Abstract
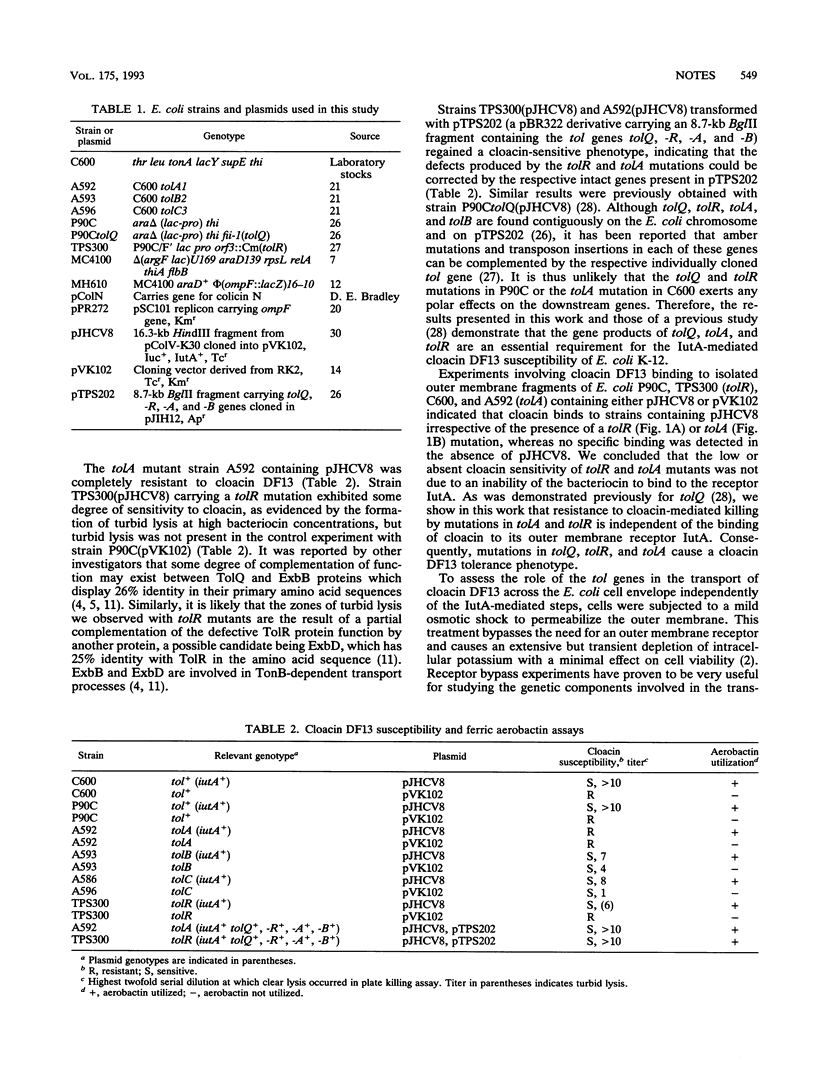
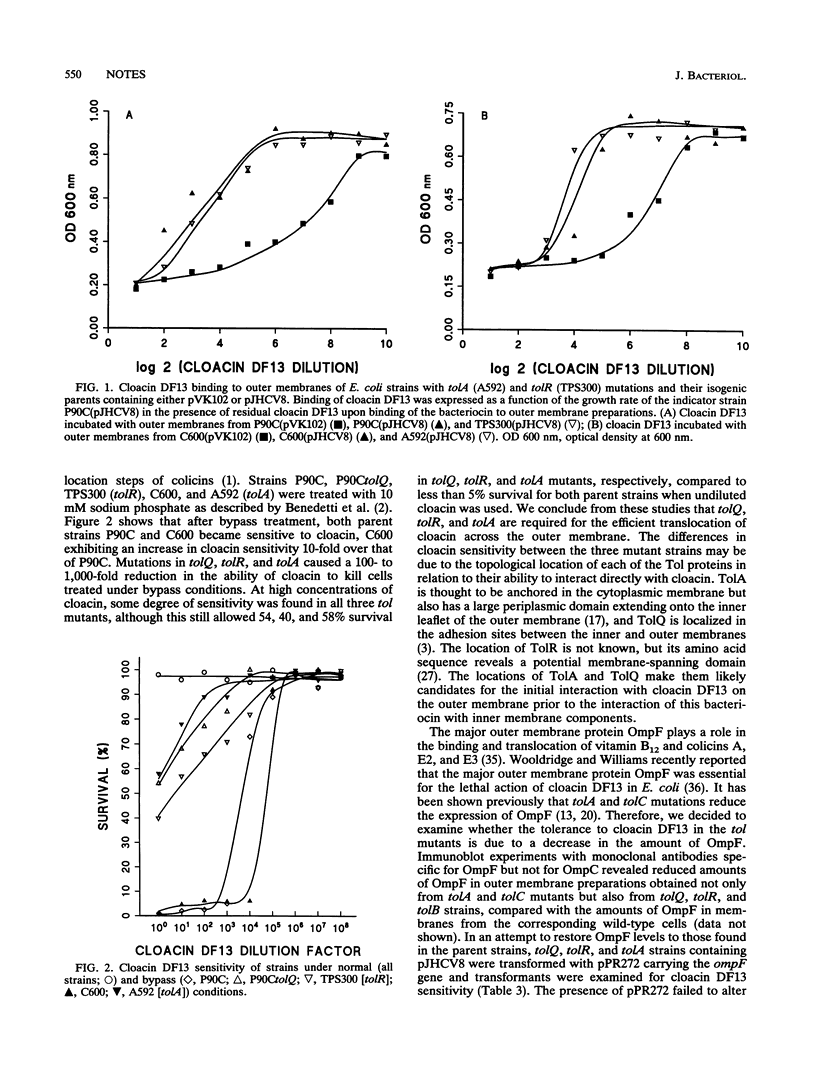
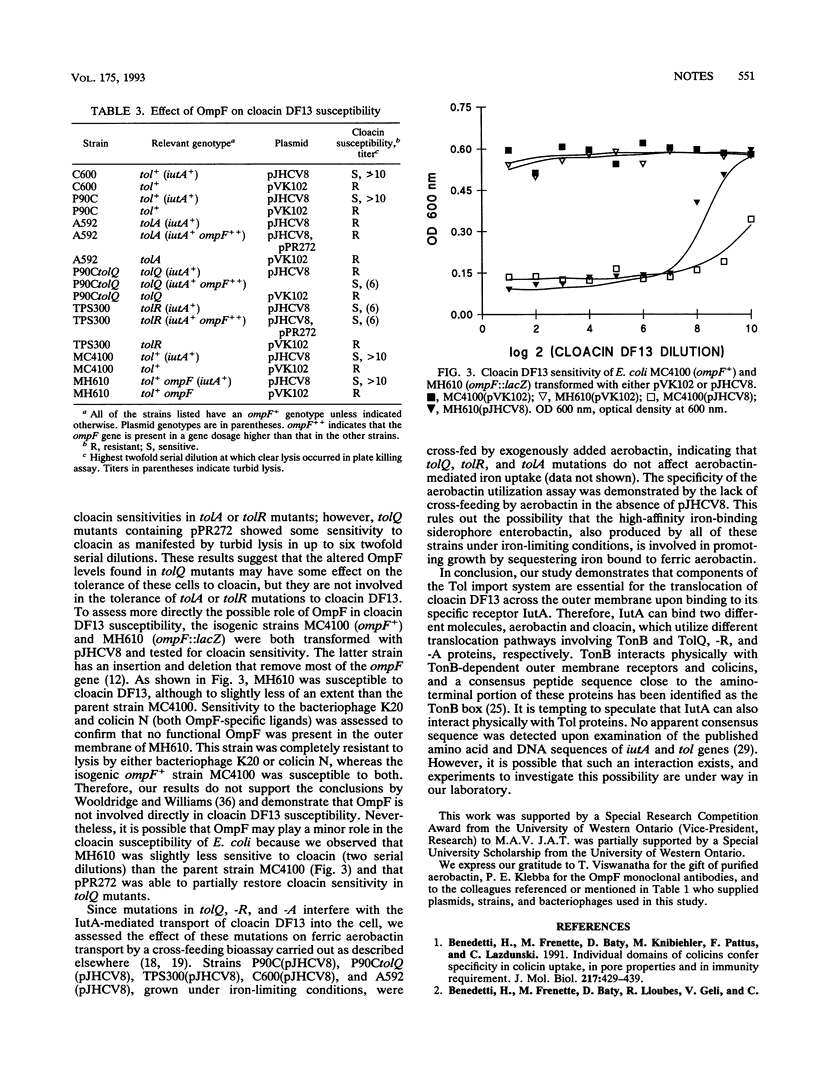
IutA is the outer membrane protein receptor for ferric aerobactin and the bacteriocin cloacin DF13. Although the same receptor is shared, ferric aerobactin transport across the outer membrane in Escherichia coli is TonB dependent, whereas cloacin DF13 transport is not. We have recently observed that tolQ is required for cloacin DF13 susceptibility (J.A. Thomas and M.A. Valvano, FEMS Microbiol. Lett. 91:107-112, 1992). In this study, we demonstrate that the genes tolQ, tolR, and tolA, but not tolB, tolC, and ompF, are required for the internalization of cloacin DF13 and they are not involved in the transport of ferric aerobactin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti H., Frenette M., Baty D., Knibiehler M., Pattus F., Lazdunski C. Individual domains of colicins confer specificity in colicin uptake, in pore-properties and in immunity requirement. J Mol Biol. 1991 Feb 5;217(3):429–439. doi: 10.1016/0022-2836(91)90747-t. [DOI] [PubMed] [Google Scholar]
- Benedetti H., Frenette M., Baty D., Lloubès R., Geli V., Lazdunski C. Comparison of the uptake systems for the entry of various BtuB group colicins into Escherichia coli. J Gen Microbiol. 1989 Dec;135(12):3413–3420. doi: 10.1099/00221287-135-12-3413. [DOI] [PubMed] [Google Scholar]
- Bourdineaud J. P., Howard S. P., Lazdunski C. Localization and assembly into the Escherichia coli envelope of a protein required for entry of colicin A. J Bacteriol. 1989 May;171(5):2458–2465. doi: 10.1128/jb.171.5.2458-2465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Günter K., Hantke K. Transport of iron across the outer membrane. Biol Met. 1991;4(1):14–22. doi: 10.1007/BF01135552. [DOI] [PubMed] [Google Scholar]
- Braun V. The structurally related exbB and tolQ genes are interchangeable in conferring tonB-dependent colicin, bacteriophage, and albomycin sensitivity. J Bacteriol. 1989 Nov;171(11):6387–6390. doi: 10.1128/jb.171.11.6387-6390.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti N. H., Williams P. H. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun. 1984 Oct;46(1):7–12. doi: 10.1128/iai.46.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K., Klaasen-Boor P. Purification and characterization of a complex between cloacin and its immunity protein isolated from Enterobacter cloacae (Clo DF13). Dissociation and reconstitution of the complex. Eur J Biochem. 1977 Feb 15;73(1):107–114. doi: 10.1111/j.1432-1033.1977.tb11296.x. [DOI] [PubMed] [Google Scholar]
- Eick-Helmerich K., Braun V. Import of biopolymers into Escherichia coli: nucleotide sequences of the exbB and exbD genes are homologous to those of the tolQ and tolR genes, respectively. J Bacteriol. 1989 Sep;171(9):5117–5126. doi: 10.1128/jb.171.9.5117-5126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Krone W. J., Luirink J., Koningstein G., Oudega B., de Graaf F. K. Subcloning of the cloacin DF13/aerobactin receptor protein and identification of a pColV-K30-determined polypeptide involved in ferric-aerobactin uptake. J Bacteriol. 1983 Nov;156(2):945–948. doi: 10.1128/jb.156.2.945-948.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone W. J., de Vries P., Koningstein G., de Jonge A. J., de Graaf F. K., Oudega B. Uptake of cloacin DF13 by susceptible cells: removal of immunity protein and fragmentation of cloacin molecules. J Bacteriol. 1986 Apr;166(1):260–268. doi: 10.1128/jb.166.1.260-268.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levengood S. K., Webster R. E. Nucleotide sequences of the tolA and tolB genes and localization of their products, components of a multistep translocation system in Escherichia coli. J Bacteriol. 1989 Dec;171(12):6600–6609. doi: 10.1128/jb.171.12.6600-6609.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marolda C. L., Valvano M. A., Crosa J. H. Polymorphism in the aerobactin-cloacin DF13 receptor genes from an enteroinvasive strain of Escherichia coli and pColV-K30 is associated only with a decrease in cloacin susceptibility. Infect Immun. 1991 Jan;59(1):357–364. doi: 10.1128/iai.59.1.357-364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marolda C. L., Valvano M. A., Lawlor K. M., Payne S. M., Crosa J. H. Flanking and internal regions of chromosomal genes mediating aerobactin iron uptake systems in enteroinvasive Escherichia coli and Shigella flexneri. J Gen Microbiol. 1987 Aug;133(8):2269–2278. doi: 10.1099/00221287-133-8-2269. [DOI] [PubMed] [Google Scholar]
- Misra R., Reeves P. R. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J Bacteriol. 1987 Oct;169(10):4722–4730. doi: 10.1128/jb.169.10.4722-4730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega B., de Graaf F. K. Enzymatic properties of cloacin DF13 and kinetics of ribosome inactivation. Biochim Biophys Acta. 1976 Mar 17;425(3):296–304. doi: 10.1016/0005-2787(76)90256-2. [DOI] [PubMed] [Google Scholar]
- Postle K. TonB and the gram-negative dilemma. Mol Microbiol. 1990 Dec;4(12):2019–2025. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Roberts M., Wooldridge K. G., Gavine H., Kuswandi S. I., Williams P. H. Inhibition of biological activities of the aerobactin receptor protein in rough strains of Escherichia coli by polyclonal antiserum raised against native protein. J Gen Microbiol. 1989 Sep;135(9):2387–2398. doi: 10.1099/00221287-135-9-2387. [DOI] [PubMed] [Google Scholar]
- Schramm E., Mende J., Braun V., Kamp R. M. Nucleotide sequence of the colicin B activity gene cba: consensus pentapeptide among TonB-dependent colicins and receptors. J Bacteriol. 1987 Jul;169(7):3350–3357. doi: 10.1128/jb.169.7.3350-3357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. P., Webster R. E. Nucleotide sequence of a gene cluster involved in entry of E colicins and single-stranded DNA of infecting filamentous bacteriophages into Escherichia coli. J Bacteriol. 1987 Jun;169(6):2667–2674. doi: 10.1128/jb.169.6.2667-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. P., Webster R. E. fii, a bacterial locus required for filamentous phage infection and its relation to colicin-tolerant tolA and tolB. J Bacteriol. 1986 Jan;165(1):107–115. doi: 10.1128/jb.165.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Valvano M. A. tolQ is required for cloacin DF13 susceptibility in Escherichia coli expressing the aerobactin/cloacin DF13 receptor IutA. FEMS Microbiol Lett. 1992 Mar 1;70(2):107–111. doi: 10.1016/0378-1097(92)90668-e. [DOI] [PubMed] [Google Scholar]
- Valvano M. A., Crosa J. H. Molecular cloning, expression, and regulation in Escherichia coli K-12 of a chromosome-mediated aerobactin iron transport system from a human invasive isolate of E. coli K1. J Bacteriol. 1988 Dec;170(12):5529–5538. doi: 10.1128/jb.170.12.5529-5538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano M. A., Silver R. P., Crosa J. H. Occurrence of chromosome- or plasmid-mediated aerobactin iron transport systems and hemolysin production among clonal groups of human invasive strains of Escherichia coli K1. Infect Immun. 1986 Apr;52(1):192–199. doi: 10.1128/iai.52.1.192-199.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tiel-Menkveld G. J., Mentjox-Vervuurt J. M., Oudega B., de Graaf F. K. Siderophore production by Enterobacter cloacae and a common receptor protein for the uptake of aerobactin and cloacin DF13. J Bacteriol. 1982 May;150(2):490–497. doi: 10.1128/jb.150.2.490-497.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. E. The tol gene products and the import of macromolecules into Escherichia coli. Mol Microbiol. 1991 May;5(5):1005–1011. doi: 10.1111/j.1365-2958.1991.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Wooldridge K. G., Williams P. H. Sensitivity of Escherichia coli to cloacin DF13 involves the major outer membrane protein OmpF. J Bacteriol. 1991 Apr;173(8):2420–2424. doi: 10.1128/jb.173.8.2420-2424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Spanjaerdt Speckman E. A., Stouthamer A. H. Mode of action of a bacteriocin produced by Enterobacter cloacae DF13. Antonie Van Leeuwenhoek. 1969;35(3):287–306. doi: 10.1007/BF02219150. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Stukart M. J., Boogerd F. C., Metselaar K. Limited proteolysis of cloacin DF13 and characterization of the cleavage products. Biochemistry. 1978 Mar 21;17(6):1137–1142. doi: 10.1021/bi00599a031. [DOI] [PubMed] [Google Scholar]
- van den Elzen P. J., Walters H. H., Veltkamp E., Nijkamp H. J. Molecular structure and function of the bacteriocin gene and bacteriocin protein of plasmid Clo DF13. Nucleic Acids Res. 1983 Apr 25;11(8):2465–2477. doi: 10.1093/nar/11.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]



