Abstract
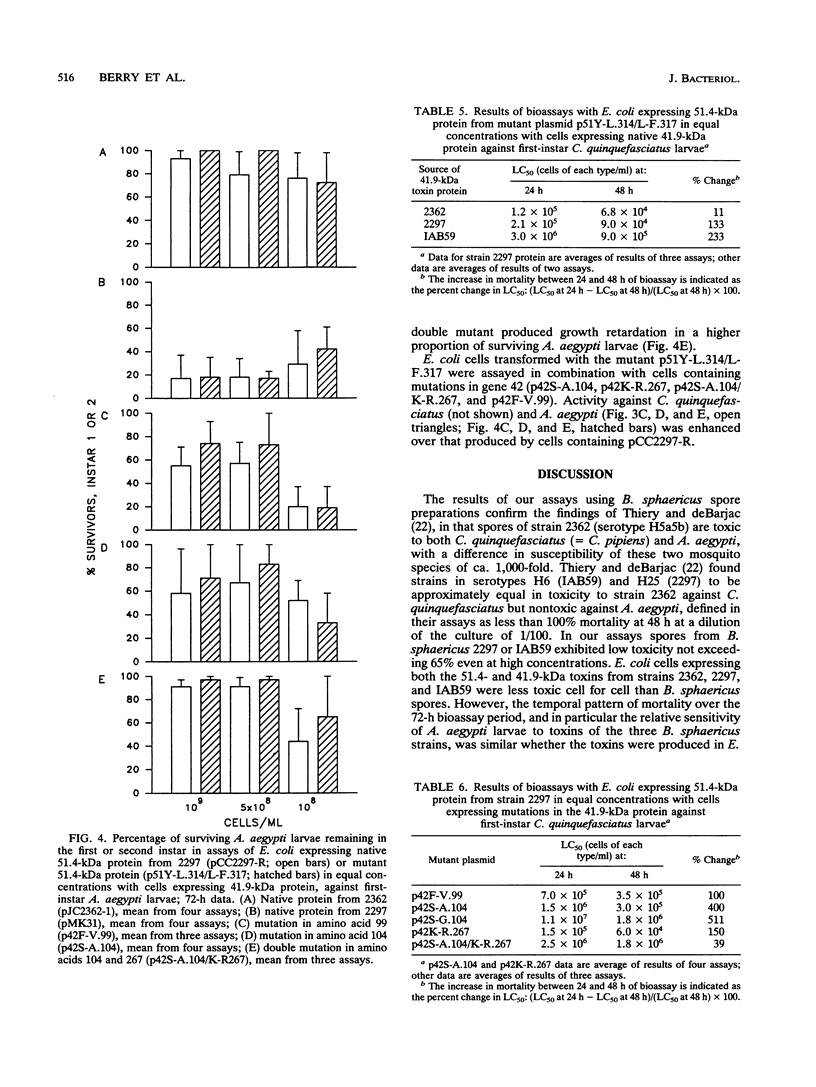
The 51.4-kDa-41.9-kDa binary toxin produced by different strains of Bacillus sphaericus shows differential activity toward Culex quinquefasciatus, Aedes atropalpus, and Aedes aegypti mosquito larvae. The patterns of larvicidal activity toward all three mosquito species and growth retardation in A. aegypti have been shown to be due to the 41.9-kDa protein. By using mutant toxins expressed in Escherichia coli, insecticidal activity and growth retardation correlated with amino acids centered around position 100 of the 41.9-kDa protein. In its response to these toxins, A. atropalpus resembled C. quinquefasciatus rather than its congener, A. aegypti.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann L., Broadwell A. H., Baumann P. Sequence analysis of the mosquitocidal toxin genes encoding 51.4- and 41.9-kilodalton proteins from Bacillus sphaericus 2362 and 2297. J Bacteriol. 1988 May;170(5):2045–2050. doi: 10.1128/jb.170.5.2045-2050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C., Hindley J. Bacillus sphaericus strain 2362: identification and nucleotide sequence of the 41.9 kDa toxin gene. Nucleic Acids Res. 1987 Jul 24;15(14):5891–5891. doi: 10.1093/nar/15.14.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C., Jackson-Yap J., Oei C., Hindley J. Nucleotide sequence of two toxin genes from Bacillus sphaericus IAB59: sequence comparisons between five highly toxinogenic strains. Nucleic Acids Res. 1989 Sep 25;17(18):7516–7516. doi: 10.1093/nar/17.18.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell A. H., Baumann L., Baumann P. The 42- and 51-kilodalton mosquitocidal proteins of Bacillus sphaericus 2362: construction of recombinants with enhanced expression and in vivo studies of processing and toxicity. J Bacteriol. 1990 May;172(5):2217–2223. doi: 10.1128/jb.172.5.2217-2223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell A. H., Clark M. A., Baumann L., Baumann P. Construction by site-directed mutagenesis of a 39-kilodalton mosquitocidal protein similar to the larva-processed toxin of Bacillus sphaericus 2362. J Bacteriol. 1990 Jul;172(7):4032–4036. doi: 10.1128/jb.172.7.4032-4036.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles J. F. Ultrastructural midgut events in Culicidae larvae fed with Bacillus sphaericus 2297 spore/crystal complex. Ann Inst Pasteur Microbiol. 1987 Jul-Aug;138(4):471–484. doi: 10.1016/0769-2609(87)90064-0. [DOI] [PubMed] [Google Scholar]
- Davidson E. W. Binding of the Bacillus sphaericus (Eubacteriales: Bacillaceae) toxin to midgut cells of mosquito (Diptera: Culicidae) larvae: relationship to host range. J Med Entomol. 1988 May;25(3):151–157. doi: 10.1093/jmedent/25.3.151. [DOI] [PubMed] [Google Scholar]
- Davidson E. W., Oei C., Meyer M., Bieber A. L., Hindley J., Berry C. Interaction of the Bacillus sphaericus mosquito larvicidal proteins. Can J Microbiol. 1990 Dec;36(12):870–878. doi: 10.1139/m90-151. [DOI] [PubMed] [Google Scholar]
- Davidson E. W. Variation in binding of Bacillus sphaericus toxin and wheat germ agglutinin to larval midgut cells of six species of mosquitoes. J Invertebr Pathol. 1989 Mar;53(2):251–259. doi: 10.1016/0022-2011(89)90015-3. [DOI] [PubMed] [Google Scholar]
- Lacey L. A., Lacey C. M., Peacock B., Thiery I. Mosquito host range and field activity of Bacillus sphaericus isolate 2297 (serotype 25). J Am Mosq Control Assoc. 1988 Mar;4(1):51–56. [PubMed] [Google Scholar]
- Oei C., Hindley J., Berry C. An analysis of the genes encoding the 51.4- and 41.9-kDa toxins of Bacillus sphaericus 2297 by deletion mutagenesis: the construction of fusion proteins. FEMS Microbiol Lett. 1990 Nov;60(3):265–273. doi: 10.1016/0378-1097(90)90315-h. [DOI] [PubMed] [Google Scholar]
- Oei C., Hindley J., Berry C. Binding of purified Bacillus sphaericus binary toxin and its deletion derivatives to Culex quinquefasciatus gut: elucidation of functional binding domains. J Gen Microbiol. 1992 Jul;138(7):1515–1526. doi: 10.1099/00221287-138-7-1515. [DOI] [PubMed] [Google Scholar]
- Sebo P., Bennardo T., de la Torre F., Szulmajster J. Delineation of the minimal portion of the Bacillus sphaericus 1593M toxin required for the expression of larvicidal activity. Eur J Biochem. 1990 Nov 26;194(1):161–165. doi: 10.1111/j.1432-1033.1990.tb19440.x. [DOI] [PubMed] [Google Scholar]
- Thanabalu T., Hindley J., Jackson-Yap J., Berry C. Cloning, sequencing, and expression of a gene encoding a 100-kilodalton mosquitocidal toxin from Bacillus sphaericus SSII-1. J Bacteriol. 1991 May;173(9):2776–2785. doi: 10.1128/jb.173.9.2776-2785.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


