Abstract
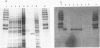
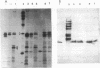
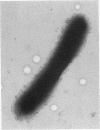
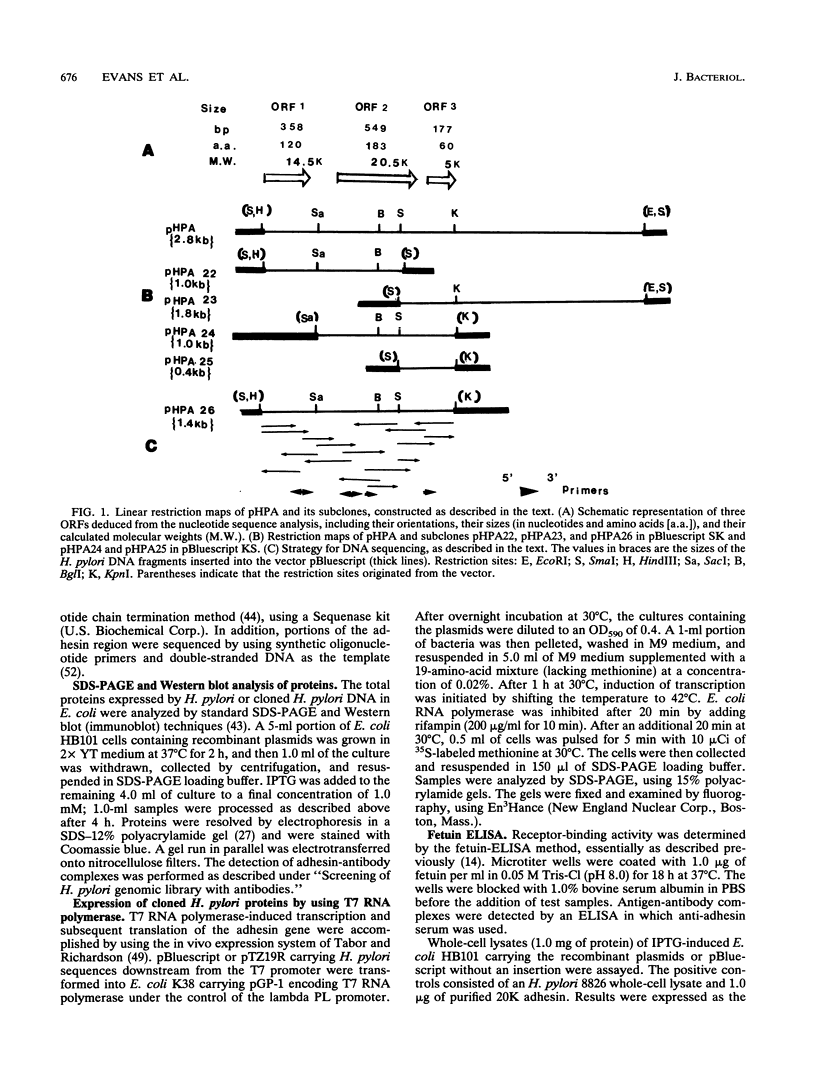
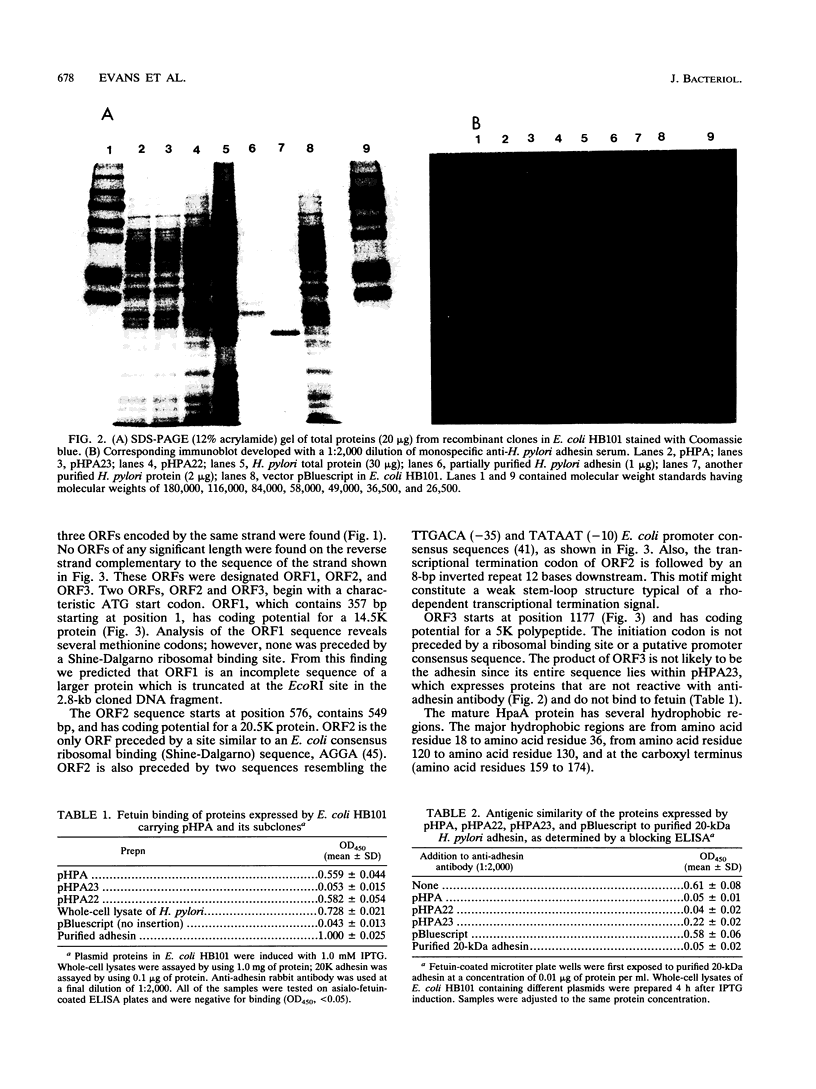
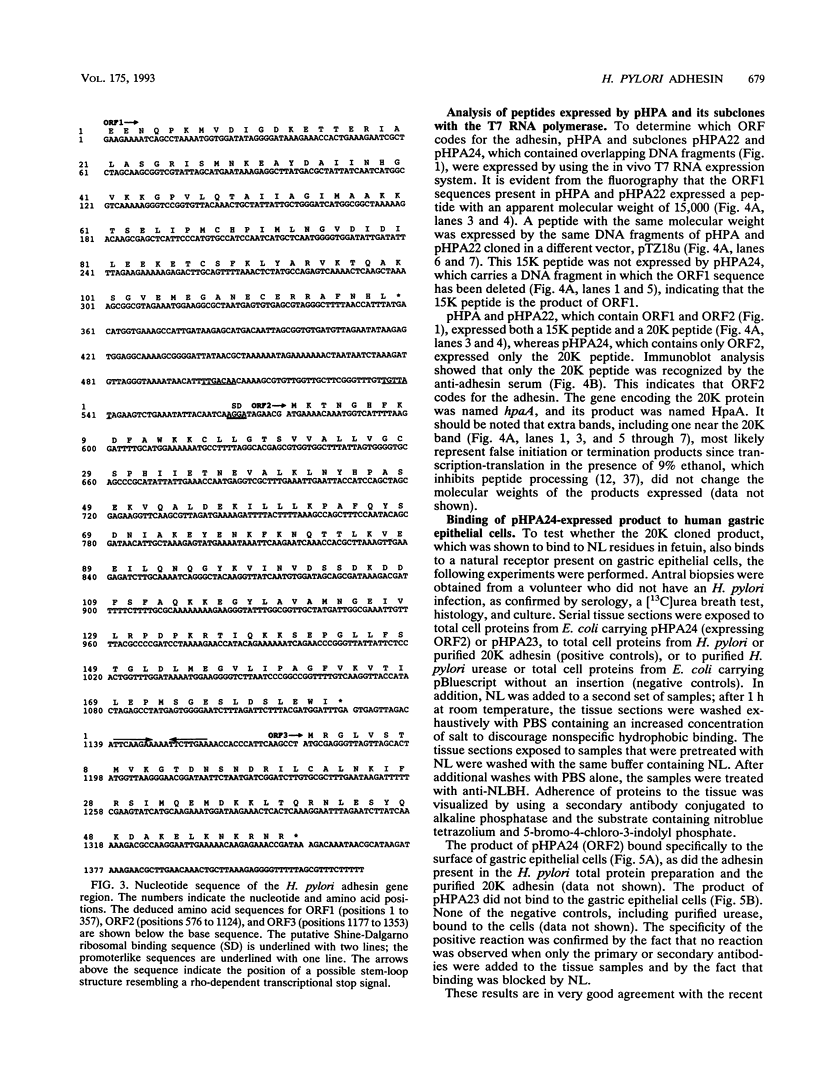
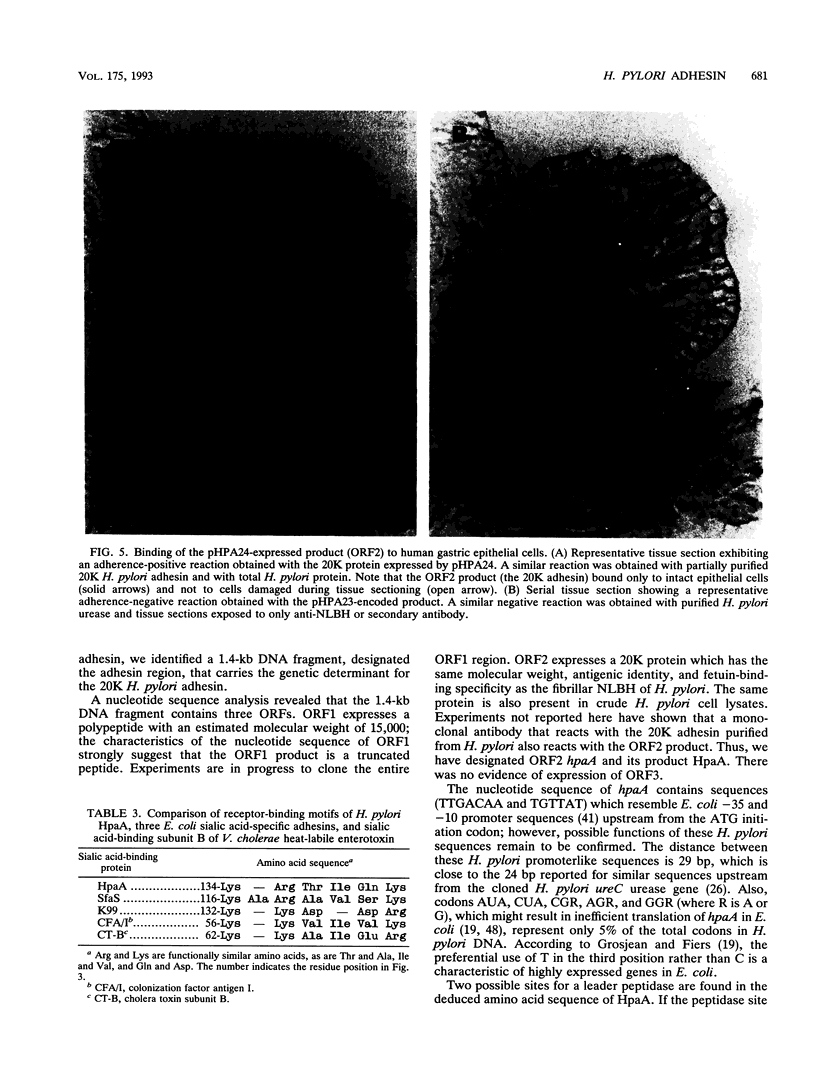
Gene hpaA, which codes for the receptor-binding subunit of the N-acetylneuraminyllactose-binding fibrillar hemagglutinin (NLBH) of Helicobacter pylori, was cloned and sequenced. The protein expressed by hpaA, designated HpaA, was identified as the adhesin subunit on the basis of its fetuin-binding activity and its reactivity with a polyclonal, monospecific rabbit serum prepared against NLBH purified from H. pylori. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis and Western blots (immunoblots) showed that the cloned adhesin has the same molecular weight (20,000) as that found on H. pylori. Also, HpaA contains a short sequence of amino acids (KRTIQK) which are all either identical or functionally similar to those which compose the sialic acid-binding motif of Escherichia coli SfaS, K99, and CFA/I. Affinity-purified antibody specific for a 12-residue synthetic peptide that included this sequence blocked the hemagglutinating activity of H. pylori and was shown by immuno-gold electron microscopy to react with almost transparent material on unstained H. pylori cells, which is consistent with previous observations concerning the location and morphology of the NLBH.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen L. P., Holck S., Elsborg L., Justesen T. The Helicobacter (Campylobacter) pylori-colonized duodenal mucosa and gastric metaplasia. APMIS. 1991 Mar;99(3):244–248. doi: 10.1111/j.1699-0463.1991.tb05145.x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J. Epidemiology and pathophysiology of Campylobacter pylori infections. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 1):S99–106. doi: 10.1093/clinids/12.supplement_1.s99. [DOI] [PubMed] [Google Scholar]
- Bode G., Malfertheiner P., Ditschuneit H. Pathogenetic implications of ultrastructural findings in Campylobacter pylori related gastroduodenal disease. Scand J Gastroenterol Suppl. 1988;142:25–39. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Båga M., Normark S., Hardy J., O'Hanley P., Lark D., Olsson O., Schoolnik G., Falkow S. Nucleotide sequence of the papA gene encoding the Pap pilus subunit of human uropathogenic Escherichia coli. J Bacteriol. 1984 Jan;157(1):330–333. doi: 10.1128/jb.157.1.330-333.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P., Fox J., Fontham E., Ruiz B., Lin Y. P., Zavala D., Taylor N., Mackinley D., de Lima E., Portilla H. Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer. 1990 Dec 15;66(12):2569–2574. doi: 10.1002/1097-0142(19901215)66:12<2569::aid-cncr2820661220>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Graham D. Y. Receptor-mediated adherence of Campylobacter pylori to mouse Y-1 adrenal cell monolayers. Infect Immun. 1989 Aug;57(8):2272–2278. doi: 10.1128/iai.57.8.2272-2278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Moulds J. J., Graham D. Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988 Nov;56(11):2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Smith K. E., Graham D. Y. Serum antibody responses to the N-acetylneuraminyllactose-binding hemagglutinin of Campylobacter pylori. Infect Immun. 1989 Mar;57(3):664–667. doi: 10.1128/iai.57.3.664-667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Burks M. F., Zupan A., Dallas W. S., Jacob C. O., Ludwig D. S. Epitopes of the cholera family of enterotoxins. Rev Infect Dis. 1987 May-Jun;9(3):544–561. doi: 10.1093/clinids/9.3.544. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Klein P. D., Evans D. J., Jr, Evans D. G., Alpert L. C., Opekun A. R., Boutton T. W. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet. 1987 May 23;1(8543):1174–1177. doi: 10.1016/s0140-6736(87)92145-3. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Hazell S. L., Lee A., Brady L., Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986 Apr;153(4):658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- Hobbs M., Dalrymple B. P., Cox P. T., Livingstone S. P., Delaney S. F., Mattick J. S. Organization of the fimbrial gene region of Bacteroides nodosus: class I and class II strains. Mol Microbiol. 1991 Mar;5(3):543–560. doi: 10.1111/j.1365-2958.1991.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Hui W. M., Ho J., Lam S. K. Pathogenetic role of Helicobacter pylori in duodenal ulcer disease. Multivariate analysis of factors affecting relapse. Dig Dis Sci. 1991 Apr;36(4):424–430. doi: 10.1007/BF01298869. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., Simons B. H., de Graaf F. K. The role of lysine-132 and arginine-136 in the receptor-binding domain of the K99 fibrillar subunit. EMBO J. 1987 Jun;6(6):1805–1808. doi: 10.1002/j.1460-2075.1987.tb02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., van den Berg P. A., Bak H. J., de Graaf F. K. Localization of lysine residues in the binding domain of the K99 fibrillar subunit of enterotoxigenic Escherichia coli. Biochim Biophys Acta. 1986 Jul 25;872(1-2):92–97. doi: 10.1016/0167-4838(86)90151-2. [DOI] [PubMed] [Google Scholar]
- Johan G., Offerhaus A., Molyvas E. N., Hoedemaeker P. J. Helicobacter pylori infection of gastric mucin cell metaplasia: the duodenum revisited. J Pathol. 1990 Nov;162(3):239–243. doi: 10.1002/path.1711620310. [DOI] [PubMed] [Google Scholar]
- Karjalainen T. K., Evans D. G., So M., Lee C. H. Molecular cloning and nucleotide sequence of the colonization factor antigen I gene of Escherichia coli. Infect Immun. 1989 Apr;57(4):1126–1130. doi: 10.1128/iai.57.4.1126-1130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne A., Cussac V., Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991 Mar;173(6):1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lingwood C. A., Law H., Pellizzari A., Sherman P., Drumm B. Gastric glycerolipid as a receptor for Campylobacter pylori. Lancet. 1989 Jul 29;2(8657):238–241. doi: 10.1016/s0140-6736(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Loffeld R. J., Willems I., Flendrig J. A., Arends J. W. Helicobacter pylori and gastric carcinoma. Histopathology. 1990 Dec;17(6):537–541. doi: 10.1111/j.1365-2559.1990.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Ludwig D. S., Holmes R. K., Schoolnik G. K. Chemical and immunochemical studies on the receptor binding domain of cholera toxin B subunit. J Biol Chem. 1985 Oct 15;260(23):12528–12534. [PubMed] [Google Scholar]
- Majewski S. I., Goodwin C. S. Restriction endonuclease analysis of the genome of Campylobacter pylori with a rapid extraction method: evidence for considerable genomic variation. J Infect Dis. 1988 Mar;157(3):465–471. doi: 10.1093/infdis/157.3.465. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P., Bode G., Stanescu A., Ditschuneit H. Gastric metaplasia and Campylobacter pylori in duodenal ulcer disease: an ultrastructural analysis. Gastroenterol Clin Biol. 1989;13(1 Pt 1):71B–74B. [PubMed] [Google Scholar]
- Marshall B. J. Campylobacter pylori: its link to gastritis and peptic ulcer disease. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 1):S87–S93. doi: 10.1093/clinids/12.supplement_1.s87. [DOI] [PubMed] [Google Scholar]
- Morris A., Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987 Mar;82(3):192–199. [PubMed] [Google Scholar]
- Morschhäuser J., Hoschützky H., Jann K., Hacker J. Functional analysis of the sialic acid-binding adhesin SfaS of pathogenic Escherichia coli by site-specific mutagenesis. Infect Immun. 1990 Jul;58(7):2133–2138. doi: 10.1128/iai.58.7.2133-2138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Hirst T. R., Hardy S. J., Holmgren J., Randall L. Synthesis of a precursor to the B subunit of heat-labile enterotoxin in Escherichia coli. J Bacteriol. 1981 Apr;146(1):325–330. doi: 10.1128/jb.146.1.325-330.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Rogers G. N., Korhonen T., Dahr W., Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986 Oct;54(1):37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez G. I., Taylor D. N., Bodhidatta L., Wongsrichanalai J., Baze W. B., Dunn B. E., Echeverria P. D., Blaser M. J. Seroprevalence of Helicobacter pylori infections in Thailand. J Infect Dis. 1990 Jun;161(6):1237–1241. doi: 10.1093/infdis/161.6.1237. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Natomi H., Zhao W. L., Okuzumi K., Sugano K., Iwamori M., Nagai Y. Identification of glycolipid receptors for Helicobacter pylori by TLC-immunostaining. FEBS Lett. 1991 May 6;282(2):385–387. doi: 10.1016/0014-5793(91)80519-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany B. L., Piotrowski J., Samanta A., VanHorn K., Murty V. L., Slomiany A. Campylobacter pylori colonization factor shows specificity for lactosylceramide sulfate and GM3 ganglioside. Biochem Int. 1989 Oct;19(4):929–936. [PubMed] [Google Scholar]
- Sørensen M. A., Kurland C. G., Pedersen S. Codon usage determines translation rate in Escherichia coli. J Mol Biol. 1989 May 20;207(2):365–377. doi: 10.1016/0022-2836(89)90260-x. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta M., Ishikawa H., Iishi H., Okuda S., Yokota Y. Reduction of gastric ulcer recurrence after suppression of Helicobacter pylori by cefixime. Gut. 1990 Sep;31(9):973–976. doi: 10.1136/gut.31.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zhang H., Scholl R., Browse J., Somerville C. Double stranded DNA sequencing as a choice for DNA sequencing. Nucleic Acids Res. 1988 Feb 11;16(3):1220–1220. doi: 10.1093/nar/16.3.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Krenn B. E., Klaasen P. Organization and expression of genes involved in the biosynthesis of K99 fimbriae. Infect Immun. 1984 Feb;43(2):508–514. doi: 10.1128/iai.43.2.508-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]