Abstract
Fat mass and tissue distribution change dramatically throughout life. Fat depot sizes reach a peak by middle or early old age, followed by a substantial decline, together with fat tissue dysfunction and redistribution in advanced old age. These changes are associated with health complications, including type 2 diabetes, atherosclerosis, dyslipidemia, thermal dysregulation, and skin ulcers, particularly in advanced old age. Fat tissue growth occurs through increases in size and number of fat cells. Fat cells turn over throughout the lifespan, with new fat cells developing from preadipocytes, which are of mesenchymal origin. The pool of preadipocytes comprises 15 to 50% of the cells in fat tissue. Since fat tissue turns over throughout life, characteristics of these cells very likely have a significant impact on fat tissue growth, plasticity, function, and distribution. The aims of this review are to highlight recent findings regarding changes in preadipocyte cell dynamics and function with aging, and to consider how inherent characteristics of these cells potentially contribute to age- and depot-dependent changes in fat tissue development and function.
Keywords: C/EBPα, PPARγ, CUGBP, Preadipocyte Differentiation, Dysdifferentiation
Introduction
Fat mass, distribution, and function undergo dramatic changes throughout the life span (Flegal et al., 1998; Kirkland et al., 1984; Kirkland et al., 1997). Fat mass reaches a peak by middle or early old age (human 40–70 years; rat 17 months), followed by a substantial decline in advanced old age (human > 70 years; rat > 24 months) (Figure 1). However, the observed decrease in total body fat with old age (Ravaglia et al., 1999), does not coincide with a decline in percent body fat, which may remain constant or increase (Kehayias et al., 1997; Petersen et al., 2003). The age-associated decline in sizes of adipose depots is accompanied by the accumulation of fat outside adipose tissue and loss of lean body mass (particularly muscle). Because of this, the proportion of body mass that is within fat depots remains constant. Ectopic fat accumulation occurs in bone marrow, muscle, liver, and at other sites, potentially contributing to age-dependent dysfunction of these tissues (Rosen and Bouxsein, 2006; Slawik and Vidal-Puig, 2006; Unger, 2005; Tchkonia et al., 2006a). For example, age-related fat infiltration in bone is associated with reduced mineral density and in muscle with development of insulin resistance, glucose intolerance, and decreased functional capacity (Hicks et al., 2005; Rosen et al., 2004; Slawik and Vidal-Puig, 2006). Thus, in old age, there is less fat where it should be and more fat where it should not be, with potential clinical consequences.
Figure 1. Diagram of age-associated changes in fat distribution.
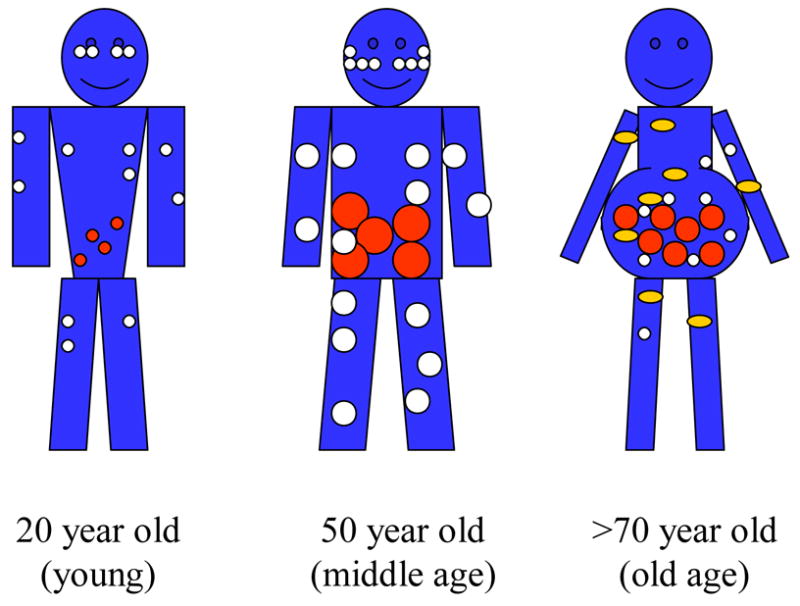
Fat mass reaches a peak by middle or early old age, followed by a substantial decline in advanced old age. Aging causes a loss of subcutaneous fat (peripherally first and then centrally), accumulation of visceral fat, and ectopic fat deposition (in muscle, liver, bone marrow, and elsewhere). White circles represent subcutaneous fat, red circles represent visceral fat, and yellow circles represent the appearance of fat in non adipose tissue.
Different fat depots undergo changes at different rates with aging (Kirkland et al., 2002). For example, with increasing age, retro-orbital and peripheral subcutaneous fat tend to be lost first, whereas visceral fat tends to be preserved (Chumlea et al., 1989; Hughs et al., 2004). A high ratio of central to peripheral fat is associated with insulin resistance and increased risk of atherosclerosis and diabetes, even in lean subjects (Cnop et al., 2003; Kahn et al., 2001). Therefore, the earlier loss of subcutaneous fat and consequent relative increase in intra-abdominal fat with aging could predispose to metabolic dysfunction and related health complications (Depres, 2006; Lakka et al., 2002; Slawik and Vidal-Puig, 2006; Unger, 2005). This indicates that depot-specific changes in fat tissue function with aging may contribute to development of age-related metabolic disorders.
The major role of white adipose tissue is to store lipids. To do this, adipocytes convert circulating cytotoxic free fatty acids into less damaging neutral triglycerides, thereby protecting other tissues from their lipotoxicity (DeFronzo et al., 2004; Tchkonia et al., 2007). Age-dependent lipotoxicity is related to a decrease in adipose tissue capacity to store free fatty acids (Slawik and Vidal-Puig, 2006). The resulting lipotoxic environment is detrimental to non-adipose tissues. This may contribute to systemic lipotoxicity, and the increased prevalence of metabolic syndrome in older populations. Metabolic syndrome, a constellation of diabetes, hypertension, dyslipidemia, atherosclerosis, and visceral obesity, affects from 24 to 40% of individuals in their 60’s and 70’s (Rodriguez et al, 2005).
What is behind fat tissue dysfunction with aging? Are depot-specific, age-related changes in fat tissue solely attributable to microenvironmental influences, such as regional variation in the abundance of non-adipose cell types (e.g., macrophages, endothelial cells), anatomic constraints, circulation, and innervation? Is the variation in aging depots due in part to changes in the inherent properties of adipocytes? The potential role of intrinsic properties of adipocytes and their precursor cells, preadipocytes, in age-related changes in fat tissue function will be addressed in this review.
Depot-dependent variation in fat tissue cell dynamics
Aging is associated with substantial redistribution of fat tissue among depots. The volume of subcutaneous fat declines first, followed much later by loss of fat in visceral depots, resulting in redistribution of fat from subcutaneous to visceral depots from late middle age until the 80’s or later. It appears that the amount of visceral fat may be a more significant risk factor than BMI by itself (Bryhni et al., 2005; Zamboni et al., 2005). Since fat cell size and number are related to insulin sensitivity, glucose and fatty acid uptake, and cytokine release, changes in function and cellular composition of fat tissue might lead to changes in metabolic state and subsequent clinical complications (Smith et al., 2006).
The age-related decline in fat depot size is a result of decreased adipocyte size and not a decrease in cell number, since new cells appear to be formed throughout the lifespan and fat cell number remains constant or increases in old age (Figures 1 and 2) (Kirkland et al., 2002). Preadipocytes are a substantial component of fat tissue, accounting for 15 to 50% of all cells (Kirkland et al., 1994; Kirkland et al., 1997). Preadipocyte gene expression and secretion profiles are distinct from adipocytes. Preadipocytes are capable of strongly influencing fat tissue function in their own right (Tchkonia et al., 2006b). For example, preadipocytes can secrete PAI, IL-6, and other proinflammatory cytokines and chemoattractant proteins to a greater extent than fat cells, affecting fat tissue cellularity and function (Chung et al., 2006).
Figure 2. Adipose tissue cell dynamics.
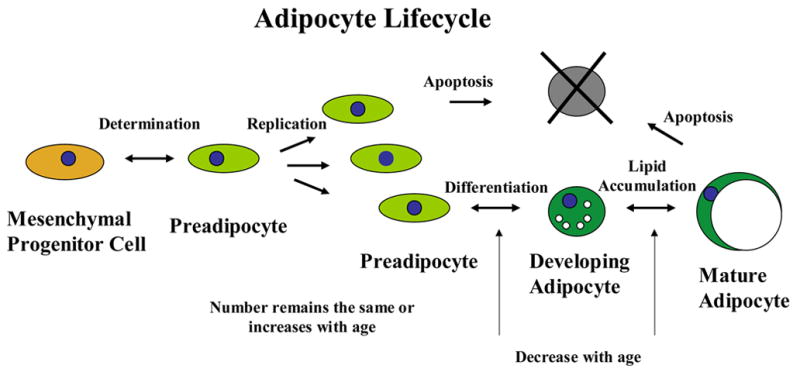
Age-related changes in transcription factor function lead to reduced differentiation of preadipocytes.
Preadipocyte capacity to replicate and differentiate declines with age (Figure 3) (Karagiannides et al., 2006; Kirkland et al, 1997). The age-related changes in preadipocyte cell dynamics could, of course, be a result of extrinsic factors, including hormonal milieu, presence of other cell types, innervation, anatomic constraints, and circulation. Recent experiments demonstrated that preadipocytes isolated from young and old rats, cultured in parallel under identical conditions, retain age-dependent cell dynamic characteristics, indicating the involvement of cell-specific, inherent mechanisms in these changes (Karagiannides et al., 2001; Karagiannides et al., 2006; Kirkland et al, 1997). Preadipocytes cultured from old animals demonstrate a decrease in lipid accumulation, lipogenic enzyme activities, and changes in differentiation-dependent gene expression (Djian et al, 1985; Hauner et al., 1989; Gregerman, 1994; Karagiannides et al., 2001; Karagiannides et al., 2006; Kirkland et al., 1984; Kirkland et al., 1997; Kirkland et al, 1990; Wang et al, 2001). The same age-related changes are evident in colonies derived from single cells after several weeks ex vivo (Kirkland et al., 1990). These findings support the hypothesis that inherent properties of preadipocytes contribute to changes in growth and function of adipose tissue with age.
Figure 3. Preadipocyte capacity for lipid accumulation declines with age.
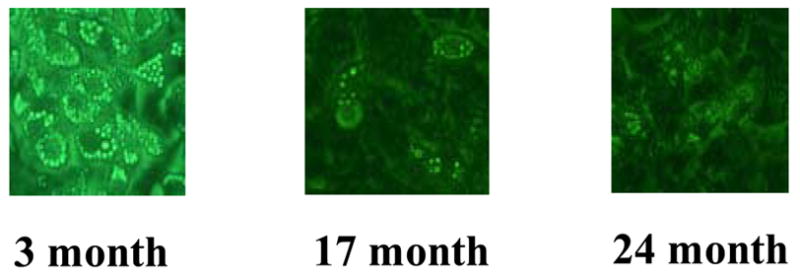
Differentiating preadipocytes isolated from young (3 month old), middle-aged (17 months), and old (24 months) Fischer 344 rat epididymal depots are shown.
Regulation of Adipogenesis
The process of preadipocyte differentiation (Figure 2) is initiated or promoted by exposure of the preadipocytes to nutrients, hormonal effectors such as insulin, glucocorticoids, and IGF-1, and paracrine and autocrine effectors, including free fatty acids and cyclic AMP (Kirkland et al., 2002; Unger, 2005). These signals activate pathways that modulate the expression and activity of a set of adipogenic transcription factors that, in turn, impact expression of a couple of thousand downstream differentiation-dependent genes (Kirkland et al., 1997; Perera et al., 2006). Adipogenesis is, to a large extent, under the control of two transcription factors: CCAAT/enhancer binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) (Figure 4) (Wu et al., 1996; Wu et al., 1999; Zuo et al., 2006). C/EBPα and PPARγ are involved in transcriptionally transactivating adipose-specific genes, including adipocyte-specific fatty acid binding protein (aP2 or fatty acid binding protein 4), adiponectin, fatty acid synthase, leptin, and glucose-specific transporter 4 (GLUT4), resulting in acquisition and maintenance of the fat cell phenotype (Gustafson et al., 2003; Kirkland et al., 1997; Wu et al., 1999).
Figure 4. Molecular mechanisms of age-related decreases in adipogenesis.
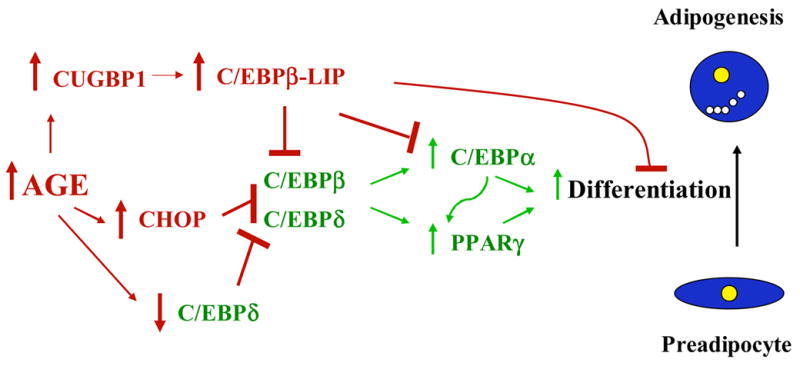
Arrows and transcription factors in green indicate adipogenic pathways. Arrows and factors in red indicate antiadipogenic pathways. Age-related activation of cellular stress response pathways, coupled with increased preadipocyte cytokine generation, could trigger increased production of the antiadipogenic factors, C/EBPβ-LIP and CHOP. C/EBPβ-LIP and CHOP inhibit the production of the adipogenic transcription factors, PPARγ and C/EBPα, resulting in impaired adipogenesis and reduced expression of differentiation-dependent genes in fat cells. Reduced adipogenesis contributes to fat cell dysfunction, reduced fat depot size, and redistribution of fat to non-adipose tissues.
C/EBPα is a member of the basic region leucine zipper (bZIP) family of transcription factors that includes other C/EBPs, cAMP response element binding protein, and activating transcription factor 2. C/EBPα acts on gene expression by forming homo- or heterodimers that transactivate CAAT response element-containing genes. Antisense C/EBPα-treated preadipocytes and C/EBPα null mice are incapable of adipogenesis, underscoring the importance of C/EBPα in fat tissue development (Lin et al., 1992; Lin et al., 1994; Wang et al., 1995). The other key transcription factor in adipose tissue differentiation, PPARγ, is a nuclear receptor belonging to the superfamily of receptors that includes the retinoid, thyroid, and steroid receptors (Chawla et al., 2001). Ligand-activated PPARγ forms a complex with the retinoid X receptor α (RXRα) and its ligand. This complex transactivates differentiation-dependent genes. The extent to which PPARγ coordinates the expression of genes directly or indirectly in adipogenesis has been demonstrated through gene array analysis of human preadipocytes in which PPARγ expression has been reduced, significantly blunting changes in expression of downstream genes (Perera et al., 2006). As with C/EBPα, small interfering RNAs (siRNAs) against PPARγ suppress differentiation of human preadipocytes (Xu et al., 2006). Furthermore, inhibition of PPARγ activity in 3T3-L1 preadipocytes by exposure to a potent and selective PPARγ antagonist or negative regulators, such as FOXO2, blocks PPARγ-induced adipogenesis (Davis et al., 2004; Zuo et al., 2006). Interestingly, PPARγ2, an isoform of PPARγ, is capable of inducing adipocyte differentiation in C/EBPα−/− fibroblasts and activating adiponectin expression in response to thiazolidinedione treatment in the absence of C/EBPα (Gustafen et al., 2003; Park et al., 2004).
The temporal process of adipocyte differentiation is activated by initial transient increases in the C/EBP isoforms, β and δ, that precede the induction of PPARγ and C/EBPα in response to adipogenic signals (Figure 4) (Tang et al., 2004). C/EBPβ modulates C/EBPα expression, and C/EBPβδ heterodimers modulate PPARγ expression (Clarke et al., 1997; Wu et al., 1996; Wu et al., 1999). Furthermore, ligand-activated PPARγ is capable of inducing C/EBPα expression by dislodging the adipogenic suppressor HDAC1 (Zuo et al., 2006). Concurrently, the down-regulation of histone deacetylases stimulates adipocyte differentiation in 3T3-L1 cells (Yoo et al 2006). C/EBPβ is also capable of independently inducing PPARγ, as shown in PPARγ (deficient) +/− mouse embryo fibroblasts, leading to subsequent activation of adipogenesis, including C/EBPα expression (Zuo et al., 2006). C/EBPα, in turn, can influence C/EBPβ protein isoform and PPARγ expression through a positive feedback loop (Burgess-Beusse et al., 1999; Clarke et al., 1997). In summary, C/EBPβ expression increases that of PPARγ, which in turn activates C/EBPα expression, leading to induction of differentiation.
The process of adipocyte differentiation is modulated by a series of anti-adipogenic regulators, including the members of the C/EBP family; C/EBPβ-LIP (liver inhibitory protein) and C/EBP homologous protein (CHOP; also called C/EBPζ or GADD153 [growth arrest and differentiation-dependent gene 153]) (Batchvarova et al., 1995; Karagiannides et al., 2001; Welm et al., 2000). These form heterodimers with other C/EBP family members that lack transactivating regions, creating dominant negative factors that act as inhibitors of adipogenesis. Upstream of these anti-adipogenic factors is the protein, (CUG) triplet repeat RNA-binding protein (CUGBP), which controls the alternative translation of adipogenic (C/EBPβ-LAP)/anti-adipogenic inhibitory (C/EBPβ-LIP) isoforms from C/EBPβ RNA, leading to increased production of the low molecular weight LIP isoform (Karagiannides et al., 2006; Timchenko et al 1999).
Adipogenesis and Aging
The capacity of preadipocytes to become fully functional mature adipocytes declines with age (Figure 3) (Karagiannides et al., 2001). Since adipocyte differentiation and maintenance of the fat cell phenotype are dependent on the transcription factors, C/EBPα and PPARγ, changes that occur with age in adipocytes may be a consequence of changes in expression of these key adipogenic transcription factors. Indeed, expression of C/EBPα, C/EBPδ, and PPARγ is substantially lower in differentiating preadipocytes isolated from old than from young rats (Karagiannides et al., 2001; Karagiannides et al., 2006). Overexpression of C/EBPα in preadipocytes from old rats restores capacity to accumulate lipid and acquire the fat cell phenotype, implying that there are changes with aging in mechanisms controlling differentiation upstream of these adipogenic transcription factors. Therefore, an important change in the differentiation process with aging is the inability to maintain adequate levels of these key adipogenic regulators (Karagiannides et al., 2001; Karagiannides et al., 2006). These declines in adipogenic transcription factor expression and activity in differentiating preadipocytes would be expected to influence the function of the adipocytes that develop from them, since continued activation of downstream target genes is required for maintenance of the normal adipocyte phenotype. For example, reduced C/EBPα expression in adipocytes contributes to impaired glucose tolerance through impairing insulin-sensitive glucose transporter 4 (GLUT4) expression (El Jack et al., 1999). Genes downstream of PPARγ, including aP2, carnitine palmitoyl transferase-1 (CPT1), and PPARγ co-activator 1α (PGC1α), are involved in regulating the pathways of fatty acid handling and mitochondrial function (Lehrke and Lazar, 2005; Slawik and Vidal-Puig, 2006). The expression and activity of PGC1α declines with age in various tissues, causing a shift from fuel oxidation to storage with accumulation of lipotoxic fatty acids, which has been associated with insulin resistance and diabetes (Ling et al., 2004; Slawik and Vidal-Puig et al., 2006; Tchkonia et al., 2007). Decline in capacity to express and maintain lineage-specific transcription factors at appropriate levels could be a general age-related phenomenon. Changes with age in lineage-specific transcription factors in bone (Moerman et al., 2004) and muscle (Lees et al., 2006) also lead to dysdifferentiation of their respective precursor cells.
The expression of adipogenic C/EBPα, C/EBPδ, and PPARγ is substantially lower in differentiating preadipocytes isolated from old than young animals, whereas the expression of anti-adipogenic factors, C/EBPβ-LIP and CHOP, increases (Figure 4) (Karagiannides et al., 2001; Karagiannides et al., 2006). Interestingly, total C/EBPβ mRNA does not change with aging. What occurs is a switch from the generation full length C/EBPβ mRNA to the production of the truncated isoform, C/EBPβ-LIP, that lacks the transactivating domain of full length isoforms. Increases in C/EBPβ-LIP and CHOP occur in response to stress induced by DNA damage, cytokines, and metabolic dysfunction (Karagiannides et al., 2001, Karagiannides et al., 2006). Both C/EBPβ-LIP and CHOP increase with aging in cultured preadipocytes, isolated fat cells, and fat tissue in vivo (Karagiannides et al., 2001). Overexpression of C/EBPβ-LIP in preadipocytes from young rats impairs adipogenesis, as occurs in preadipocytes isolated from old rats (Karagiannides et al., 2001). Furthermore, since C/EBPβ is capable of independently inducing the expression of PPARγ (Zuo et 2006), the inhibitory activity of C/EBPβ-LIP could account for some of the decrease in PPARγ expression with age (Figure 4).
What is the mechanism for the increase in C/EBPβ-LIP translation with aging or stress? CUG triplet repeat binding protein 1 (CUGBP1), which binds to C/EBPβ mRNA causing the preferential translation of C/EBPβ-LIP isoform (Timchenko et al., 1999; Welm et al., 2000), is elevated in aged preadipocytes (Karagiannides et al., 2006). Age-related changes in CUGBP translational regulation of production of C/EBP isoforms have been found in other tissues (Timchenko et al., 2006). Age-mediated induction of CUGBP1 significantly increases translation of truncated isoforms of C/EBPβ in cultured hepatocytes and in the liver (Timchenko et al., 2006).
The increase in CUGBP1 abundance and in vitro binding activity that occurs with aging predisposes undifferentiated preadipocytes to resist adipogenesis. CUGBP1 siRNA interference causes a reduction in C/EBPβ-LIP and an increase in adipogenesis in preadipocytes isolated from old animals, whereas CUGBP1 overexpression increases C/EBPβ-LIP, subsequently inhibiting the expression of C/EBPα and lipid accumulation in differentiating rat preadipocytes (Karagiannides et al., 2006). The effect of CUGBP1 on differentiation is extremely time and stage sensitive, occurring shortly before the initiation of adipogenesis (Karagiannides et al., 2006). CUGBP1 abundance and activity increases in undifferentiated preadipocytes with aging, contributing to decreased ability to differentiate and fat tissue dysfunction (Karagiannides et al., 2006).
One of the stimuli that can increase CUGBP activity is TNFα, which increases in fat tissue with age (Morin et al., 1997). TNFα is produced by both macrophages (Weisberg et al., 2003) and preadipocytes (Chung et al., 2004; Curat et al., 2006). TNFα impacts adipose tissue by interfering with preadipocyte differentiation, and causes lipolysis, decreased fat cell size, and reduced insulin responsiveness (Hube et al., 1999). The effects of TNFα on preadipocytes appear to be fat depot-dependent. Preadipocytes cloned from different human fat depots respond with different sensitivity to TNFα, with those isolated from omental depots being the most susceptible to apoptosis (Tchkonia et al., 2005). TNFα receptor knockout mice have enlarged fat depots (Uysal et al., 1998). Furthermore, TNFα inhibits C/EBPα and PPARγ expression and activity in differentiating preadipocytes (Stephens et al., 1992; Zhang et al., 1996). In agreement with this, TNFα induces C/EBPβ-LIP expression (Baldwin et al., 2004) and up-regulates CUGBP1 expression (Karagiannides et al., 2006). TNFα also causes serine phosphorylation of IRS-1, resulting in impaired insulin/IGF-1 signaling (Hotamisligil et al., 1996), which is required for adipogenesis. TNFα is known to increase CHOP (Xu et al., 2004). Thus TNFα inhibits adipogenesis through multiple mechanisms. Whether elevated TNFα in fat tissue is a result of increased expression of this cytokine by preadipocytes or is a result of increased macrophage infiltration remains to be elucidated.
Preadipocytes from different fat depots are distinct cell types
Aging is associated with reduction of fat depot sizes, redistribution of fat among depots, and deposition of fat outside fat depots. Visceral fat accumulation and loss of subcutaneous fat is a common phenomenon associated with aging. This shift toward an increase in central fat could be a key factor in the development of metabolic disorders (Bryhni et al., 2005; Kirkland et al., 2002). Since new mature adipocytes appear throughout adulthood, regionally distinct cell dynamic properties of preadipocytes could contribute.
We identified that human subcutaneous, mesenteric, and omental preadipocytes isolated from the same subjects and cultured under identical conditions maintained fat depot-specific characteristics even after many population doublings ex vivo (Tchkonia et al., 2005; Tchkonia et al., 2006b). This indicates existence of inherent mechanisms contributing to depot-specific properties. Subcutaneous preadipocytes exhibit higher replicative potential, adipogenic transcription factor expression, lipid accumulation, and less TNFα-induced apoptosis than omental preadipocytes. We found that two subtypes of preadipocytes exist with different capacities for replication, differentiation, and susceptibility to TNFα-induced apoptosis (Tchkonia et al., 2005). These subtypes can convert one to another and the ratio of the subtypes varies among fat depots. Subcutaneous fat is rich in preadipocytes of the rapidly replicating and differentiating subtype, whereas the slowly replicating and differentiating subtype is abundant in omental depots. Interestingly, mesenteric preadipocyte cell-dynamic characteristics are distinct from omental cells, indicating that visceral fat is not homogeneous. Regionally distinct cell dynamic properties, as well as subtype abundance, are preserved in depot-specific cell strains derived from single preadipocytes by stably expressing human telomerase reverse transcriptase (hTERT) (Tchkonia et al., 2006b). Differences in preadipocyte cell dynamic properties and in preadipocyte subtype abundance among depots could affect the capacities of different fat depots to alter in size and function in response to aging and changes in nutritional, paracrine, and hormonal states.
Not only do preadipocytes isolated from subcutaneous, mesenteric, and omental depots maintain depot-specific dynamic properties, they also exhibit unique patterns of gene expression, supporting the contention that depot-specific characteristics inherent to preadipocytes contribute to regional differences in function (Tchkonia et al., 2007). Comparison of gene expression profiles using genome-wide arrays indicate similar patterns of developmental gene expression in primary and single cell-derived hTERT clones across fat depots. Over 900 transcripts were identified that are differentially expressed among depots (Tchkonia et al., 2007). Adipogenic transcription factors (PPARγ2 and C/EBPα) and their downstream targets (aP2, adipsin, sterol regulatory element binding protein 1, perilipin, hormone sensitive lipase, apolipoprotein E, lipoprotein lipase, and glycerol-3-phosphate dehydrogenase) are differentially expressed in a coordinated, depot-specific manner. Remarkably, developmental genes are prominent among the transcripts differentially expressed in undifferentiated preadipocytes from different depots (Tchkonia et al., 2007). Some of these developmental regulators, for example, homeobox genes, regulate differentiation, replication, and apoptosis and may contribute to the regional differences of cell dynamics associated with aging. The inherent regional differences in their expression as well as regional variation in preadipocyte cell dynamics may result from differences in epigenetic patterning among depots. Interestingly, based on our preliminary findings, depot-specific expression patterns in rat preadipocytes persist and are amplified with aging (unpublished observation).
The capacity of human preadipocytes to differentiate and express adipogenic transcription factors declines with successive cell divisions, as does telomere length (Tchkonia et al., 2006b). Preventing telomerase shortening by stably expressing hTERT forestalls the decline in capacity for adipogenesis with increasing numbers of cell divisions. We found that omental preadipocytes replicate more slowly than subcutaneous cells (Tchkonia et al., 2001) and that telomeres are shorter in omental than subcutaneous preadipocytes (Tchkonia et al., 2006b). Thus, one mechanism potentially contributing to the earlier loss of subcutaneous than visceral fat with aging is the more extensive replicative history and, therefore, greater relative decline in capacity for adipogenesis, in subcutaneous compared to omental preadipocytes.
Increased accumulation of fat outside fat depots with aging
Aging is associated with the accumulation of lipids outside fat depots, including in muscle, liver, and bone, potentially contributing to dysfunction of these organs. For example, increased fat accumulation is related to the development of insulin resistance and glucose intolerance in muscle and reduced mineral density in bone (Rosen et al., 2004; Slawik and Vidal-Puig, 2006). What mechanisms are responsible for age-related accumulation of fat outside fat depots?
The age-associated decline in fat mass and adipose tissue ability to store lipids could contribute to the redistribution of fat into other tissues. Fat loss and redistribution also occurs in lipodystrophic syndromes. Examples of these, such as familial partial lipodystrophy and highly active retroviral therapy (HAART), are associated with the accumulation of lipid in the liver and other tissues and subsequent development of diabetes (Capeau et al., 2005; Domingo et al., 2005). Another model of adipose tissue loss is transgenic mice in which SREBP-1c, a transcription factor involved in lipid biosynthesis (Sewter et al., 2002), is over-expressed, blocking development of fat tissue (Shimomura et al., 1998). Again, loss of adipose tissue in this model results in lipid accumulation in liver and development of diabetes (Shimomura et al., 1998). Interestingly, SREBP-1c expression is inhibited by TNFα, one of the age-associated cytokines (Sewter et al., 2002). Therefore, extensive adipose tissue loss can result in increased fat accumulation outside fat depots with clinical consequences.
Another mechanism for the accumulation of fat in non-adipose tissue with age could be the dysdifferentiation of multipotent mesenchymal progenitors, which are in muscle, bone marrow, and other tissues, into adipocyte-like cells instead of cell types appropriate for their tissues. Muscle satellite cells, osteoblast precursors, and other mesenchymal progenitors can acquire features of adipocytes, including the ability to express aP2 and PPARγ2, accumulate lipid, and develop the appearance of adipocytes (Kirkland et al., 2002). Of mesenchymal cell types, macrophages most closely resemble preadipocytes. Both cell types can express aP2, PPARγ and many of the same cytokines (Charriere et al., 2003; Kirkland et al., 2002). Increased expression of aP2 by macrophages is associated with accelerated development of atherosclerosis in the apolipoprotein E deficient mouse (Makowski et al., 2001). Like other mesenchymal progenitor cells, preadipocytes themselves isolated from old rats do not differentiate into fully functional fat cells and instead assume a partial adipocyte phenotype (Karagiannides et al., 2001; Karagiannides et al., 2006). Dysdifferentiation of mesenchymal progenitors into mesenchymal adipocyte-like default (or MAD) cells in muscle, marrow, fat tissue, and elsewhere, could result from age-associated stress response pathway activation (Kirkland et al., 2002).
Summary
Aging is associated with impaired adipose tissue function. Changes in cell dynamics of the fat cell progenitor pool could contribute significantly. Evidence is mounting that the capacities of preadipocytes for replication, differentiation, and resistance to apoptosis decline with aging. Along with extrinsic microenvironmental factors, inherent, genetic or epigenetic properties of preadipocytes may underlie age- and depot-associated changes in fat tissue. Genetic or epigenetic properties of preadipocytes may set the stage for the pace and extent of age-related changes in adipose tissue function. Inherent aging changes in preadipocyte function may reflect a general aging phenomenon across mesenchymal tissues, since the same trends are observed in muscle and bone progenitors. The relative contribution of inherent and microenvironmental mechanisms to age-related changes in fat tissue characteristics and metabolic function remain to be elucidated. Future research will continue to address this, yet a growing body of evidence is mounting indicating the importance of the inherent changes in preadipocyte properties to age-related metabolic dysfunction.
Acknowledgments
Supported by NIH grants AG13925, DK56891, and AG23960.
Abbreviations used
- C/EBP
CCAAT enhancer binding protein
- PPAR
peroxisome proliferator activated receptor
- RXRα
retinoid X receptor α
- LIP
liver inhibitory protein
- LAP
liver activating protein
- CHOP
C/EBP homologous protein
- CUGBP
GUG triplet binding protein
- aP2
adipocyte fatty acid binding protein
- PGC1
PPARγ coactivator-1
- CPT1
carnitine palmitoyl transferase-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, Digby JE, Sewter CP, Lazar MA, Chatterjee VK, O’Rahilly S. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest. 1997;100:3149–3153. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin BR, Timchenko NA, Zahnow CA. Epidermal growth factor receptor stimulation activates the RNA binding protein CUG-BP1 and increases expression of C/EBPbeta-LIP in mammary epithelial cells. Mol Cell Biol. 2004;24:3682–3691. doi: 10.1128/MCB.24.9.3682-3691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchvarova N, Wang XZ, Ron D. Inhibition of adipogenesis by the stress-induced protein CHOP (Gadd153) EMBO J. 1995;14:4654–4661. doi: 10.1002/j.1460-2075.1995.tb00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryhni B, Jenssen TG, Olafsen K, Bendikssen A. Oxidative and nonoxidative glucose disposal in elderly vs younger men with similar and smaller body mass indices and waist circumferences. Metabolism. 2005;54:748–755. doi: 10.1016/j.metabol.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Burgess-Beusse B, Timchenko NA, Darlington GJ. CCAAT/enhancer binding protein alpha (C/EBPalpha) is an important mediator of mouse C/EBPbeta protein isoform production. Hepatology. 1999;29:597–601. doi: 10.1002/hep.510290245. [DOI] [PubMed] [Google Scholar]
- Capeau J, Magre J, Lascols O, Caron M, Bereziat V, Vigouroux C, Bastard JP. Diseases of adipose tissue: genetic and acquired lipodystrophies. Biochem Soc Trans. 2005;33:1073–1077. doi: 10.1042/BST0331073. [DOI] [PubMed] [Google Scholar]
- Charriere G, Cousin B, Arnaud E, Andre M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa J, Evans R, Mangelsdorf D. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chumlea WC, Rhyne RL, Garry PJ, Hunt WC. Changes in anthropometric indices of body composition with age in a healthy elderly population. Am J Human Biol. 1989;1:457–462. doi: 10.1002/ajhb.1310010408. [DOI] [PubMed] [Google Scholar]
- Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- Clarke SL, Robinson CE, Gimble JM. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor gamma 2 promoter. Biochem Biophys Res Commun. 1997;240:99–103. doi: 10.1006/bbrc.1997.7627. [DOI] [PubMed] [Google Scholar]
- Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- Davis KE, Moldes M, Farmer SR. The forkhead transcription factor FoxC2 inhibits white adipocyte differentiation. J Biol Chem. 2004;279:42453–42461. doi: 10.1074/jbc.M402197200. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract Suppl. 2004;143:9–21. doi: 10.1111/j.1368-504x.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- Djian P, Phillips M, Green H. The activation of specific gene transcription in the adipose conversion of 3T3 cells. J Cell Physiol. 1985;124:554–556. doi: 10.1002/jcp.1041240327. [DOI] [PubMed] [Google Scholar]
- Domingo P, Vidal F, Domingo JC, Veloso S, Sambeat MA, Torres F, Sirvent JJ, Vendrell J, Matias-Guiu X, Richart C HIV-FRS Study Group. Tumour necrosis factor alpha in fat redistribution syndromes associated with combination antiretroviral therapy in HIV-1-infected patients: potential role in subcutaneous adipocyte apoptosis. Eur J Clin Invest. 2005;35:771–780. doi: 10.1111/j.1365-2362.2005.01576.x. [DOI] [PubMed] [Google Scholar]
- El Jack AK, Hamm JK, Pilch PF, Farmer SR. Reconstitution of insulin-sensitive glucose transport in fibroblasts requires expression of both PPARgamma and C/EBPalpha. J Biol Chem. 1999;274:7946–7951. doi: 10.1074/jbc.274.12.7946. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Troiano RP, Ballard-Barbash R. Aim for a healthy weight: what is the target? J Nutr. 2001;131:440S–450S. doi: 10.1093/jn/131.2.440S. [DOI] [PubMed] [Google Scholar]
- Garg A. Lipodystrophies. Am J Medicine. 2000;108:143–152. doi: 10.1016/s0002-9343(99)00414-3. [DOI] [PubMed] [Google Scholar]
- Gustafson B, Jack MM, Cushman SW, Smith U. Adiponectin gene activation by thiazolidinediones requires PPAR gamma 2, but not C/EBP alpha-evidence for differential regulation of the aP2 and adiponectin genes. Biochem Biophys Res Commun. 2003;308:933–939. doi: 10.1016/s0006-291x(03)01518-3. [DOI] [PubMed] [Google Scholar]
- Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer EF. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest. 1989;84:1663–1670. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, Tylavsky FA. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol. 2005;60:1420–1424. doi: 10.1093/gerona/60.11.1420. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Hube F, Hauner H. The role of TNF-alpha in human adipose tissue: prevention of weight gain at the expense of insulin resistance? Horm Metab Res. 1999;31:626–631. doi: 10.1055/s-2007-978810. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Roubenoff R, Wood M, Frontera WR, Evans WJ, Fiatarone Singh MA. Anthropometric assessment of 10-y changes in body composition in the elderly. Am J Clin Nutr. 2004;80:475–482. doi: 10.1093/ajcn/80.2.475. [DOI] [PubMed] [Google Scholar]
- Kahn S, Prigeon R, Schwartz R, Fujimoto W, Knopp R, Brunzell R, Porte D. Obesity, body fat distribution, insulin sensitivity and islet Beta-cell function as explanations for metabolic diversity. J Nutr. 2001;131:354S–360S. doi: 10.1093/jn/131.2.354S. [DOI] [PubMed] [Google Scholar]
- Karagiannides I, Tchkonia T, Dobson DE, Steppan CM, Cummins P, Chan G, Salvatori K, Hadzopoulou-Cladaras M, Kirkland JL. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol. 2001;280:R1772–R1780. doi: 10.1152/ajpregu.2001.280.6.R1772. [DOI] [PubMed] [Google Scholar]
- Karagiannides I, Thomou T, Tchkonia T, Pirtskhalava T, Kypreos KE, Cartwright A, Dalagiorgou G, Lash TL, Farmer SR, Timchenko NA, Kirkland JL. Increased CUG triplet repeat-binding protein-1 predisposes to impaired adipogenesis with aging. J Biol Chem. 2006;281:23025–23033. doi: 10.1074/jbc.M513187200. [DOI] [PubMed] [Google Scholar]
- Kehayias JJ, Fiatarone MA, Zhuang H, Roubenoff R. Total body potassium and body fat: relevance to aging. Am J Clin Nutr. 1997;66:904–910. doi: 10.1093/ajcn/66.4.904. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Dax EM. Adipose hormone responsiveness and aging in the rat. J Amer Geriatr Soc. 1984;32:219–228. doi: 10.1111/j.1532-5415.1984.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Hollenberg CH, Gillon WS. Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am J Physiol. 1990;258:C206–C210. doi: 10.1152/ajpcell.1990.258.2.C206. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Hollenberg CH, Kindler S, Gillon WS. Effects of age and anatomic site on preadipocyte number in rat fat depots. J Gerontol. 1994;49:B31–B35. doi: 10.1093/geronj/49.1.b31. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Dobson DE. Preadipocyte function and aging: links between age-related changes in cell dynamics and altered fat cell function. J Amer Geriatr Soc. 1997;45:959–967. doi: 10.1111/j.1532-5415.1997.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37:757–767. doi: 10.1016/s0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. J Am Med Assoc. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Lees SJ, Rathbone CR, Booth FW. Age-associated decrease in muscle precursor cell differentiation. Am J Physiol Cell Physiol. 2006;290:C609–615. doi: 10.1152/ajpcell.00408.2005. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Lin FT, Lane MD. Antisense CCAAT/enhancer binding protein RNA suppresses co-ordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Devel. 1992;6:533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- Lin FT, Lane MD. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci USA. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest. 2004;114:1518–1526. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nature Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler CU, Siddle K, Prins JB. Human preadipocytes display a depot-specific susceptibility to apoptosis. Diabetes. 1998;47:1365–1368. doi: 10.2337/diab.47.8.1365. [DOI] [PubMed] [Google Scholar]
- Park BH, Qiang L, Farmer SR. Phosphorylation of C/EBP{beta} at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol Cell Biol. 2004;24:8671–8680. doi: 10.1128/MCB.24.19.8671-8680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RJ, Marcusson EG, Koo S, Kang X, Kim Y, White N, Dean NM. Identification of novel PPARgamma target genes in primary human adipocytes. Gene. 2006;15(369):90–99. doi: 10.1016/j.gene.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Boschi F, Cicognani A, Gasbarrini G. Measurement of body fat in healthy elderly men: a comparison of methods. J Gerontol. 1999;54:M70–M76. doi: 10.1093/gerona/54.2.m70. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore Longitudinal Study of Aging. Metabolism. 2005;54:542–547. doi: 10.1016/j.metabol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Ackert-Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, Horton LG, Delahunty KM, Beamer WG, Sipos J, Clemmons D, Nelson T, Bouxsein ML, Horowitz M. Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone. 2004;35:1046–58. doi: 10.1016/j.bone.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- Sewter C, Berger D, Considine RV, Medina G, Rochford J, Ciaraldi T, Henry R, Dohm L, Flier JS, O’Rahilly S, Vidal-Puig AJ. Human obesity and type 2 diabetes are associated with alterations in SREBP1 isoform expression that are reproduced ex vivo by tumor necrosis factor-alpha. Diabetes. 2002;51:1035–41. doi: 10.2337/diabetes.51.4.1035. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Devel. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawik M, Vidal-Puig AJ. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev. 2006:144–64. doi: 10.1016/j.arr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Smith J, Al-Amri M, Dorairaj P, Sniderman A. The adipocyte life cycle hypothesis. Clin Sci. 2006;110:1–9. doi: 10.1042/CS20050110. [DOI] [PubMed] [Google Scholar]
- Stephens JM, Pekala PH. Transcriptional repression of the C/EBP-alpha and GLUT4 genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. Regulation is coordinate and independent of protein synthesis. J Biol Chem. 1992;267:13580–13584. [PubMed] [Google Scholar]
- Tang QQ, Zhang JW, Daniel Lane M. Sequential gene promoter interactions of C/EBPbeta, C/EBPalpha, and PPARgamma during adipogenesis. Biochem Biophys Res Commun. 2004;319:235–239. doi: 10.1016/j.bbrc.2004.04.176. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, Kirkland JL. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, Koo A, Stevenson M, Chinnappan D, Cartwright A, Jensen MD, Kirkland JL. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol. 2005;288:E267–E277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Corkey BE, Kirkland JL. Current views of the fat cell as an endocrine cell: lipotoxicity. Endocrine Updates. 2006a;26:105–118. [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, Kirkland JL. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006b;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- Timchenko NA, Welm AL, Lu X, Timchenko LT. CUG repeat binding protein (CUGBP1) interacts with the 5′ region of C/EBPbeta mRNA and regulates translation of C/EBPbeta isoforms. Nucleic Acids Res. 1999;27:4517–4525. doi: 10.1093/nar/27.22.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko LT, Salisbury E, Wang GL, Nguyen H, Albrecht JH, Hershey JW, Timchenko NA. Age-specific CUGBP1-eIF2 complex increases translation of CCAAT/enhancer-binding protein beta in old liver. J Biol Chem. 2006;281:32806–32819. doi: 10.1074/jbc.M605701200. [DOI] [PubMed] [Google Scholar]
- Unger RH. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie. 2005;87:57–64. doi: 10.1016/j.biochi.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Hotamisligil GS. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-alpha-mediated insulin resistance in genetic obesity. Endocrinology. 1998;139:4832–4838. doi: 10.1210/endo.139.12.6337. [DOI] [PubMed] [Google Scholar]
- Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBPalpha knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- Wang ZW, Pan WT, Lee Y, Kakuma T, Zhou YT, Unger RH. The role of leptin resistance in the lipid abnormalities of aging. FASEB J. 2001;15:108–114. doi: 10.1096/fj.00-0310com. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welm AL, Mackey SL, Timchenko LT, Darlington GJ, Timchenko NA. Translational induction of LIP during acute phase response leads to repression of C/EBP alpha mRNA. J Biol Chem. 2000;275:27406–27413. doi: 10.1074/jbc.M002343200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bucher NLR, Farmer SR. Induction of peroxisome proliferator-activated receptorgamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBP-beta, C/EBP-delta and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBPalpha and PPARgamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- Xu LG, Li LY, Shu HB. TRAF7 potentiates MEKK3-induced AP1 and CHOP activation and induces apoptosis. J Biol Chem. 2004;279:17278–17282. doi: 10.1074/jbc.C400063200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Mirmalek-Sani SH, Yang X, Zhang J, Oreffo RO. The use of small interfering RNAs to inhibit adipocyte differentiation in human preadipocytes and fetal-femur-derived mesenchymal cells. Exp Cell Res. 2006;312:1856–1864. doi: 10.1016/j.yexcr.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Yoo EJ, Chung JJ, Choe SS, Kim KH, Kim JB. Down-regulation of histone deacetylases stimulates adipocyte differentiation. J Biol Chem. 2006;281(10):6608–6615. doi: 10.1074/jbc.M508982200. [DOI] [PubMed] [Google Scholar]
- Zamboni M, Mazzali G, Zoico E, Harris TB, Meigs JB, Di Francesco V, Fantin F, Bissoli L, Bosello O. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes. 2005;29:1011–1029. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- Zhang B, Berger J, Hu E, Szalkowski D, White-Carrington S, Spiegelman BM, Moller DE. Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol Endocrinol. 1996;10:1457–1466. doi: 10.1210/mend.10.11.8923470. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Qiang L, Farmer SR. Activation of CCAAT/enhancer-binding protein (C/EBP) alpha expression by C/EBP beta during adipogenesis requires a peroxisome proliferator-activated receptor-gamma-associated repression of HDAC1 at the C/EBP alpha gene promoter. J Biol Chem. 2006;281:7960–7967. doi: 10.1074/jbc.M510682200. [DOI] [PubMed] [Google Scholar]


