Abstract
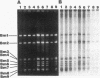
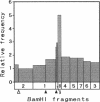
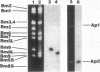
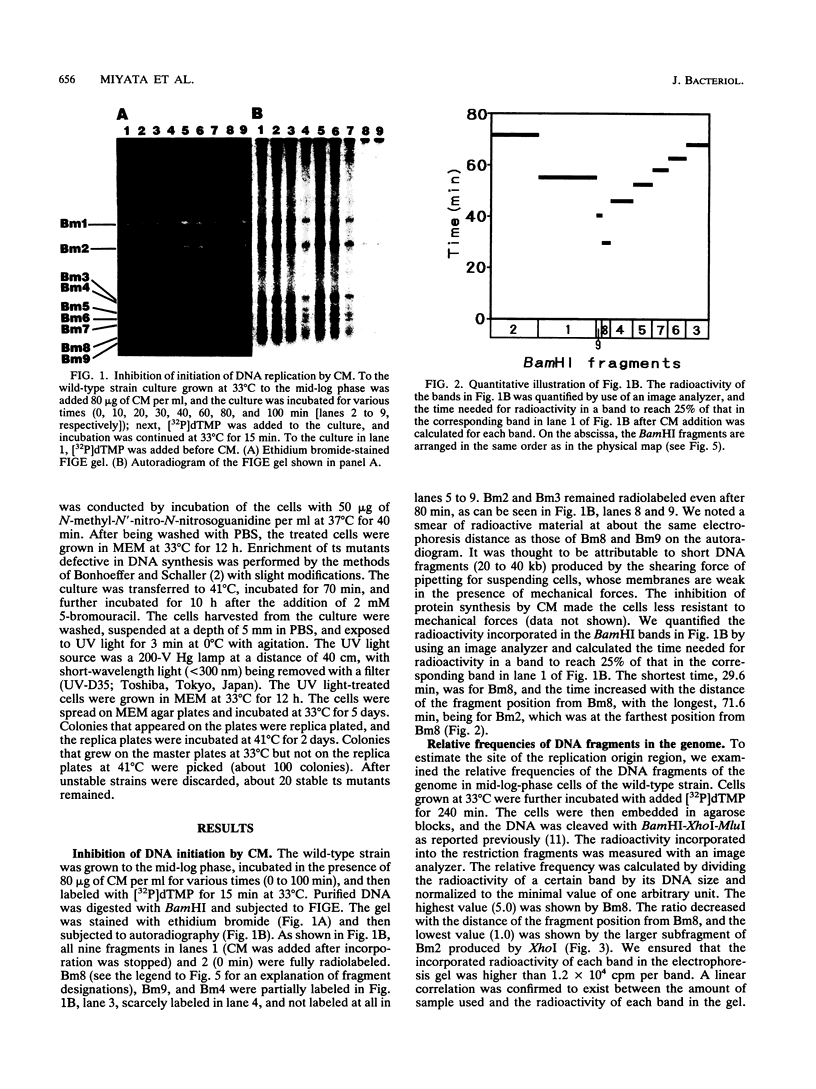
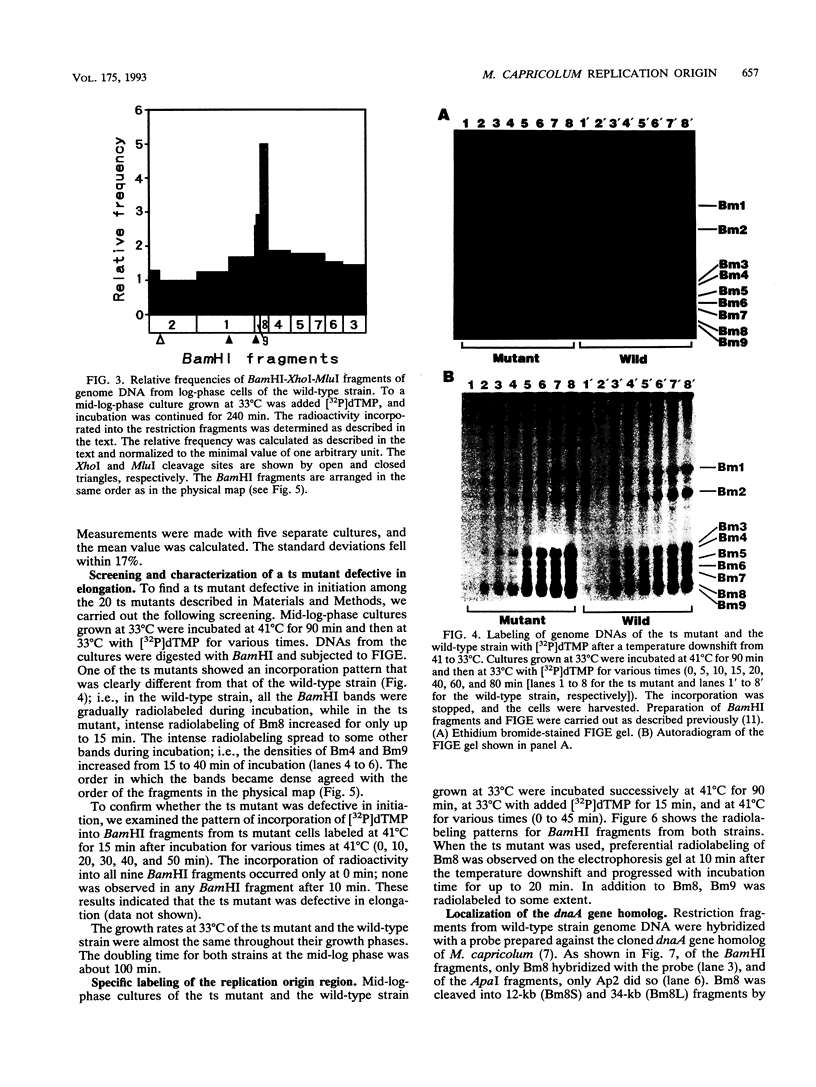
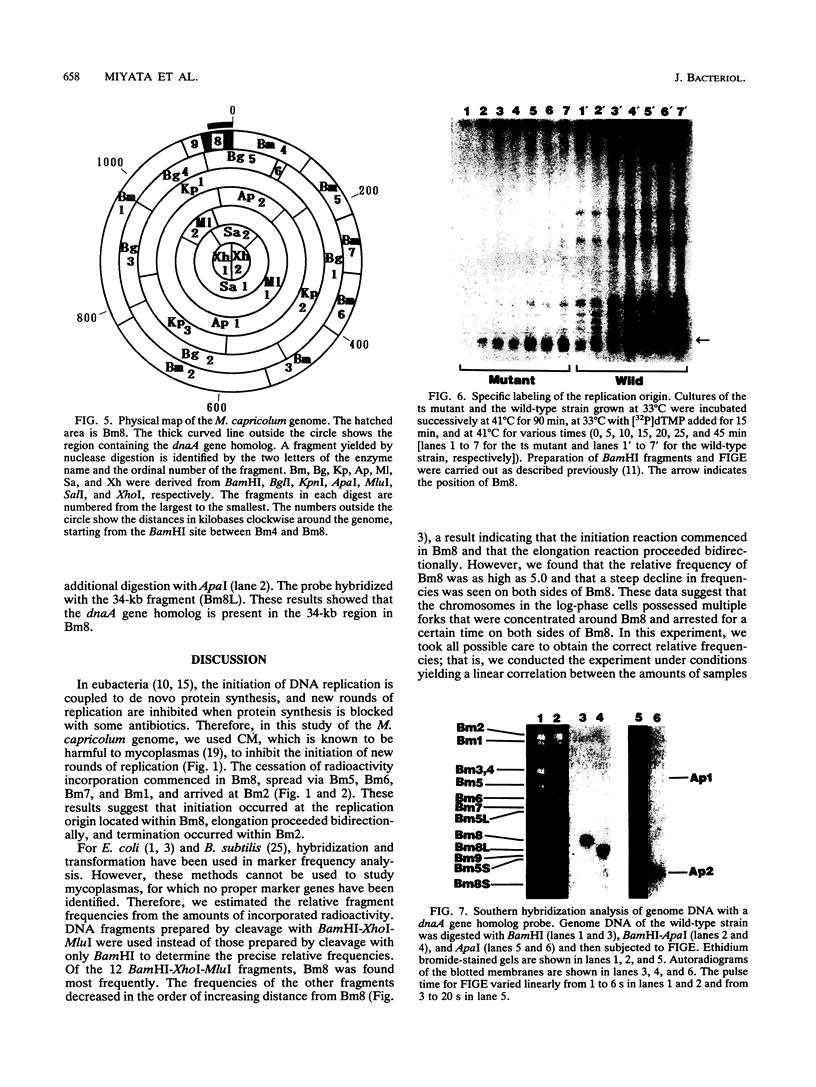
Four lines of evidence argue that the replication origin of the Mycoplasma capricolum genome lies within the 46-kb BamHI fragment bordered by two BamHI sites of the total of nine BamHI sites that have been located on the physical map (M. Miyata, L. Wang, and T. Fukumura, FEMS Microbiol. Lett. 79:329-334, 1991). First, this fragment lost its labeling in preference to other fragments when log-phase cultures were incubated in the presence of chloramphenicol for various times to inhibit the initiation of new rounds of replication and then further incubated with radioactive dTMP to allow DNA elongation to continue. Second, the relative frequencies of various restriction fragments of the genome DNA from exponentially growing cells decreased with increasing distance from the putative origin. Third, preferential labeling occurred when radioactive dTMP was added to cultures of a DNA elongation-defective, temperature-sensitive mutant with a simultaneous temperature downshift. Fourth, the M. capricolum homolog of the dnaA gene, which is located near the replication origin in many other bacteria, was found in the 46-kb fragment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONHOEFFER F., SCHALLER H. A METHOD FOR SELECTIVE ENRICHMENT OF MUTANTS BASED ON THE HIGH UV SENSITIVITY OF DNA CONTAINING 5-BROMOURACIL. Biochem Biophys Res Commun. 1965 Jun 18;20:93–97. [PubMed] [Google Scholar]
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- Dingwall A., Shapiro L. Rate, origin, and bidirectionality of Caulobacter chromosome replication as determined by pulsed-field gel electrophoresis. Proc Natl Acad Sci U S A. 1989 Jan;86(1):119–123. doi: 10.1073/pnas.86.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K. Mycoplasmal genetics. Annu Rev Microbiol. 1990;44:81–104. doi: 10.1146/annurev.mi.44.100190.000501. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fujita M. Q., Yoshikawa H., Ogasawara N. Structure of the dnaA region of Micrococcus luteus: conservation and variations among eubacteria. Gene. 1990 Sep 1;93(1):73–78. doi: 10.1016/0378-1119(90)90138-h. [DOI] [PubMed] [Google Scholar]
- Kohiyama M. DNA synthesis in temperature sensitive mutants of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:317–324. doi: 10.1101/sqb.1968.033.01.036. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Miyata M., Wang L., Fukumura T. Physical mapping of the Mycoplasma capricolum genome. FEMS Microbiol Lett. 1991 Apr 15;63(2-3):329–333. doi: 10.1016/0378-1097(91)90107-l. [DOI] [PubMed] [Google Scholar]
- Muto A. The genome structure of Mycoplasma capricolum. Isr J Med Sci. 1987 May;23(5):334–341. [PubMed] [Google Scholar]
- Neale G. A., Mitchell A., Finch L. R. Pathways of pyrimidine deoxyribonucleotide biosynthesis in Mycoplasma mycoides subsp. mycoides. J Bacteriol. 1983 Apr;154(1):17–22. doi: 10.1128/jb.154.1.17-22.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki M., Smith C. L. Tracking bacterial DNA replication forks in vivo by pulsed field gel electrophoresis. Nucleic Acids Res. 1989 May 11;17(9):3479–3490. doi: 10.1093/nar/17.9.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle L. E., Corcoran L. N., Cocks B. G., Bergemann A. D., Whitley J. C., Finch L. R. Pulsed-field electrophoresis indicates larger-than-expected sizes for mycoplasma genomes. Nucleic Acids Res. 1988 Jul 11;16(13):6015–6025. doi: 10.1093/nar/16.13.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle L. E., Finch L. R. A physical map of the genome of Mycoplasma mycoides subspecies mycoides Y with some functional loci. Nucleic Acids Res. 1988 Jul 11;16(13):6027–6039. doi: 10.1093/nar/16.13.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Molecular biology and genetics of mycoplasmas (Mollicutes). Microbiol Rev. 1985 Dec;49(4):419–455. doi: 10.1128/mr.49.4.419-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Yoshikawa H. The chromosome of Bacillus subtilis. I. Theory of marker frequency analysis. Genetics. 1965 Oct;52(4):747–757. doi: 10.1093/genetics/52.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Massy B., Patte J., Louarn J. M., Bouché J. P. oriX: a new replication origin in E. coli. Cell. 1984 Jan;36(1):221–227. doi: 10.1016/0092-8674(84)90092-8. [DOI] [PubMed] [Google Scholar]