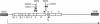Abstract
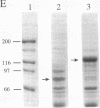
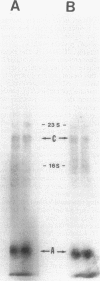
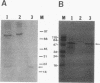
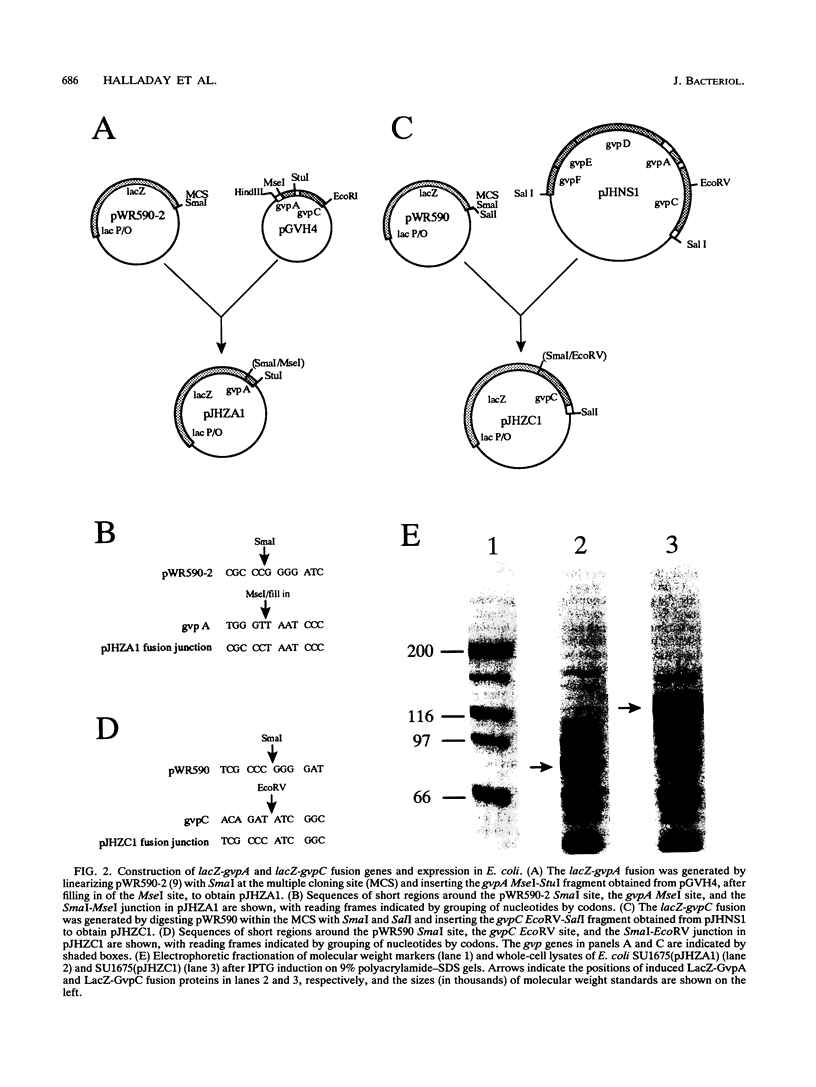
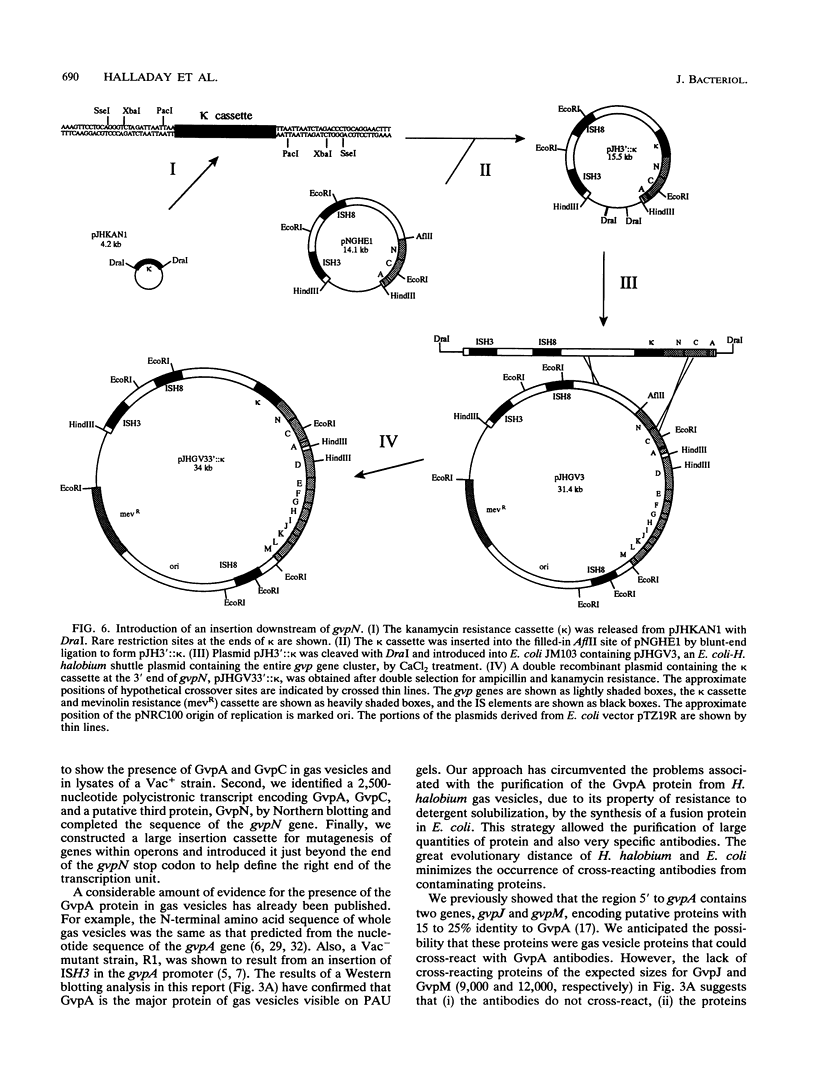
The extreme halophile Halobacterium halobium synthesizes intracellular gas-filled vesicles that confer buoyancy. A cluster of 13 genes on the 200-kb endogenous plasmid pNRC100 has been implicated in the biosynthesis of gas vesicles. Here, we show that two gas vesicle proteins are encoded by genes in the rightward operon, gvpA and gvpC, by Western blotting (immunoblotting) analysis with antibodies directed against LacZ-GvpA and LacZ-GvpC fusion proteins. Our results are consistent with previous data showing that the gvpA gene product is the major gas vesicle protein and demonstrate for the first time that the gvpC gene product is also present in H. halobium gas vesicles. Northern (RNA) blotting analysis showed two RNA species, an abundant 0.35-kb transcript of gvpA and a minor 2.5-kb transcript of gvpAC, and a third gene 3' to gvpAC, named gvpN. The gvpN gene encodes a hypothetical acidic protein with a molecular weight of 39,000 and a nucleotide binding motif. We used a site-directed mutagenesis method involving double recombination in Escherichia coli to insert a kanamycin resistance cassette just beyond the stop codon of gvpN. Introduction of the mutated gene cluster into an H. halobium mutant with a deletion of the entire gas vesicle gene cluster resulted in gas vesicle-positive transformants; this result suggests that gvpN is the last gene of the rightward gas vesicle transcription unit. We discuss the design and utility of the kanamycin resistance cassette for the mutagenesis of other genes in large operons.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaseio U., Pfeifer F. Transformation of Halobacterium halobium: development of vectors and investigation of gas vesicle synthesis. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6772–6776. doi: 10.1073/pnas.87.17.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline S. W., Doolittle W. F. Efficient transfection of the archaebacterium Halobacterium halobium. J Bacteriol. 1987 Mar;169(3):1341–1344. doi: 10.1128/jb.169.3.1341-1344.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R., Pfennig N. Comparative study of the structure of gas vacuoles. J Bacteriol. 1969 Nov;100(2):1049–1061. doi: 10.1128/jb.100.2.1049-1061.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerval T., Houmard J., Guglielmi G., Csiszar K., Tandeau de Marsac N. A developmentally regulated gvpABC operon is involved in the formation of gas vesicles in the cyanobacterium Calothrix 7601. Gene. 1987;54(1):83–92. doi: 10.1016/0378-1119(87)90350-7. [DOI] [PubMed] [Google Scholar]
- DasSarma S., Damerval T., Jones J. G., Tandeau de Marsac N. A plasmid-encoded gas vesicle protein gene in a halophilic archaebacterium. Mol Microbiol. 1987 Nov;1(3):365–370. doi: 10.1111/j.1365-2958.1987.tb01943.x. [DOI] [PubMed] [Google Scholar]
- DasSarma S. Mechanisms of genetic variability in Halobacterium halobium: the purple membrane and gas vesicle mutations. Can J Microbiol. 1989 Jan;35(1):65–72. doi: 10.1139/m89-010. [DOI] [PubMed] [Google Scholar]
- Dassarma S., Halladay J. T., Jones J. G., Donovan J. W., Giannasca P. J., de Marsac N. T. High-frequency mutations in a plasmid-encoded gas vesicle gene in Halobacterium halobium. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6861–6865. doi: 10.1073/pnas.85.18.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L. H., Stepień P. P., Tso J. Y., Brousseau R., Narang S., Thomas D. Y., Wu R. Synthesis of human insulin gene. VIII. Construction of expression vectors for fused proinsulin production in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):251–254. doi: 10.1016/0378-1119(84)90186-0. [DOI] [PubMed] [Google Scholar]
- Halladay J. T., Ng W. L., DasSarma S. Genetic transformation of a halophilic archaebacterium with a gas vesicle gene cluster restores its ability to float. Gene. 1992 Sep 21;119(1):131–136. doi: 10.1016/0378-1119(92)90078-4. [DOI] [PubMed] [Google Scholar]
- Hayes P. K., Buchholz B., Walsby A. E. Gas vesicles are strengthened by the outer-surface protein, GvpC. Arch Microbiol. 1992;157(3):229–234. doi: 10.1007/BF00245155. [DOI] [PubMed] [Google Scholar]
- Hayes P. K., Lazarus C. M., Bees A., Walker J. E., Walsby A. E. The protein encoded by gvpC is a minor component of gas vesicles isolated from the cyanobacteria Anabaena flos-aquae and Microcystis sp. Mol Microbiol. 1988 Sep;2(5):545–552. doi: 10.1111/j.1365-2958.1988.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Hayes P. K., Walsby A. E., Walker J. E. Complete amino acid sequence of cyanobacterial gas-vesicle protein indicates a 70-residue molecule that corresponds in size to the crystallographic unit cell. Biochem J. 1986 May 15;236(1):31–36. doi: 10.1042/bj2360031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne M., Englert C., Wimmer C., Pfeifer F. A DNA region of 9 kbp contains all genes necessary for gas vesicle synthesis in halophilic archaebacteria. Mol Microbiol. 1991 May;5(5):1159–1174. doi: 10.1111/j.1365-2958.1991.tb01889.x. [DOI] [PubMed] [Google Scholar]
- Jones J. G., Hackett N. R., Halladay J. T., Scothorn D. J., Yang C. F., Ng W. L., DasSarma S. Analysis of insertion mutants reveals two new genes in the pNRC100 gas vesicle gene cluster of Halobacterium halobium. Nucleic Acids Res. 1989 Oct 11;17(19):7785–7793. doi: 10.1093/nar/17.19.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. G., Young D. C., DasSarma S. Structure and organization of the gas vesicle gene cluster on the Halobacterium halobium plasmid pNRC100. Gene. 1991 Jun 15;102(1):117–122. doi: 10.1016/0378-1119(91)90549-q. [DOI] [PubMed] [Google Scholar]
- Konopka A. E., Staley J. T., Lara J. C. Gas vesicle assembly in Microcyclus aquaticus. J Bacteriol. 1975 Jun;122(3):1301–1309. doi: 10.1128/jb.122.3.1301-1309.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz M. J., Ballou C. E. Analysis of Halobacterium halobium gas vesicles. J Bacteriol. 1973 Jun;114(3):1058–1067. doi: 10.1128/jb.114.3.1058-1067.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. L., Kothakota S., DasSarma S. Structure of the gas vesicle plasmid in Halobacterium halobium: inversion isomers, inverted repeats, and insertion sequences. J Bacteriol. 1991 Mar;173(6):1958–1964. doi: 10.1128/jb.173.6.1958-1964.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C. W., Kapp J. A., Benacerraf B. Regulation by the H-2 gene complex of macrophage-lymphoid cell interactions in secondary antibody responses in vitro. J Exp Med. 1976 Aug 1;144(2):371–381. doi: 10.1084/jem.144.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surek B., Pillay B., Rdest U., Beyreuther K., Goebel W. Evidence for two different gas vesicle proteins and genes in Halobacterium halobium. J Bacteriol. 1988 Apr;170(4):1746–1751. doi: 10.1128/jb.170.4.1746-1751.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac N., Mazel D., Bryant D. A., Houmard J. Molecular cloning and nucleotide sequence of a developmentally regulated gene from the cyanobacterium Calothrix PCC 7601: a gas vesicle protein gene. Nucleic Acids Res. 1985 Oct 25;13(20):7223–7236. doi: 10.1093/nar/13.20.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toeckenius W., Kunau W. H. Further characterization of particulate fractions from lysed cell envelopes of Halobacterium halobium and isolation of gas vacuole membranes. J Cell Biol. 1968 Aug;38(2):337–357. doi: 10.1083/jcb.38.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Waaland J. R., Branton D. Gas vacuole development in a blue-green alga. Science. 1969 Mar 21;163(3873):1339–1341. doi: 10.1126/science.163.3873.1339. [DOI] [PubMed] [Google Scholar]
- Walsby A. E., Hayes P. K. Gas vesicle proteins. Biochem J. 1989 Dec 1;264(2):313–322. doi: 10.1042/bj2640313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]