Abstract
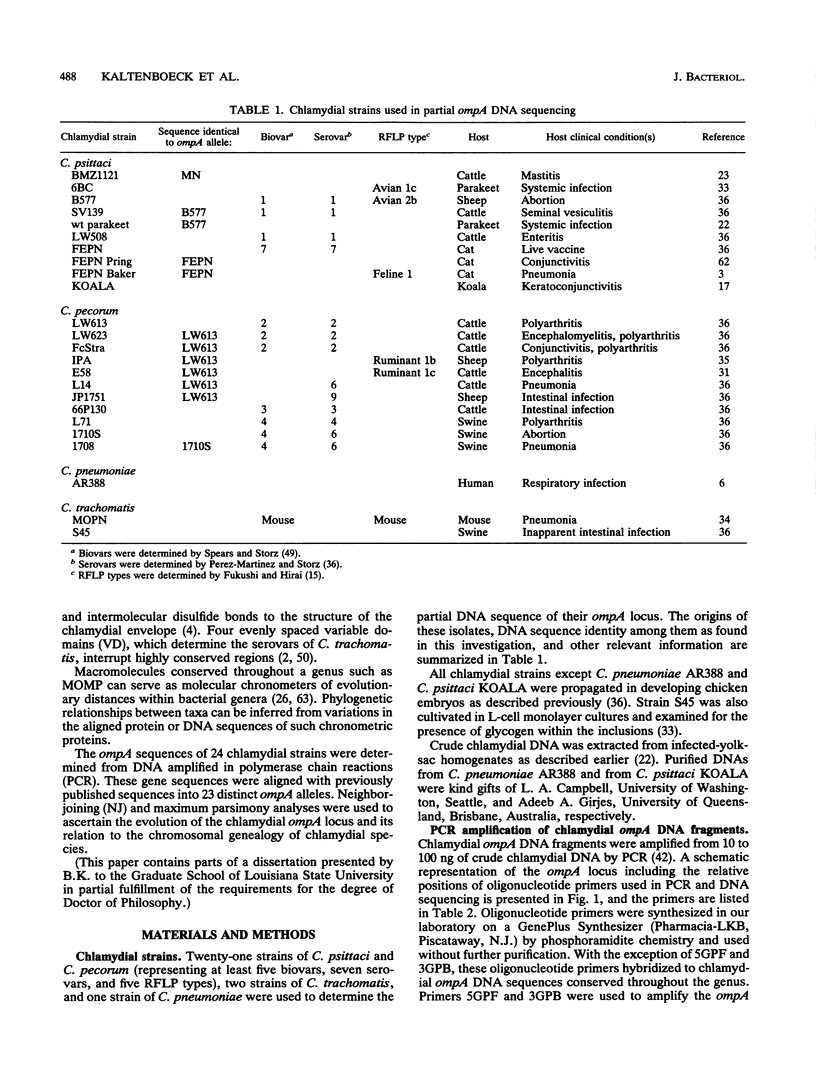
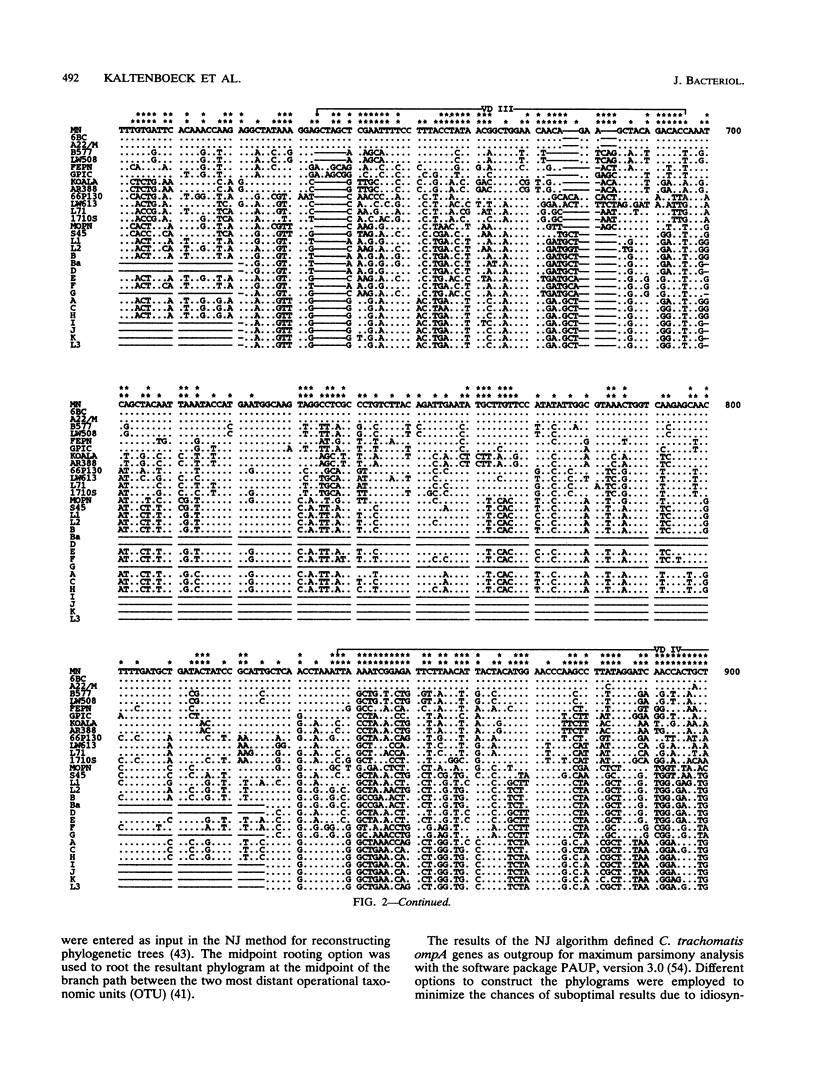
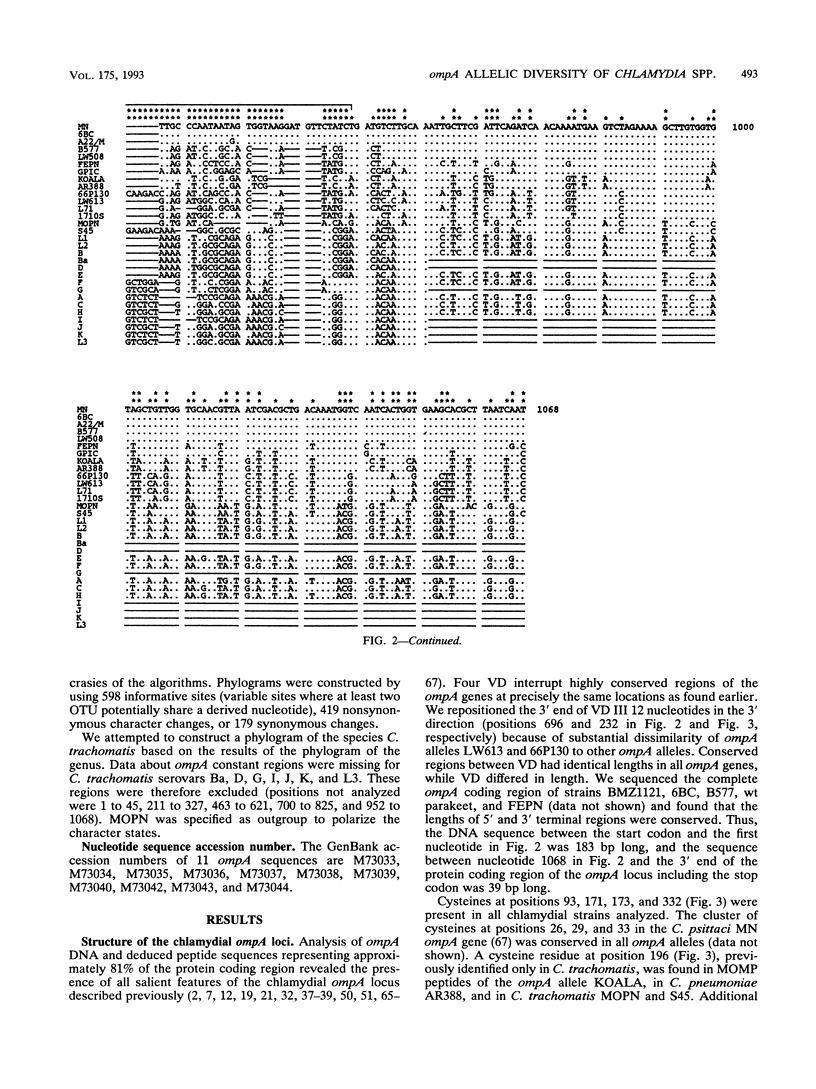
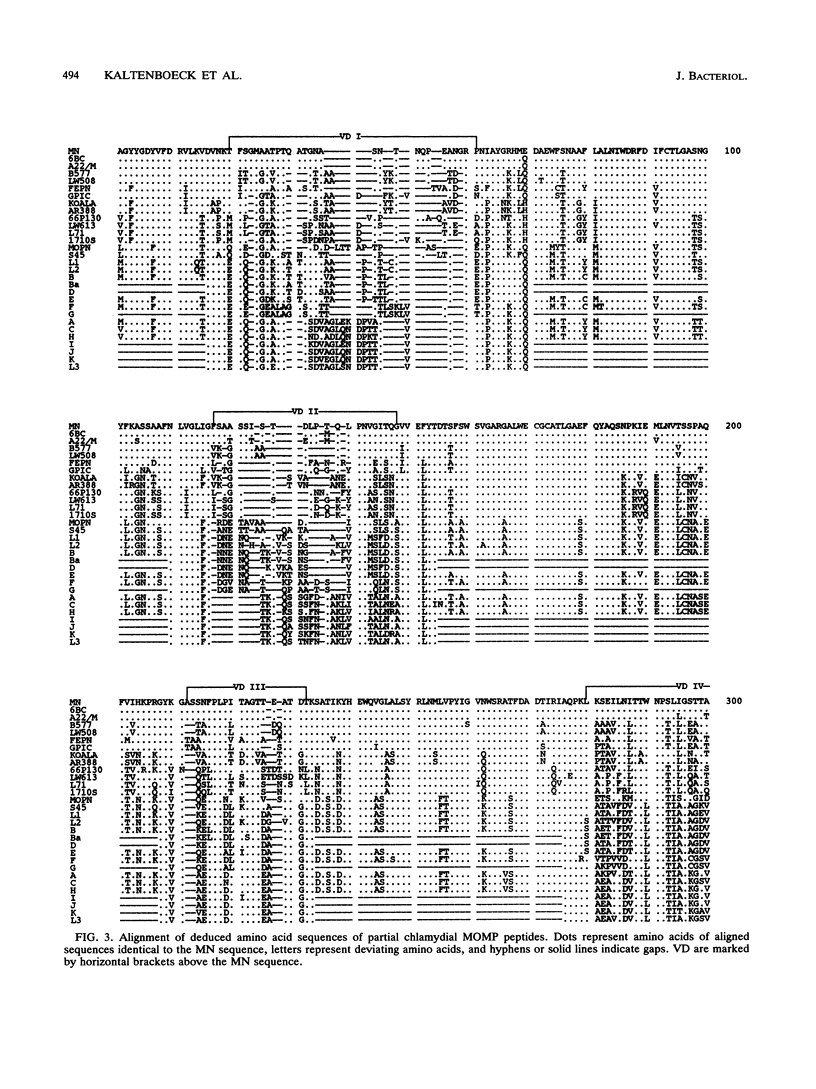
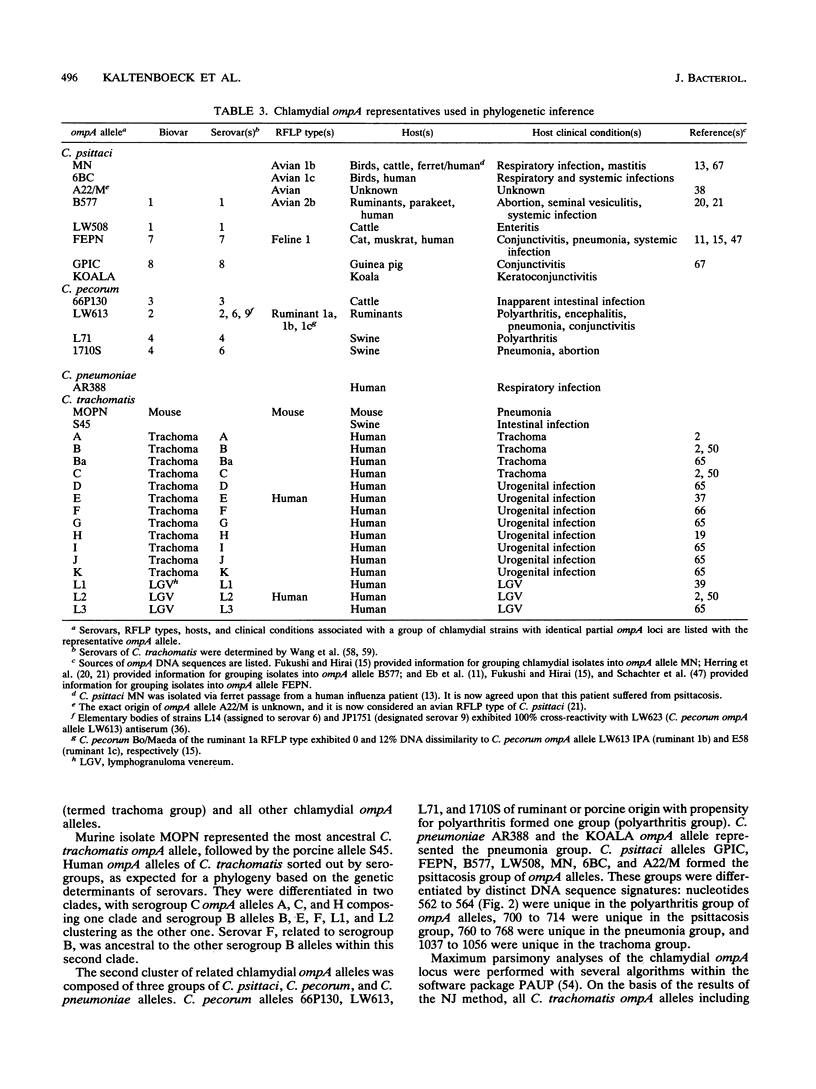
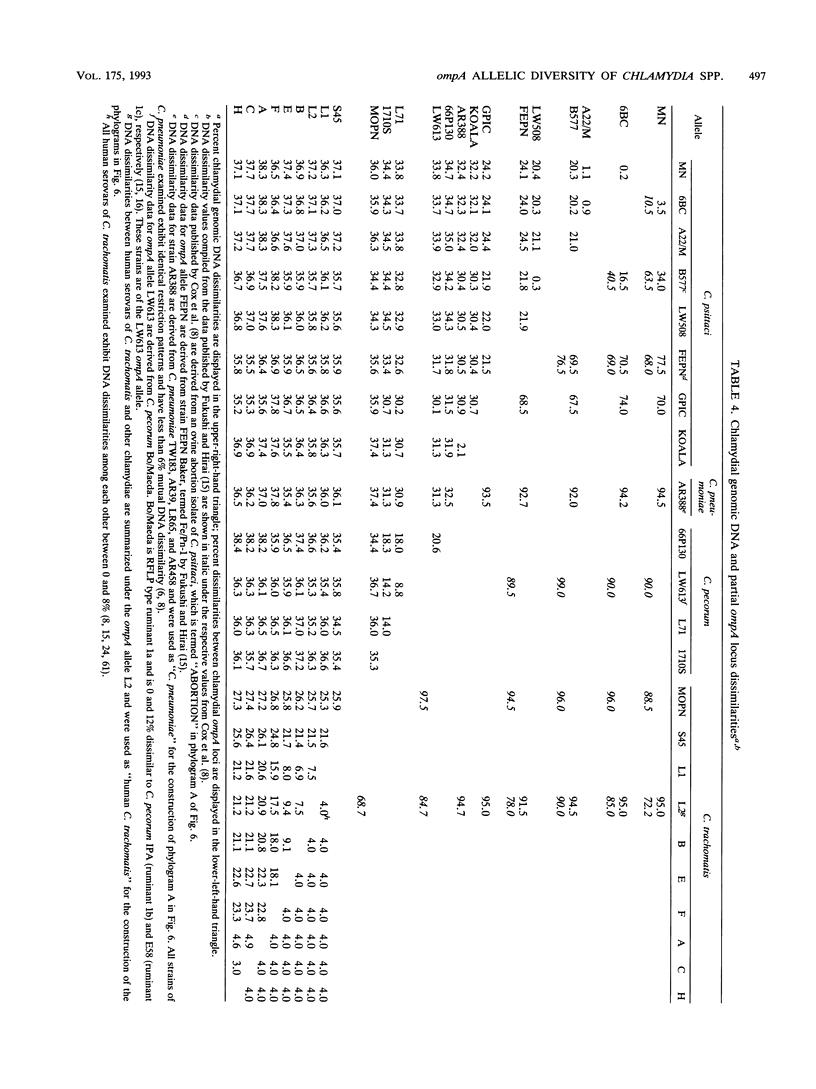
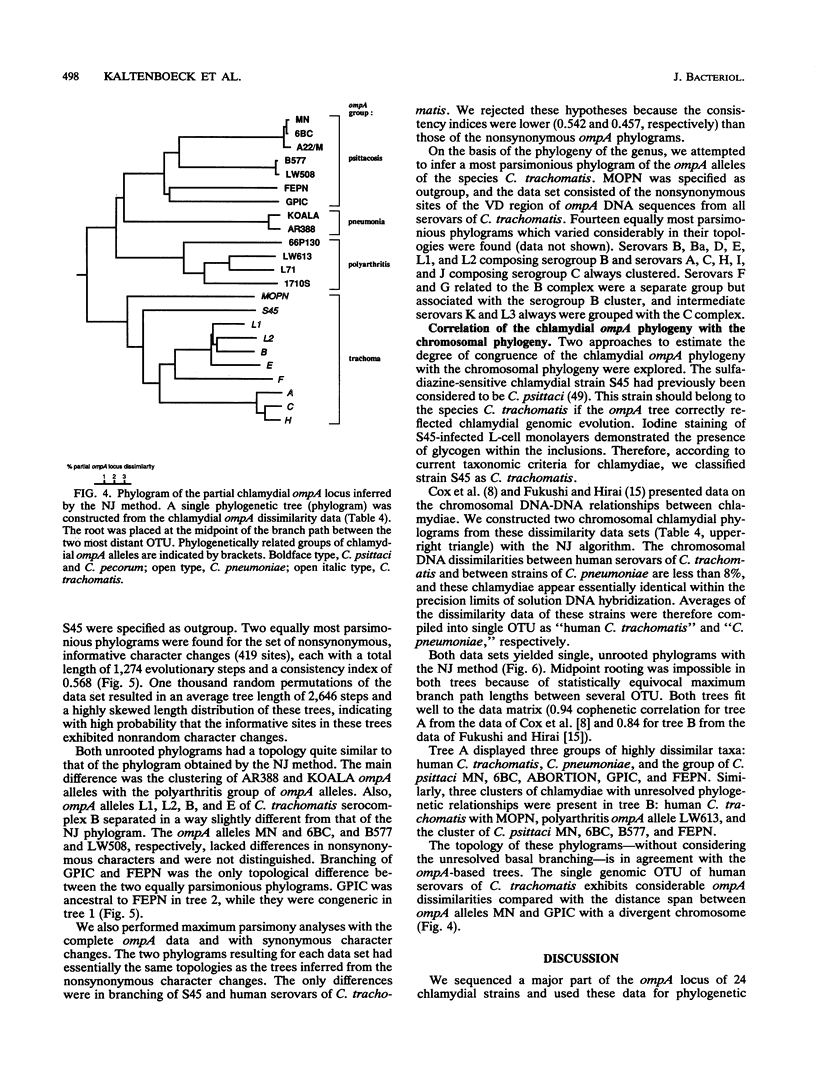
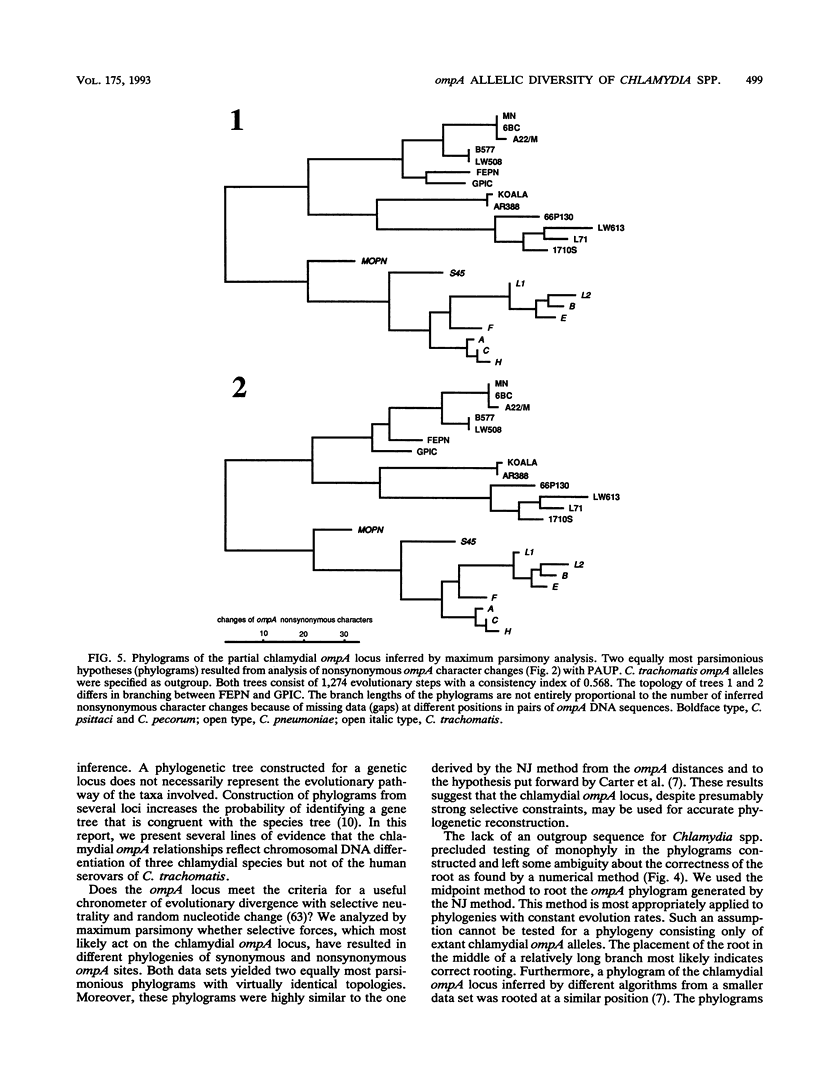
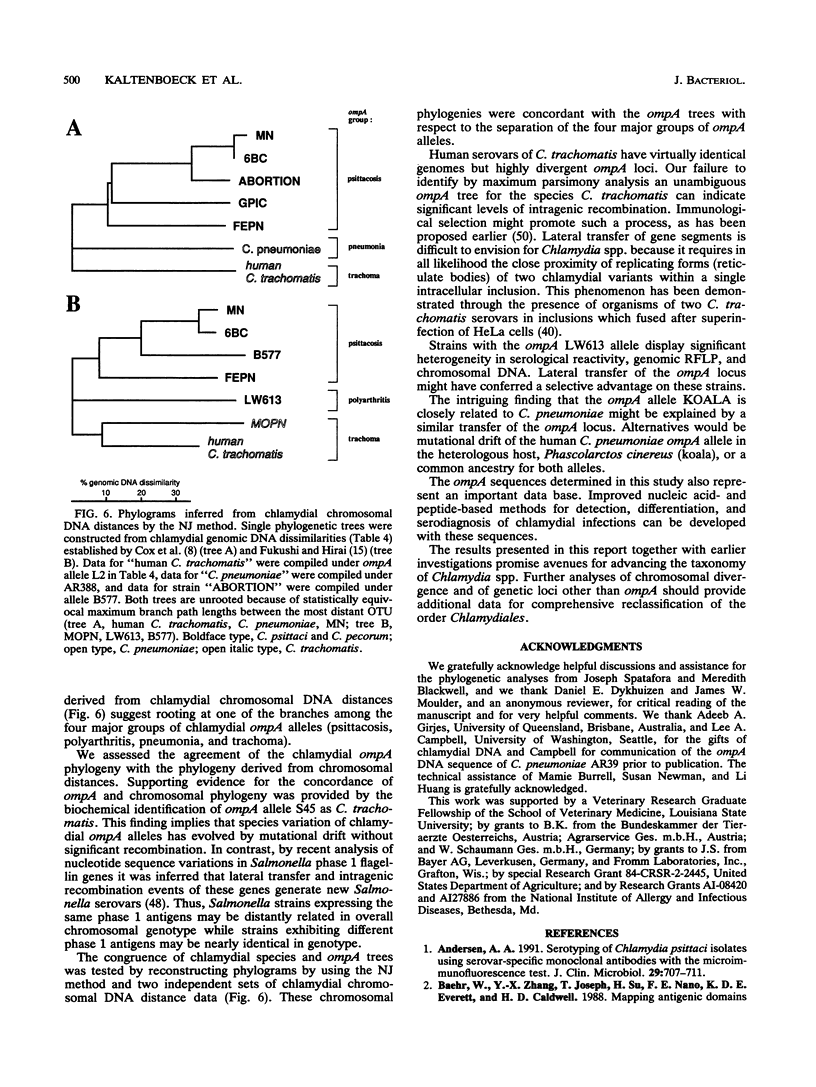
DNA sequences coding for 81% of the ompA gene from 24 chlamydial strains, representing all chlamydial species, were determined from DNA amplified by polymerase chain reactions. Chlamydial strains of serovars and strains with similar chromosomal restriction fragment length polymorphism had identical ompA DNA sequences. The ompA sequences were segregated into 23 different ompA alleles and aligned with each other, and phylogenetic relationships among them were inferred by neighbor-joining and maximum parsimony analyses. The neighbor-joining method produced a single phylogram which was rooted at the branch between two major clusters. One cluster included all Chlamydia trachomatis ompA alleles (trachoma group). The second cluster was composed of three major groups of ompA alleles: psittacosis group (alleles MN, 6BC, A22/M, B577, LW508, FEPN, and GPIC), pneumonia group (Chlamydia pneumoniae AR388 with the allele KOALA), and polyarthritis group (ruminant and porcine chlamydial alleles LW613, 66P130, L71, and 1710S with propensity for polyarthritis). These groups were distinguished through specific DNA sequence signatures. Maximum parsimony analysis yielded two equally most parsimonious phylograms with topologies similar to the ompA tree of neighbor joining. Two phylograms constructed from chlamydial genomic DNA distances had topologies identical to that of the ompA phylogram with respect to branching of the chlamydial species. Human serovars of C. trachomatis with essentially identical genomes represented a single taxonomic unit, while they were divergent in the ompA tree. Consistent with the ompA phylogeny, the porcine isolate S45, previously considered to be Chlamydia psittaci, was identified as C. trachomatis through biochemical characteristics. These data demonstrate that chlamydial ompA allelic relationships, except for human serovars of C. trachomatis, are cognate with chromosomal phylogenies.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. A. Serotyping of Chlamydia psittaci isolates using serovar-specific monoclonal antibodies with the microimmunofluorescence test. J Clin Microbiol. 1991 Apr;29(4):707–711. doi: 10.1128/jcm.29.4.707-711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Ohlin A., Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984 May;44(2):479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Grayston J. T. Characterization of the new Chlamydia agent, TWAR, as a unique organism by restriction endonuclease analysis and DNA-DNA hybridization. J Clin Microbiol. 1987 Oct;25(10):1911–1916. doi: 10.1128/jcm.25.10.1911-1916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. W., al-Mahdawi S. A., Giles I. G., Treharne J. D., Ward M. E., Clark I. N. Nucleotide sequence and taxonomic value of the major outer membrane protein gene of Chlamydia pneumoniae IOL-207. J Gen Microbiol. 1991 Mar;137(3):465–475. doi: 10.1099/00221287-137-3-465. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D. E., Green L. Recombination in Escherichia coli and the definition of biological species. J Bacteriol. 1991 Nov;173(22):7257–7268. doi: 10.1128/jb.173.22.7257-7268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eb F., Orfila J., Milon A., Géral M. F. Intérêt épidémiologique du typage par immunofluorescence de Chlamydia psittaci. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):77–93. [PubMed] [Google Scholar]
- Everett K. D., Andersen A. A., Plaunt M., Hatch T. P. Cloning and sequence analysis of the major outer membrane protein gene of Chlamydia psittaci 6BC. Infect Immun. 1991 Aug;59(8):2853–2855. doi: 10.1128/iai.59.8.2853-2855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi H., Hirai K. Genetic diversity of avian and mammalian Chlamydia psittaci strains and relation to host origin. J Bacteriol. 1989 May;171(5):2850–2855. doi: 10.1128/jb.171.5.2850-2855.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi H., Hirai K. Immunochemical diversity of the major outer membrane protein of avian and mammalian Chlamydia psittaci. J Clin Microbiol. 1988 Apr;26(4):675–680. doi: 10.1128/jcm.26.4.675-680.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi H., Hirai K. Proposal of Chlamydia pecorum sp. nov. for Chlamydia strains derived from ruminants. Int J Syst Bacteriol. 1992 Apr;42(2):306–308. doi: 10.1099/00207713-42-2-306. [DOI] [PubMed] [Google Scholar]
- Girjes A. A., Hugall A. F., Timms P., Lavin M. F. Two distinct forms of Chlamydia psittaci associated with disease and infertility in Phascolarctos cinereus (koala). Infect Immun. 1988 Aug;56(8):1897–1900. doi: 10.1128/iai.56.8.1897-1900.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton P. T., Malinowski D. P. Nucleotide sequence of the major outer membrane protein gene from Chlamydia trachomatis serovar H. Nucleic Acids Res. 1989 Oct 25;17(20):8366–8366. doi: 10.1093/nar/17.20.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Anderson I. E., McClenaghan M., Inglis N. F., Williams H., Matheson B. A., West C. P., Rodger M., Brettle P. P. Restriction endonuclease analysis of DNA from two isolates of Chlamydia psittaci obtained from human abortions. Br Med J (Clin Res Ed) 1987 Nov 14;295(6608):1239–1239. doi: 10.1136/bmj.295.6608.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Tan T. W., Baxter S., Inglis N. F., Dunbar S. Sequence analysis of the major outer membrane protein gene of an ovine abortion strain of Chlamydia psittaci. FEMS Microbiol Lett. 1989 Nov;53(1-2):153–158. doi: 10.1016/0378-1097(89)90383-2. [DOI] [PubMed] [Google Scholar]
- Kaltenboeck B., Kousoulas K. G., Storz J. Detection and strain differentiation of Chlamydia psittaci mediated by a two-step polymerase chain reaction. J Clin Microbiol. 1991 Sep;29(9):1969–1975. doi: 10.1128/jcm.29.9.1969-1975.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenboeck B., Spatafora J. W., Zhang X., Kousoulas K. G., Blackwell M., Storz J. Efficient production of single-stranded DNA as long as 2 kb for sequencing of PCR-amplified DNA. Biotechniques. 1992 Feb;12(2):164, 166, 168-71. [PubMed] [Google Scholar]
- Kingsbury D. T., Weiss E. Lack of deoxyribonucleic acid homology between species of the genus Chlamydia. J Bacteriol. 1968 Oct;96(4):1421–1423. doi: 10.1128/jb.96.4.1421-1423.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. G., Ochman H., Hartl D. L. Molecular and evolutionary relationships among enteric bacteria. J Gen Microbiol. 1991 Aug;137(8):1911–1921. doi: 10.1099/00221287-137-8-1911. [DOI] [PubMed] [Google Scholar]
- Lusher M., Storey C. C., Richmond S. J. Plasmid diversity within the genus Chlamydia. J Gen Microbiol. 1989 May;135(5):1145–1151. doi: 10.1099/00221287-135-5-1145. [DOI] [PubMed] [Google Scholar]
- Mantel N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967 Feb;27(2):209–220. [PubMed] [Google Scholar]
- McClenaghan M., Herring A. J., Aitken I. D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984 Aug;45(2):384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClenaghan M., Honeycombe J. R., Bevan B. J., Herring A. J. Distribution of plasmid sequences in avian and mammalian strains of Chlamydia psittaci. J Gen Microbiol. 1988 Mar;134(3):559–565. doi: 10.1099/00221287-134-3-559. [DOI] [PubMed] [Google Scholar]
- Nigg C. AN UNIDENTIFIED VIRUS WHICH PRODUCES PNEUMONIA AND SYSTEMIC INFECTION IN MICE. Science. 1942 Jan 9;95(2454):49–50. doi: 10.1126/science.95.2454.49-a. [DOI] [PubMed] [Google Scholar]
- Perez Melgosa M., Kuo C. C., Campbell L. A. Sequence analysis of the major outer membrane protein gene of Chlamydia pneumoniae. Infect Immun. 1991 Jun;59(6):2195–2199. doi: 10.1128/iai.59.6.2195-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martinez J. A., Storz J. Antigenic diversity of Chlamydia psittaci of mammalian origin determined by microimmunofluorescence. Infect Immun. 1985 Dec;50(3):905–910. doi: 10.1128/iai.50.3.905-910.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Markoff B. A., de la Maza L. M. The major outer membrane protein nucleotide sequence of Chlamydia trachomatis, serovar E. Nucleic Acids Res. 1990 Jun 11;18(11):3414–3414. doi: 10.1093/nar/18.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderhof J. C., Barnes R. C. Fusion of inclusions following superinfection of HeLa cells by two serovars of Chlamydia trachomatis. Infect Immun. 1989 Oct;57(10):3189–3193. doi: 10.1128/iai.57.10.3189-3193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia. I. Isolates of ovine origin. Infect Immun. 1974 Jan;9(1):92–94. doi: 10.1128/iai.9.1.92-94.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia: isolates of bovine origin. Infect Immun. 1975 May;11(5):904–907. doi: 10.1128/iai.11.5.904-907.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Ostler H. B., Meyer K. F. Human infection with the agent of feline pneumonitis. Lancet. 1969 May 31;1(7605):1063–1065. doi: 10.1016/s0140-6736(69)91703-6. [DOI] [PubMed] [Google Scholar]
- Smith N. H., Beltran P., Selander R. K. Recombination of Salmonella phase 1 flagellin genes generates new serovars. J Bacteriol. 1990 May;172(5):2209–2216. doi: 10.1128/jb.172.5.2209-2216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears P., Storz J. Biotyping of Chlamydia psittaci based on inclusion morphology and response to diethylaminoethyl-dextran and cycloheximide. Infect Immun. 1979 Apr;24(1):224–232. doi: 10.1128/iai.24.1.224-232.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Schoolnik G. K. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988 Mar 1;167(3):817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Morrison R. P., Watkins N. G., Caldwell H. D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990 Jul 1;172(1):203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Takashima I., Hashimoto N. Immunotyping of Chlamydia psittaci by indirect immunofluorescence antibody test with monoclonal antibodies. Microbiol Immunol. 1988;32(3):251–263. doi: 10.1111/j.1348-0421.1988.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Timms P., Eaves F. W., Girjes A. A., Lavin M. F. Comparison of Chlamydia psittaci isolates by restriction endonuclease and DNA probe analyses. Infect Immun. 1988 Jan;56(1):287–290. doi: 10.1128/iai.56.1.287-290.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Barnes R. C., Stephens R. S., Grayston J. T. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J Infect Dis. 1985 Oct;152(4):791–800. doi: 10.1093/infdis/152.4.791. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Hatch T. P., Woese C. R. Eubacterial origin of chlamydiae. J Bacteriol. 1986 Aug;167(2):570–574. doi: 10.1128/jb.167.2.570-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Schramek S., Wilson N. N., Newman L. W. Deoxyribonucleic Acid Heterogeneity Between Human and Murine Strains of Chlamydia trachomatis. Infect Immun. 1970 Jul;2(1):24–28. doi: 10.1128/iai.2.1.24-28.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J., Gruffydd-Jones T. J., Richmond S., Paul I. D. Isolation of Chlamydia psittaci from cases of conjunctivitis in a colony of cats. Vet Rec. 1984 Apr 7;114(14):344–346. doi: 10.1136/vr.114.14.344. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Manning D. S., Caldwell H. D. Multiple tandem promoters of the major outer membrane protein gene (omp1) of Chlamydia psittaci. Infect Immun. 1990 Sep;58(9):2850–2855. doi: 10.1128/iai.58.9.2850-2855.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Morrison S. G., Caldwell H. D., Baehr W. Cloning and sequence analysis of the major outer membrane protein genes of two Chlamydia psittaci strains. Infect Immun. 1989 May;57(5):1621–1625. doi: 10.1128/iai.57.5.1621-1625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Morrison S. G., Caldwell H. D. The nucleotide sequence of major outer membrane protein gene of Chlamydia trachomatis serovar F. Nucleic Acids Res. 1990 Feb 25;18(4):1061–1061. doi: 10.1093/nar/18.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]