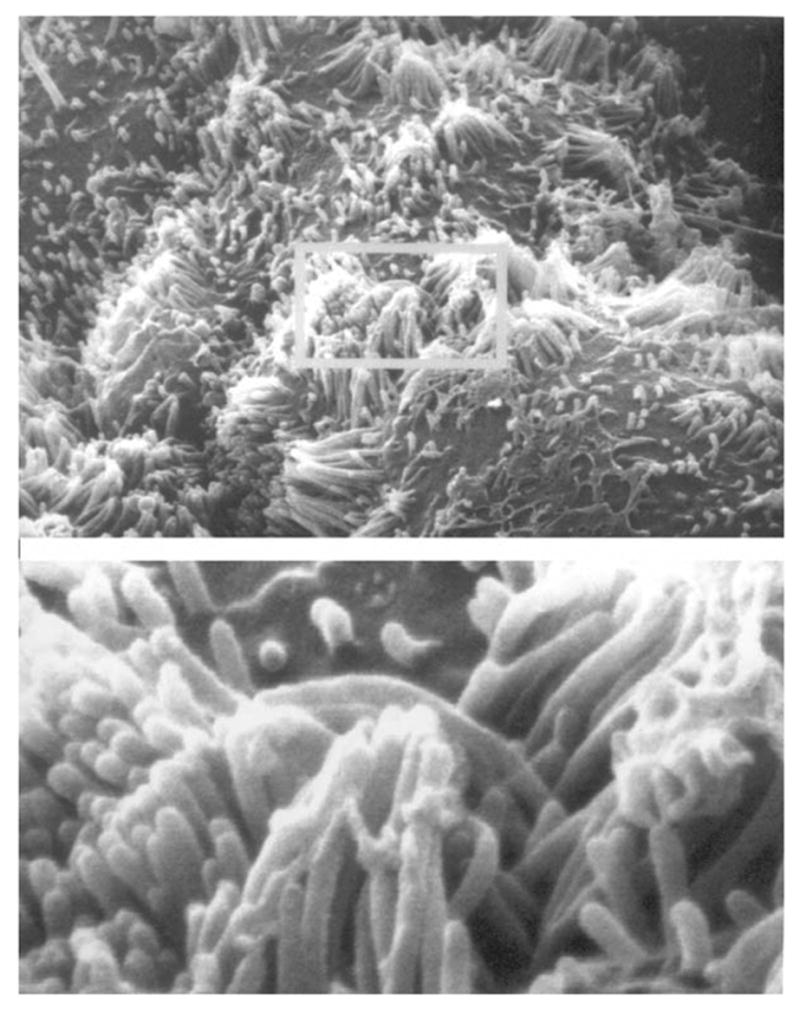
Scanning electron microscopy (SEM) of primary renal proximal tubule cells. A monolayer of primary rabbit kidney proximal tubule cells was examined by SEM at ×6000 magnification (insert is ×30,000).
Table 1.
Properties of Primary Rabbit Kidney Proximal Tubule Cell Cultures
|
These monolayer cultures can be used for a large number of purposes, ranging from infection with viruses, and subsequent viral production, to transfection with plasmid DNAs containing oncogenes for subsequent cell immortalization. The confluent monolayers are amenable to biochemical studies when cultured on plastic, as well as electrophysiologic studies when cultured on permeable supports. Now that many of the differentiated functions of the renal proximal tubule cells have been defined and appropriate genes have been cloned, the primary cultures are amenable for molecular biology studies concerning the control of the expression of differentiated function.
2. Materials
One of the most critical steps involved in the initial setup prior to culturing renal proximal tubule cells includes the preparation of the basal medium (see Note 1). As the cells are grown serum-free, additional medium supplements are required, including insulin, transferrin, and hydrocortisone. In addition, antibiotics can be added to the medium as a preventative against bacterial growth (see Note 2). Finally, implements required for the dissection of the kidney and purification of renal proximal tubules must be sterilized prior to use.
Milli-Q reagent-grade water.
Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F12 (F12) (50:50 mixture). Mix 10 L DMEM (with 4.5 g/L D-glucose and L-glutamine, and without either sodium pyruvate or sodium bicarbonate) and 10 L of Ham’s F12 medium to obtain a 50:50 mixture (DMEM/F12).
Peristaltic pump.
Kanamycin. Sterile aliquots of 1000X concentrated kanamycin (100 mg/mL) are prepared by dissolving 5.0 g of kanamycin in 50 mL Milli-Q water containing 0.425 g NaCl. The solution is sterilized through a 0.22-μm syringe filter, aliquoted into 5-mL sterile polystyrene tubes, and stored at −20°C. Aliquots in use are stored at 4°C.
Penicillin. Sterile aliquots of 1000X concentrated penicillin (92,000 IU/mL) are prepared by dissolving 3.05 g penicillin in 50 mL Milli-Q water containing 0.425 g NaCl/50 mL. The solution is sterilized through a syringe filter (0.22 μm), aliquoted into 5-mL sterile polystyrene tubes, and stored at −20°C. Aliquots being used are stored at 4°C.
Streptomycin. Sterile aliquots of 1000X concentrated streptomycin (200 μg/mL) are prepared by dissolving 10 g streptomycin in 50 mL Milli-Q water. The solution is sterilized through a syringe filter (0.22 μm) and aliquoted into 5-mL sterile polystyrene tubes. The aliquots are stored at −20°C for up to 1 yr. Aliquots in use are stored at 4°C.
Na+ bicarbonate.
HEPES.
Basal media. The basal medium consists of DMEM/F12 supplemented with 15 mM HEPES buffer (pH 7.4) and 20 mM sodium bicarbonate (DMEM/F12). The medium is sterilized using a Millipak filter unit with a 0.22-μm pore size. A Millipore peristaltic pump is utilized to pump the medium from a reservoir (a 20-L carboy), through the Millipak filter, into sterile medium bottles. Individual bottles of medium are stored frozen at −20°C, until ready for use. After thawing, the medium is kept at 4°C for up to 2 wk.
Antibiotic-supplemented basal media. This medium is used throughout the tubule isolation protocol. It is prepared by adding 92 IU penicillin and 200 μg/mL streptomycin to the basal medium (see Note 2).
Complete growth media. The basal medium is supplemented with 5 μg/mL bovine insulin, 5 μg/mL human transferrin, and 5 × 10−8 M hydrocortisone on the day of use. The sterile stock solutions of 5 mg/mL bovine insulin, 5 mg/mL human transferrin, and 10−3 M hydrocortisone are prepared for this purpose. In addition, the growth medium also contains 92 IU penicillin and 0.1 mg/mL kanamycin (see Note 2).
Bovine insulin: Bovine insulin (Sigma, cat. no. I5500) is solubilized in 0.01 N HCl at a concentration of 5 mg/mL, sterilized by passage through a 0.22-μμ filter, and distributed into sterile 12, 75-mm polystyrene tubes using 1-mL aliquots. Insulin is kept at 4°C and can be used for up to 1 yr as long as sterility is maintained.
Human transferrin. Human apo-transferrin (iron poor, > 97% pure; Sigma, cat. no. T2252) is prepared at a concentration of 5 mg/mL in water, sterilized using a 0.22-μm filter unit, and distributed into sterile polystyrene tubes. Individual aliquots are kept at −20°C. Aliquots of transferrin can be frozen and thawed up to four times.
Hydrocortisone: Hydrocortisone (Sigma, cat. no. H4001) is solubilized in 100% ethanol at 10−3 M, aliquoted into sterile 5-mL polypropylene tubes, and stored at 4°C for 3–4 m.
Phosphate-buffered saline (PBS).
Sodium hydroxide (NaOH).
Ferrous sulfate.
Potassium nitrate.
Oxygen-saturated water.
Iron oxide solution (0.5% w/v). While perfusing the kidney, a 0.5% iron oxide solution (w/v), prepared as described by Cook and Pickering (25) is required for removal of the contaminating glomeruli. The solution is prepared by dissolving 2.6 g sodium hydroxide and 20 g potassium nitrate in 100 mL of oxygen-saturated water. Ferrous sulfate (9 g) is dissolved in 100-mL aliquots of oxygen-saturated water. These two 100-mL solutions are mixed in a flask and boiled for 20 min. The resulting black precipitate (iron oxide) is washed 5–10 times with Milli-Q water. During each wash, the precipitate is first resuspended in water and is then brought down to the bottom of the flask using a strong magnet. The wash water is then decanted away. After the final wash, the iron oxide is resuspended in 1 L of 0.9% NaCl, distributed into 250-mL bottles, and autoclaved. Immediately prior to use, a portion of the iron oxide solution is diluted fourfold in PBS.
NaCl.
EDTA–trypsin in PBS (Invitrogen Corp.). A trypsin solution is prepared for subculturing. Sterile 0.25% trypsin–1 mM (ethylenedinitrilo)tetraacetic acid (EDTA) in PBS (Invitrogen Corp.), used for the trypsinization of renal proximal tubule cell cultures, is prepared by diluting a 10X concentrate (Invitrogen Corp.) into PBS. Trypsin solutions are filter-sterilized, aliquoted, and frozen at −20°C. Aliquots for immediate use are maintained at 4°C.
Soybean trypsin inhibitor (Invitrogen Corp.). A soybean trypsin inhibitor solution is prepared to inhibit proteases in collagenase. In addition, soybean trypsin inhibitor is used to inhibit trypsin action after trypsinization has come to an end (a necessity when using serum-free medium, unlike the case with medium containing serum, which itself inhibits trypsin action). A 0.1% soybean trypsin inhibitor solution is prepared in PBS. Soybean trypsin inhibitor solutions are filter-sterilized, aliquoted, and frozen at −20°C. Aliquots for immediate use are maintained at 4°C.
Collagenase Class 4 (162 U/mg; Worthington, cat. no. 4188): A collagenase preparation that permits the outgrowth of cells from nephron segments is prepared in basal medium at 10 mg/mL and filter-sterilized. The collagenase solution is prepared on the day of use (see Note 3).
New Zealand white rabbits, 4–5 lb.
100% CO2
Curved-nose scissors.
100-mm-Diameter glass Petri dishes (2).
Heavy suture thread.
Kelly hemostat (5-1/2 in. straight end).
20-Gage metal needle (blunt ended with a file).
50-mL Glass syringe.
15-mL Dounce homogenizer (Type A, loose pestle).
Metal spatula (9 in.).
2-in. Magnetic stir bar.
1000-mL Beaker.
Nylon nitrex screening fabric, both 253 μm and 85 μm (TETCO, Inc., Depew, NY).
4-in. Plastic embroidery hoops (two sets).
Sieves are prepared for the purification of renal proximal tubules and glomeruli from a suspension of nephron segments obtained from disrupted renal cortical tissue in the procedure described in Subheading 3.1.5. The sieving procedure involves the use of Nylon nitrex screening fabric (both 253 μm and 85 μm) cut to a size permitting them to be held in place in a 4-in.-diameter plastic embroidery hoop.
Disposable tubes: 50 mL polypropylene; 5 mL polystyrene; 5 mL polypropylene.
Tissue culture dishes (35 mm in diameter).
Transwells (Corning, cat. no. 3450-Clear), 24 mm in diameter, 0.4 μm pore size.
Filtration apparatuses (0.22 μm).
3. Methods
The methods described outline (1) the purification of renal proximal tubule cells and (2) the culturing of the primary cells.
3.1. Purification of Renal Proximal Tubule Cells
Immediately prior to the cell culture procedure, implements must be sterilized. A sterile collagenase solution must be prepared and growth factors must be added to the basal medium. Subsequently, renal proximal tubules are purified as outlined here for immediate use for primary cell cultures.
3.1.1. Preparation of Medium, Reagents, and Implements Prior to Primary Culture
Implements to be used for primary renal proximal tubule cell culture are wrapped appropriately in aluminum foil and sterilized in an autoclave (1 h at 135°C, 35 psi in a Castle autoclave). Included among the implements are two sets of forceps, curved-nose scissors, a hemostat, the blunt-ended needle (18 gage), a metal spatula, suture, two magnetic stir bars (2 in. long), a 1000-mL beaker, two 100-mm-diameter glass Petri dishes, a 15-mL Dounce tissue homogenizer with a loose pestle (type A), and a 50-mL glass syringe (see Note 4).
The Nylon mesh placed within embroidery hoops is sterilized by prior soaking (overnight) in a 2-L polypropylene beaker containing 95% ethanol. One 253-μm mesh and one 85-μm mesh are used per kidney. If both kidneys are utilized for culturing, then two 253-μm meshes and two 85-μm meshes should be prepared (see Note 4).
Prepare the sterile collagenase solution (see Materials, item 24).
Prepare the antibiotic-supplemented basal media and the complete growth medium for isolation and subsequent culture, respectively, of the tubules.
3.1.2. Initial Dissection of Kidneys
A rabbit is sacrificed in a container filled with 100% CO2 (see Note 4).
Prior to the removal of each kidney, the ureter is removed. The renal artery and vein are separated with forceps, so as to facilitate the insertion of the needle into the renal artery, for perfusion of the kidney (as described in Subheading 3.1.3.).
Each kidney is then removed (with the renal artery and vein intact) using sterile scissors and placed in a sterile 50-mL conical tube containing ice-cold antibiotic-supplemented basal medium. The tubes containing the left and right arteries are labeled, as the length of the artery varies with the kidney (the left kidney has the longer artery, which can be somewhat easier to insert the needle for perfusion, as described in Subheading 3.1.3.).
The kidneys are kept ice cold prior to perfusion, by placing the 50-mL tubes in ice until the kidneys are used for culturing.
3.1.3. Perfusion of Kidneys
Each kidney is placed in a 100-mm-diameter glass Petri dish and washed with ice-cold antibiotic-supplemented basal medium. A sterile, blunt-ended needle (18 gage) is inserted into the renal artery, and the artery is sutured. A hemostat is also used to keep the needle in place in the renal artery.
A 50-mL syringe is then connected to the needle, and the kidney is perfused with 30–40 mL of ice-cold PBS (to remove the blood).
After the blood is removed (the kidney becomes blanche in color), the kidney is perfused with 30–40 mL of PBS containing iron oxide (such that the kidney becomes gray–black in color). For this purpose, the iron oxide is diluted fourfold in PBS.
3.1.4. Removal of Renal Cortex and Homogenization
To remove the renal capsule, the kidney is grasped with two pairs of sterile forceps (4-1/2-in. straight, toothed-end). One of the forceps is used to pierce the renal capsule and gradually peel it off the kidney. Using the remaining forceps, the kidney is immediately transferred into another sterile 100-mm glass Petri dish containing 1–2 mL of ice-cold antibiotic-supplemented basal medium. At this point, great care must be taken with regard to sterility.
Slices of the renal cortex are removed from the kidney using a sterile, curve-nosed scissors (see Note 5).
The slices of the renal cortex are transferred into a sterile 15-mL Dounce homogenizer containing antibiotic-supplemented basal medium. Although ice-cold medium is preferred at this point, successful cultures can still be obtained after carrying out the procedure at room temperature. The tissue is disrupted with four to five strokes of a loose pestle (type A). After tissue disruption, the homogenizer can be covered with the lid of a sterile tissue culture dish, to maintain sterility while setting up the sieves for the next purification step (see Note 6).
3.1.5. Purification of Renal Proximal Tubules and Glomeruli by Sieving
The nephron segments are separated using the Nylon mesh sieves. Toward this end, a 85-μm sieve is placed over a sterile 1000-mL beaker, sitting directly on the mouth of the beaker. Then, the wider 253-μm sieve is placed over the 85-μm sieve.
The sieves are washed with 100–200 mL of antibiotic-supplemented basal medium (DMEM/F12) to remove the ethanol while maintaining sterility.
The suspension of disrupted tissue in the Dounce homogenizer (which contains tubule segments and glomeruli) is poured over the top sieve. Subsequently, 700–800 mL of antibiotic-supplemented basal medium is slowly poured over the sieves. During this process, care is taken so as to wash the tubules and glomeruli through appropriate sieves. Undisrupted material remains on the top of the first sieve 253 μm. Proximal tubules and glomeruli collect on the top of the second sieve 85 μm, because of their large diameter. Narrower tubule segments and debris pass into the beaker.
The tubules and glomeruli are removed from the top of the 85-μm sieve with a sterile metal spatula (preferably with a rounded end) and are transferred into a sterile 50-mL plastic conical tube containing 40 mL antibiotic-supplemented basal medium.
3.1.6. Removal of Glomeruli and Disruption of the Basement Membrane
The glomeruli in the tubule suspension are removed by placing a sterile magnetic stir bar in the 50-mL conical tube (see Note 7). The glomeruli (which are covered with iron oxide) are attracted to the stir bar. Then, the tubule suspension is carefully poured from the conical tube (without the stir bar) into another 50-mL conical tube.
In order to disrupt the basement membrane, soybean trypsin inhibitor (0.1 mL of a 0.1% solution) followed by collagenase (0.2 mL of a 10 mg/mL solution) is added to the tubule suspension (to obtain final concentrations of 0.0025 and 0.050 mg/mL, respectively). The tubules are incubated with collagenase for 2 min at 23°C (see Note 8).
The collagenase treatment is stopped by centrifugation of the tube containing the tubules in a desktop centrifuge for 5 min at 500 rpm (21g).
The pellet containing the renal proximal tubules is resuspended in 40 mL antibiotic-supplemented basal medium and centrifuged once again for 5 min at 500 rpm (21g).
3.2. Primary Renal Proximal Tubule Cell Cultures
As many as 60 confluent monolayers in 35-mm culture dishes can be obtained from the purified renal proximal tubules, following the procedure outlined here (see Note 9).
3.2.1. Plating of Renal Proximal Tubules for Monolayer Cell Culture
After centrifugation, the renal proximal tubules are suspended in 30 mL of complete growth medium in order to obtain 60 confluent monolayers in 35-mm culture dishes.
Prior to adding the purified renal proximal tubules to the 35-mm culture dishes, 1.5 mL of complete growth medium is added to each dish.
The tubule suspension is inoculated into the media-containing 35-mm diameter tissue culture dishes at 0.5 mL/dish using a 5-mL pipet (see Note 10).
The culture dishes are placed in a 5% CO2/95% air humidified environment at 37°C.
The culture medium is changed the day after plating the tubules (to remove debris and unattached nephron segments). The medium is changed routinely every 2 d thereafter. Initially, cells grow out from the nephron segments that attach to the culture dish surface. Subsequently, monolayers form and can become confluent following 6–7 d in culture (see Notes 11–14).
3.2.2. Plating of Proximal Tubules Into Transwells
As an alternative to preparing monolayer cultures on plastic dishes, primary cultures can also be prepared on transwells or other semipermeable supports. Attachment of tubules and growth to confluence can be obtained on 3450-Clear transwells (24 mm in diameter; 0.4 μm pore size), which also permit visualization of the monolayers through an inverted microscope. Growth of tubules on transwells permits studies requiring accessibility to either the basolateral or the apical membrane.
After centrifugation, the tubules are suspended in 24 mL complete growth medium
Prior to the addition of the tubules to the culture dishes, 2.6 mL of complete growth medium is added to the bottom of each of the six wells present within a plate containing transwells.
Tubules are added to each well of the transwells in a total volume of 1.5 mL, taking care to have an equal distribution of material in each transwell (see Note 15). After 2 d in culture, medium is changed every other day.
3.2.3. Passaging of Primary Cultures
Primary rabbit kidney proximal tubule cells on plastic dishes can be subcultured using EDTA–trypsin. Confluent first-passage cultures can be obtained if care is taken to minimize the trypsinization period. In some cases, proximal tubule monolayers can even be obtained following a second passage into plastic dishes.
In order to obtain first-passage cells, the culture medium is removed by aspiration and the cells are washed with PBS.
A solution of EDTA–trypsin is then added. The majority of the EDTA–trypsin solution is immediately removed, so that only a film of trypsin covers the cells, providing a gentler trypsinization.
The cells are transferred to a 37°C incubator for a short time (as short as 1 min) and examined under an inverted microscope at ×100 magnification to determine whether the cells have detached from the dish surface. Trypsin action is stopped by the addition of an equimolar concentration of soybean trypsin inhibitor (0.5 mL/dish). Basal medium is added to bring the volume to 5 mL (see Note 16).
The cells are removed from the dish into a 12-mL plastic centrifuge tube, followed by centrifugation for 5 min at 500 rpm (21g). The cells are resuspended in complete growth medium and inoculated into plastic tissue culture dishes. Confluent monolayers may be obtained following a 1:1, a 1:2, or a 1:4 passage (see Notes 16 and 17).
Acknowledgments
Dr. Thaddeus Szczesny of the State University of New York at Buffalo is thanked for his preparation of transmission electron micrographs and Dr. Peter Bush of the State University of New York at Buffalo is thanked for his assistance in scanning electron microscopy. This work was supported by NHLBI grant no. 1RO1HL69676-01 to MT.
Footnotes
Water to be used for the preparation of sterile medium and other sterile reagents is purified with a Milli-Q reagent-grade water system as previously described (4,5). The feed water for the Milli-Q reagent-grade water system is obtained from a Millipore reverse osmosis system. A separate set of glassware, bottles, and stir bars is utilized for the preparation of medium, as is the case with all other tissue culture solutions. Glassware and other implements are to be washed using a phosphate-free detergent such as 7X (ICN Biomedical, Costa Mesa, CA).
The medium used during the purification of the renal proximal tubules contains both penicillin and streptomycin, to kill micro-organisms associated with the kidney preparation. However, the medium used for the growth and maintenance of primary renal proximal tubule cells should not be supplemented with streptomycin, which is a nephrotoxin. Streptomycin apparently has no deleterious effects during the 1- to 2-h period during which the proximal tubules are being purified and two antibiotics in combination are more effective than only one to prevent the growth of micro-organisms. However, our culture results indicate that over more extended incubation periods at 37°C nephrotoxic effects of streptomycin are indeed elicited. Streptomycin not only impedes the initial attachment of nephron segments to the culture dish but also affects the initial outgrowth of cells from the nephron segments. If penicillin is to be added, a final concentration of 0.92 × 105 IU/L is to be employed. We have successfully cultured primary proximal tubule cells in medium that is completely antibiotic-free. As a second antibiotic, kanamycin can be added at a final concentration of 100 μg/mL.
Because of lot-to-lot differences, each lot of collagenase should be tested to evaluate whether a particular lot promotes the outgrowth of cells from renal proximal tubules or has deleterious effects.
If proximal tubule cells are to be isolated from a different animal than the rabbit, then several different implements would be required. If a mouse or a rat kidney is to be used, then the kidneys of these animal would be perfused in situ, using a sterile Terumo Surflo winged infusion set (19G × 3/4 in. needle and 12-in. tubing). In addition, meshes that differ in size from that utilized with the rabbit kidney would need to be identified. The use of iron oxide to perfuse was originally described by Meezan et al. (8) for the isolation of glomeruli from rat kidneys.
The medulla can be distinguished from the cortex after perfusion with iron oxide, as only the cortex becomes gray–black in color following this procedure. Care should be taken not to remove slices of the medulla, as during subsequent processing, the medullary slices cause difficulty in the homogenization step and also result in the presence of a large quantity of debris in the final cell cultures (which might be prohibitive to cell growth).
During homogenization of the renal cortex, renal tubule fragments are released. Because the renal proximal tubules and glomeruli are wider in diameter than distal tubules and loops of Henle, the proximal tubules and glomeruli do not pass through the second sieve (85-μm) used during the sieving process. Thus, a purified preparation of renal proximal tubules and glomeruli is obtained after sieving.
Iron oxide is used to remove glomeruli from the preparation of purified proximal tubules and glomeruli. After perfusion of the iron oxide through the renal artery, the iron particles become associated with glomeruli, but are too large to pass through the glomeruli and enter the lumen of the nephron. After homogenization of the kidney, a suspension of purified nephron segments is obtained. The suspension is further enriched with renal proximal tubules and glomeruli following the sieving procedure (as described in Subheading 3.1.5.). The glomeruli in this suspension are selectively attracted to a magnetic stir bar. The glomeruli are then removed with the magnet, leaving a suspension of purified proximal tubules. If a substantial number of glomeruli with iron oxide remain after removing the magnetic stir bar, a second sterile magnetic stir bar can be added to the suspension of renal proximal tubules and, once again, the second magnet can be removed. However, care must be taken not to lose tubules during this process.
Empirically, we have observed that the use of collagenase is required if the outgrowth of epithelial cells from the tubules is to occur in vitro. Soybean trypsin inhibitor is used to inhibit proteases in collagenase. In addition, soybean trypsin inhibitor inhibits trypsin action after cells in treated cultures have detached from the culture dish. This is a necessity when using serum-free medium. In contrast, serum-supplemented medium contains serum components, which inhibit trypsin action.
During the process of pipetting the proximal tubules into culture dishes, care should be taken to keep the tubules in suspension, as they are denser than isolated cells in suspension and could rapidly settle even while pipetting. Thus, the tubule suspension should be shaken to resuspend after each pipetting. Alternatively, to maintain uniformity, the tubule suspension can be placed in a sterile bottle with a sterile stir bar and continuously stirred at a medium speed (a setting of 3–4) using a stirring motor. These steps should be carried out in a tissue culture hood. Tubules can be plated in larger-diameter dishes, including 60-mm-diameter dishes (30 dishes/kidney preparation), as well as 100-mm-diameter dishes (20 dishes/kidney preparation). Cultures prepared in this manner are particularly useful for such applications as Northern analysis and enzyme activity measurements. For growth studies (where low plating densities are desired), up to 200 mL of medium can be used to suspend the renal proximal tubules obtained from a single rabbit kidney and, thus, many more cultures in 35-mm dishes can be obtained. Ultimately, confluent monolayers can even be obtained from such cultures plated at lower densities.
If primary rabbit kidney proximal tubule cell cultures do not grow to confluence, a number of problems might have occurred. First, enough tubules must be added to the culture dishes in order to obtain confluent monolayers (If desired the protein content, approx 0.5 mg protein/mL tubule suspension) can be determined by the Bradford method (26) immediately after obtaining the suspension of purified renal proximal tubules). The tubules can easily be lost if they are not carefully harvested at the end of the sieving procedure.
Second, cell cultures obtained from kidneys of young adults (as opposed to older animals) are the most successful. A third point of concern is the tissue culture medium. The purity of the water is critical in defined medium studies. Loss of purity because of contamination (from a dirty pH probe, for example) could result in medium that does not support cell growth. Our laboratory determines pH using samples of the medium rather than placing the probe in the medium to be used for tissue culture studies. In addition, a set of glassware is used in medium preparation that is specifically designated for that purpose.
Another point of caution is the hormone supplements. Improper preparation or storage of the growth supplements might be deleterious to cell growth. The growth stimulatory effect of insulin might be lost if the stock solution is frozen. Furthermore, the medium supplements should be added to the medium immediately prior to use for tissue culture, as these supplements are not necessarily stable in the tissue culture medium.
Care should be taken that the incubator be maintained at a constant temperature of 37°C and in a constant 5% CO2/95% air environment. Animal cell growth in the absence of serum is more sensitive to shifts in temperature and to changes in the medium pH than in the presence of serum. The addition of HEPES buffer to the medium alleviates this latter problem to some extent.
Finally, the primary rabbit kidney proximal tubule cells are less adherent to plastic dishes than many other cell types. Thus, the cells might detach during their manipulation for cell growth studies or for transport studies (for example). The problem of adhesion might be alleviated by growing the cultures on tissue culture dishes coated with basement membrane components such as laminin, or Type IV collagen, with Matrigel, or with such biomaterials as silica (9). Renal proximal tubule cell cultures on plastic cell culture dishes can readily be passaged once. Although one or two additional passages can be obtained, confluence is obtained with difficulty. Proximal tubule cell cultures grow more rapidly and achieve a higher saturation density and a higher passage number on laminin-coated dishes.
Care must be taken at this step to evenly distribute the tubules throughout the surface of each transwell (otherwise confluent monolayers that completely cover the transwell will not be obtained). Chopstick electrodes (Millipore Corp.), or another similar apparatus, are used to test the transepithelial resistance when confluence is indeed achieved, so as to determine if an electrically tight monolayer has been obtained.
Trypsinization can also be conducted at room temperature. The cells are loosely attached to the bottom of the culture dish and are readily detached by an incubation with trypsin as short as 5 min. Trypsinization at room temperature is gentler to the cells and does not result in the type of cell damage and death that can occur with these delicate cultures at 37°C.
When passaging primary cultures on a 1:4 basis, we have been able to reproducibly obtain confluent first-passage cultures within 1 wk. Similarly, second-passage cultures are readily obtained within 1 wk when passaging on a 1:4 basis. However, following the third passage, cultures do not readily undergo many cell divisions. Thus, in order to obtain confluent monolayers after more than two passages, either more cells must be used originating from larger dishes (e.g., passaging 1:4 from a 100-mm dish into a 35-mm dish) and/or lower passage ratios can be utilized (i.e., 1:1 or 1:2). Other options that might permit a greater success during passages include the addition of growth factors (such as epidermal growth factor, 10 ng/mL, or fibroblast growth factor, 50 ng/mL) to the culture medium, as well as coating culture dishes with such matrix components as laminin or collagen IV.
References
- 1.Taub M, Chuman L, Saier MH, Jr, Sato G. Growth of Madin Darby Canine Kidney epithelial cell (MDCK) line in hormone-supplemented serum-free medium. Proc Natl Acad Sci USA. 1979;76:3338–3342. doi: 10.1073/pnas.76.7.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuman L, Fine LG, Cohen AI, Saier MH., Jr Continuous growth of proximal tubular epithelial cells in hormone-supplemented serum-free medium. J Cell Biol. 1982;94:506–510. doi: 10.1083/jcb.94.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taub M, Sato G. Growth of functional primary cultures of kidney epithelial cells in defined medium. J Cell Physiol. 1979;105:369–378. doi: 10.1002/jcp.1041050220. [DOI] [PubMed] [Google Scholar]
- 4.Chung SD, Alavi N, Livingston D, Hiller S, Taub M. Characterization of primary rabbit kidney cultures that express proximal tubule functions in a hormonally defined medium. J Cell Biol. 1982;95:118–126. doi: 10.1083/jcb.95.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taub M. Primary culture of proximal tubule cells in defined medium. J Tissue Culture Methods. 1985;9:67–72. [Google Scholar]
- 6.Waqar MA, Seto J, Chung SD, Hiller-Grohol S, Taub M. Phosphate uptake by primary renal proximal tubule cells grown in hormonally defined medium. J Cell Physiol. 1985;124:411–423. doi: 10.1002/jcp.1041240309. [DOI] [PubMed] [Google Scholar]
- 7.Brendel K, Meezan E. Isolation and properties of a pure preparation of proximal kidney tubules obtained without collagenase treatment. Fed Proc. 1975;34:803. [Google Scholar]
- 8.Meezan EK, Brendel J, Ulreich J, Carlson EC. Properties of a pure metabolically active glomerular preparation from rat kidneys. I Isolation. J Pharm Exp Ther. 1973;187:332–341. [PubMed] [Google Scholar]
- 9.Taub M, Axelson E, Park JH. Improved method for primary cell cultures: use of tissue culture dishes with a colloidal silica surface. Biotechniques. 1998;25:990–994. doi: 10.2144/98256st01. [DOI] [PubMed] [Google Scholar]
- 10.Sakhrani LM, Badie-Dezfooly B, Trizna W, et al. Transport and metabolism of glucose by renal proximal tubular cells in primary culture. Am J Physiol. 1984;246:F757–F764. doi: 10.1152/ajprenal.1984.246.6.F757. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo S, Fukatsu A, Taub ML, Caldwell PRB, Brentjens JR, Andres G. Nephrotoxic glomerulonephritis induced in the rabbit by antiendothelial antibodies. J Clin Invest. 1987;79:1798–1811. doi: 10.1172/JCI113021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang IS, Goldinger JM, Hong SK, Taub M. The preparation of basolateral membranes that transport p-aminohippurate from primary cultures of rabbit kidney proximal tubule cells. J Cell Physiol. 1987;135:481–487. doi: 10.1002/jcp.1041350316. [DOI] [PubMed] [Google Scholar]
- 13.Fine LG, Sakhrani LM. Proximal tubular cells in primary culture. Miner Electrolyte Metab. 1986;12:51–57. [PubMed] [Google Scholar]
- 14.Park SH, Taub M, Han HJ. Regulation of phosphate uptake in primary cultured rabbit renal proximal tubule cells by glucocorticoids: evidence for nongenomic as well as genomic mechanisms. Endocrinology. 2001;142:710–720. doi: 10.1210/endo.142.2.7934. [DOI] [PubMed] [Google Scholar]
- 15.Han HJ, Lee YH, Park SH, Taub M. Estradiol-17β stimulates phosphate uptake and is mitogenic for primary rabbit renal proximal tubule cells. Exp Nephrol. 2002;10:355–364. doi: 10.1159/000065300. [DOI] [PubMed] [Google Scholar]
- 16.Han HJ, Park SH, Koh HJ, Taub M. Mechanism of regulation of Na+ transport by angiotensin II in primary renal cells. Kidney Int. 2000;57:2457–2467. doi: 10.1046/j.1523-1755.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 17.Aleo MD, Taub ML, Olson JR, Kostyniak PJ. Primary cultures of rabbit renal proximal tubule cells: II. Selected phase I and phase II metabolic capacities. Toxicol In Vitro. 1990;4:727–733. doi: 10.1016/0887-2333(90)90041-q. [DOI] [PubMed] [Google Scholar]
- 18.Aleo MD, Taub ML, Kostyniak PJ. Primary cultures of rabbit renal proximal tubule cells: III Comparative cytotoxicity of inorganic and organic mercury. J Toxicol Appl Pharm. 1992;112:310–317. doi: 10.1016/0041-008x(92)90201-3. [DOI] [PubMed] [Google Scholar]
- 19.Aleo MD, Taub ML, Olson JR, Nickerson PA, Kostyniak PJ. Primary cultures of rabbit renal proximal tubule cells as an in vitro model of nephrotoxicity: effects of 2 mercurials. In: Goldberg AM, editor. In vitro Toxicology: Approaches to Validation. Vol. 5. Mary Ann Liebert, Inc.; New York: 1987. pp. 211–225. [Google Scholar]
- 20.Wang Y, Taub M. Insulin and other regulatory factors modulate the growth and the phosphoenolpyruvate carboxykinase (PEPCK) activity of primary rabbit kidney proximal tubule cells in serum free medium. J Cell Physiol. 1991;147:374–382. doi: 10.1002/jcp.1041470224. [DOI] [PubMed] [Google Scholar]
- 21.Jung JC, Lee SM, Kadakia N, Taub M. Growth and function of primary rabbit kidney proximal tubule cells in glucose-free serum-free medium. J Cell Physiol. 1992;150:243–250. doi: 10.1002/jcp.1041500204. [DOI] [PubMed] [Google Scholar]
- 22.Han HJ, Jung JC, Taub M. Response of primary rabbit kidney proximal tubule cells to estrogens. J Cell Physiol. 1999;178:35–43. doi: 10.1002/(SICI)1097-4652(199901)178:1<35::AID-JCP5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Springate J, Davies S, Chen K, Taub M. Toxicity of ifosfamide and its metabolite chloroacetaldehyde in cultured renal tubule cells. In Vitro Cell Dev Biol. 1999;35:314–317. doi: 10.1007/s11626-999-0080-y. [DOI] [PubMed] [Google Scholar]
- 24.Zaki EL, Springate JE, Taub M. Comparative toxicity of ifosfamide metabolites and protective effect of mesna and amifostine in cultured renal tubule cells. Toxicol In Vitro. 2003;17:397–402. doi: 10.1016/s0887-2333(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 25.Cook WF, Pickering GW. A rapid method for separating glomeruli from rabbit kidney. Nature. 1958;182:1103–1104. doi: 10.1038/1821103a0. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]


