Abstract
Antagonists of bombesin/gastrin-releasing peptide (BN/GRP) have been developed to inhibit the stimulatory effects of BN/GRP on the mitogenesis of tumor cells such as human small-cell lung carcinoma (SCLC). The mode of action of these antagonists is not completely understood. In this study, we evaluated the effect of BN/GRP antagonist RC-3095 on receptors for BN/GRP and epidermal growth factor (EGF) in H-128 human SCLC line xenografted into nude mice. Treatment with RC-3095, administered s.c. at a dose of 20 μg/day per animal for 4 weeks caused a 70% reduction in tumor volume and weight. Membrane receptors for BN/GRP and EGF were characterized in untreated and treated animals. In the control group, [125I-Tyr4]BN was bound to a single class of specific, high affinity binding sites with a dissociation constant (Kd) = 6.55 ± 0.93 nM and maximal binding capacity (Bmax) = 512.8 ± 34.8 fmol/mg membrane protein. Therapy with RC-3095 decreased the concentration of BN/GRP receptors on H-128 SCLC tumor membranes. Specific, high affinity binding sites for EGF with Kd = 1.78 ± 0.26 nM and Bmax = 216.8 ± 19.6 fmol/mg membrane protein were also found on the untreated H-128 SCLC tumors. Treatment with RC-3095 significantly decreased Bmax of receptors for EGF. Our results indicate that the suppression of growth of H-128 SCLC by BN antagonist RC-3095 is accompanied by a decrease in the number of receptors for both BN/GRP and EGF. These observations are in agreement with the results obtained in other experimental cancers. The findings on antagonist RC-3095 reinforce the view that both BN/GRP and EGF receptors participate in a cascade of events involved in the growth of SCLC and other cancers. Although the complete mechanisms of action of antagonist RC-3095 remain to be elucidated, the antitumor effect could be the result of the fall in the EGF receptor number, which might lead to a decrease in EGF receptor autophosphorylation.
Keywords: lung carcinoma, tumor regression, down-regulation of binding sites
Lung cancer is the leading cause of cancer-related deaths in the developed world. Small-cell lung carcinoma (SCLC) constitutes ≈25% of all lung carcinomas and follows a rapid and aggressive clinical course (1). Surgery, radiation, and chemotherapy are of limited effectiveness in the treatment of lung carcinomas, and new therapeutic strategies must be explored. The involvement of growth factors such as epidermal growth factor (EGF), insulin-like growth factor I and II (IGF I and II), and bombesin/gastrin-releasing peptide (BN/GRP)-like peptides in lung cancer biology has been extensively studied in the past decade (2–8). BN/GRP-like peptides have been shown to function as potent mitogens in vitro and are implicated as autocrine growth factors in the pathogenesis and progression of SCLCs (2, 3, 9, 10). Most SCLCs express mRNA for GRP (11, 12). BN-like peptides exert their function by binding to high affinity receptors on the surface of target cells. Four BN receptor subtypes have been characterized to date. They are the GRP-preferring subtype (11), the neuromedin B (NMB)-preferring subtype (13), the BRS-3 subtype (14), and the recently cloned and characterized fourth receptor subtype, BB4 (15). Because of the high homology between BN-like peptides, there is a potential for interaction between ligands and receptors.
The findings that BN and GRP can function as autocrine growth factors and promote the proliferation of various cancers expressing BN/GRP receptors suggest that their receptor antagonists might inhibit the tumor growth. Such analogs can occupy the specific receptors but are unable to activate signal transduction pathways. A series of short-chain BN (6–14) antagonists with a reduced peptide bond between positions 13 and 14 were synthesized in our laboratory (16) and were tested for inhibition of various tumors. Some of these antagonists inhibited tumor growth in different animal cancer models and in nude mice bearing xenografts of human cancer cell lines (9, 17–27). Thus, antagonists of BN/GRP, such as RC-3095, inhibited the in vivo growth of pancreatic, colorectal, and gastric cancers and other tumors, apparently by greatly reducing the maximal binding capacity of EGF receptors (9, 17–27). It is well established that a receptor/ligand complex can regulate another receptor, a phenomenon that is known as heterologous regulation. Such modulation of receptors results in an up-regulation or a down-regulation of the binding site.
In the present study, we investigated the presence and binding characteristics of receptors for BN/GRP and EGF in membranes of H-128 human SCLC. We also evaluated the effects of our synthetic BN/GRP antagonist RC-3095 on the growth of xenografts of the H-128 SCLC cell line in athymic nude mice and the changes in characteristics of membrane receptors for BN/GRP and EGF after treatment with this antagonist. It was hoped that the investigation of the correlation between tumor growth inhibition and receptor levels might provide some insight into cellular mechanisms of action of BN/GRP antagonists on SCLC.
MATERIALS AND METHODS
Chemicals.
GRP(14–27) and BN/GRP antagonist RC-3095 [D-Tpi6,13Ψ14,CH2-NH,Leu14]BN-(6–14) were synthesized by solid-phase methods and characterized in our laboratory (16). RC-3095 acetate (D-22213) made by Asta Medica (Frankfurt/Main, Germany) was used for chronic experiments. NMB and [Tyr4]BN were purchased from Bachem. Recombinant human EGF and radioisotope 125I-labeled sodium were purchased from R & D Systems and Amersham, respectively. All other chemicals, unless otherwise mentioned, were obtained from Sigma. For s.c. administration, RC-3095 was dissolved in 0.1% dimethyl sulfoxide in saline.
Animals.
Five- to 6-week-old male athymic (NCr nu/nu) nude mice were obtained from the National Cancer Institute (Bethesda, MD). The mice were housed in sterile cages under laminar flow hoods in a temperature-controlled room with a 12-hr light/12-hr dark schedule and were fed autoclaved chow and water ad libitum. Their care was in accord with institutional guidelines.
Cell Line.
The human SCLC cell line H-128 was obtained from the American Type Culture Collection. The cell line was maintained in RPMI 1640 medium, supplemented with 4 mM l-glutamine, 50 units of penicillin G sodium per ml, 50 μg of streptomycin sulfate per ml, 0.125 μg of amphotericin B per ml, and 20% fetal bovine serum at 37°C in a humidified 95% air/5% carbon dioxide atmosphere. Cells were passaged weekly and routinely monitored for mycoplasma contamination using a detection kit (Boehringer-Mannheim). All culture media components were purchased from GIBCO.
Experimental Protocol.
H-128 cells growing exponentially were implanted into male nude mice by s.c. injection of 1 × 107 cells into the right flank of five mice. Tumor xenografts resulting after 7 weeks were aseptically dissected and mechanically minced; 3 mm3 pieces of tumor tissue were transplanted s.c. by trocar needle into 30 mice under methoxyflurane (Metofane; Pitman–Moore, Mundelein, IL) anesthesia. Two weeks after transplantation, when tumors in most mice had grown to a volume of ≈12 mm3, the mice with tumors were randomized and divided into 2 experimental groups of 10 animals each. The control group was injected s.c. with saline only. Group 2 received RC-3095 acetate (D-22213) by daily s.c. injections at a dose of 20 μg/day per animal. The compounds were injected s.c. at a distance of ≈5 mm from the tumor. The treatment with BN/GRP antagonist RC-3095 lasted 4 weeks.
Tumors were measured weekly with microcalipers, and tumor volume was calculated as length × width × height × 0.5236 (9). At the end of the treatment period, treated animals and control mice were anesthetized with methoxyflurane and killed by dacapitation. Tumors were carefully removed, cleaned, weighed, and frozen on dry ice, then samples were stored at -70°C until the analyses of receptors.
BN and EGF Binding Studies in Tumor Membranes.
[Tyr4]BN was radiolabeled with 125I-labeled sodium using an Enzymobead iodination kit (Bio-Rad) (specific activity, 1700–2000 Ci/mmol; 1 Ci = 37 GBq) as described (28). 125I-labeled EGF (1200–1500 Ci/mmol) was purchased from Amersham. Preparation of tumor membranes was carried out as described (29). Receptor binding of BN and EGF were performed as reported (18, 29) using sensitive in vitro ligand competition assays based on binding of radiolabeled [Tyr4]BN and EGF to tumor membrane homogenates. Briefly, membrane homogenates containing 50–100 μg protein were incubated in duplicate or triplicate with 50–80,000 cpm radioligand and increasing concentrations (10−12–10−6 M) of nonradioactive peptides as competitors in a total volume of 150 μl assay buffer. At the end of incubations, the reactions were terminated by adding 250 μl ice-cold assay buffer to the tubes, and the bound ligand was separated from free ligand by centrifugation at 4500 × g for 10 min at 4°C. The pellet was washed twice with 500 μl ice-cold buffer, and the radioactivity in the pellet of each tube was counted in a gamma counter (Micromedic Systems, Huntsville, AL). Protein concentration was determined by the method of Bradford (30) using a Bio-Rad protein assay kit.
Mathematical Analysis of the Binding Data.
Specific ligand-binding capacities and affinities were calculated by the ligand-pc computerized curve-fitting program of Munson and Rodbard (31) as modified by McPherson (32). To determine the types of receptor binding, dissociation constants (Kd values), and the maximal binding capacity of receptors (Bmax), binding data for BN and EGF were also analyzed by the Scatchard method (33).
Statistical Method.
Statistical analyses of the experimental data on tumor growth and receptors were performed using Duncan’s new multiple range test (34).
RESULTS
Effect of RC-3095 on Growth of H-128 SCLC in Nude Mice.
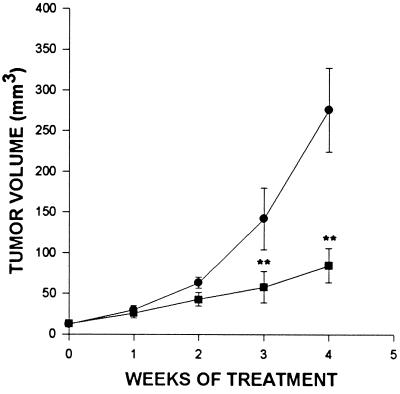
The effects of treatment with BN/GRP antagonist RC-3095 on body and tumor weights in nude mice implanted s.c. with H-128 SCLC as well as initial and final tumor volumes, are shown in Table 1. Tumor growth throughout the experiment is illustrated in Fig. 1. At the end of the experiment, there were no significant differences in body weights between groups. The growth of SCLC H-128 tumors in animals treated with RC-3095 was significantly (P < 0.01) inhibited within 21 days from the start of the experiment (Fig. 1). The mean tumor volume and weight were significantly (P < 0.01) reduced in animals receiving RC-3095 for 4 weeks to 84.6 ± 21.1 mm3 and 79 ± 51 mg, compared with those in the control group, which were 275.7 ± 51.4 mm3 and 290 ± 134 mg, respectively (Table 1).
Table 1.
Effect of treatment with BN/GRP antagonist RC-3095 on body and tumor weight and tumor volume in nude mice bearing xenografts of the human SCLC H-128 cell line
| Treatment | Tumor volume, mm3
|
Body weight, g | Tumor weight, mg | |
|---|---|---|---|---|
| Initial | Final | |||
| Control | 12.2 ± 0.8 | 275.7 ± 51.4 | 25.8 ± 2.1 | 290 ± 134 |
| RC-3095 | 12.9 ± 1.0 | 84.6 ± 21.1** | 24.5 ± 1.4 | 79 ± 51** |
, P < 0.01 vs. control.
Figure 1.
Tumor volumes in nude mice bearing H-128 human SCLC (•, control) during treatment with BN/GRP antagonist RC-3095 (▪), administered s.c. at a dose of 20 μg/day per animal. Vertical bars represent SE. ∗∗, P < 0.01 vs. control.
Membrane Receptors for BN/GRP.
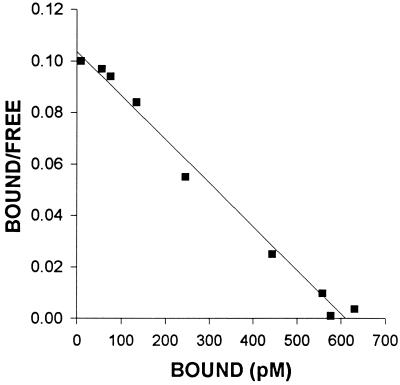
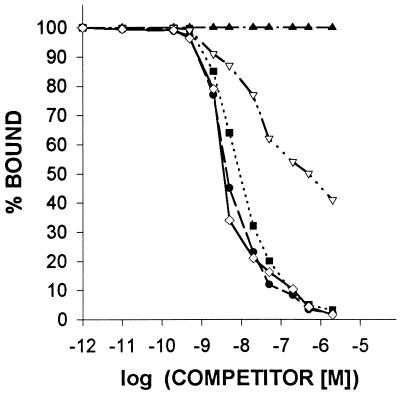
The characteristics of binding of [125I-Tyr4]BN to the membrane receptors on the H-128 SCLC cells were determined using ligand competition assays. The displacement of labeled [Tyr4]BN and the Scatchard analysis of these data (Fig. 2) indicated that in membranes of H-128 tumors, labeled peptide was bound to the single class of high affinity, low-capacity binding sites (Kd = 6.55 ± 0.93 nM; Bmax = 512.8 ± 34.8 fmol/mg membrane protein). To determine the specificity of the binding sites for BN/GRP in membranes of H-128 SCLC, several peptides structurally related or unrelated to BN/GRP were tested for their ability to inhibit [125I-Tyr4]BN binding. The binding of radiolabeled [Tyr4]BN was completely displaced by increasing concentrations (10−12–10−6) of [Tyr4]BN, GRP(14–27), and BN/GRP antagonist RC-3095, but none of the structurally or functionally unrelated peptides tested inhibited binding of labeled [Tyr4]BN at 10−6 M (Fig. 3). [Tyr4]BN, RC-3095, and GRP(14–27) exhibited an essentially equal high affinity for the receptor subtype, whereas NMB was less effective by more than two orders of magnitude in displacing [125I-Tyr4]BN binding.
Figure 2.
Representative example of Scatchard plots of [125I-Tyr4]BN binding to the membrane fraction isolated from untreated H-128 SCLC tumors. Specific binding was determined as described. Each point represents mean of triplicate determinations.
Figure 3.
Ligand-binding specificity of [Tyr4]BN binding. Competition for binding of radiolabeled [Tyr4]BN to membrane fractions of human SCLC H-128 tumors was determined in the presence of increasing concentrations of EGF (▴), human growth hormone-releasing hormone (▴), somatostatin (▴), [D-Trp6]-luteinizing hormone-releasing hormone (▴), NMB (▿), [Tyr4]BN (⋄), RC-3095 (•), and GRP(14–27) (▪). One hundred percent specific binding is defined as the difference between binding in the absence and in the presence of 10−5 M [Tyr4]BN. Each point represents the mean of at least three experiments, each performed in duplicate.
After treatment with BN/GRP antagonist RC-3095, the maximal binding capacity of high affinity receptors for [Tyr4]BN showed a significant decrease of about 55% compared with controls (P < 0.01) (Table 2). The treatment with RC-3095 did not significantly influence the affinity of the BN/GRP receptors (Table 2).
Table 2.
Effect of treatment with BN/GRP antagonist RC-3095 on binding of BN and EGF in membranes of SCLC H-128 tumors
| Treatment | BN
|
EGF
|
||
|---|---|---|---|---|
| Kd, nM | Bmax, fmol/mg protein | Kd, nM | Bmax, fmol/mg protein | |
| Control | 6.55 ± 0.93 | 512.8 ± 34.8 | 1.78 ± 0.26 | 216.8 ± 19.6 |
| RC-3095 | 6.91 ± 0.22 | 228.9 ± 22.9** | 0.86 ± 0.17* | 52.9 ± 2.08** |
Binding characteristics were obtained from 10-point displacement experiments. All values represent mean ± SE of 3-4 independent experiments, each done in duplicate or triplicate.
, P < 0.05 vs. control.
, P < 0.01 vs. control.
Membrane Receptors for EGF.
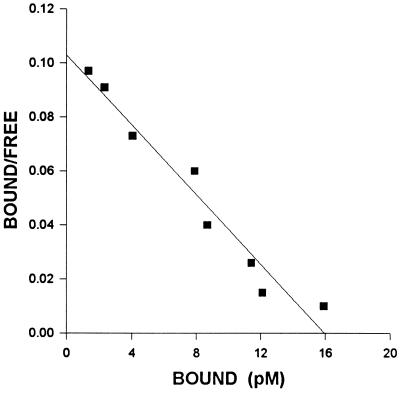
High affinity binding sites for EGF were also found in the membrane fractions of H-128 SCLC cells. The specific binding was reversible, and only one class of high affinity receptors was found (Fig. 4). In control tumors analyzed by complete displacement, these receptors exhibited a mean dissociation constant (Kd) of 1.78 ± 0.26 nM and a mean maximal binding capacity (Bmax) of 216.8 ± 19.6 fmol/mg membrane protein (Table 2). To demonstrate the specificity of EGF binding to membranes of H-128 cells, competitive inhibition studies were also performed (data not shown). Treatment with BN/GRP antagonist RC-3095 produced a down-regulation as shown by a significant reduction of ≈76% in the concentration of EGF binding sites. There was a parallel increase in binding affinity of EGF receptors compared with controls (Table 2).
Figure 4.
Representative example of Scatchard plots of [125I]EGF binding to the membrane fraction isolated from H-128 SCLC tumors after treatment with RC-3095. Specific binding was determined as described. Each point represents the mean of triplicate determinations.
DISCUSSION
The involvement of BN/GRP-like peptides in the pathogenesis of SCLC is well established (2, 3, 11). SCLC can secrete BN-like peptides including GRP and NMB, which function like autocrine growth factors and stimulate the growth of this malignancy (2, 11). The present study demonstrates that the potent BN/GRP antagonist RC-3095 significantly inhibits the growth of the human SCLC H-128 cell line xenografted into nude mice. This observation is in accord with our previous investigations in the H-69 SCLC line (9). A consequent aim of our study was to identify and characterize BN/GRP and EGF receptors on H-128 SCLC tumors, untreated and after chronic therapy with RC-3095, using specific radioligand binding assays. The findings presented here demonstrate that H-128 SCLC tumors grown in nude mice possess specific receptors for BN/GRP. The analyses of displacement studies indicate that in membranes of H-128 tumors, the radiolabeled [Tyr4]BN was bound to one class of binding sites with high affinity (Kd = 6.55 nM) and low binding capacity (Bmax = 512.8 fmol/mg membrane protein). These receptor proteins recognized BN/GRP peptides in a specific manner as ascertained by the evaluation of a variety of unlabeled compounds. Because the affinity of [Tyr4]BN, GRP(14–27), and RC-3095 was >100 times higher than that of neuromedin B, it was concluded that H-128 tumors contain primarily the BN/GRP receptor subtype, although many SCLCs express NMB receptors (11). Previous studies have shown that high affinity receptors for BN/GRP are present in several other SCLC cell lines (3, 9–11, 35, 36).
Receptors for EGF with high affinity (Kd=1.78 nM) and a mean Bmax of 216.8 fmol/mg membrane protein were also found in membrane fractions of H-128 tumors. The demonstration of high affinity binding sites for EGF in membranes of H-128 SCLC tumors is in agreement with findings previously reported by us and by other groups on the presence of receptors for EGF in various human lung cancer cell lines (7, 9, 37).
We observed that chronic treatment of nude mice with BN/GRP antagonist RC-3095 produced a significant down-regulation of binding sites for both BN/GRP and EGF in H-128 tumors. Previously we reported that BN/GRP antagonists, including RC-3095, induced a marked reduction of BN/GRP and EGF receptors in membranes of H-69 SCLC tumors (9). We have also shown that inhibition of growth of other experimental cancers, including mammary, pancreatic, prostatic, and colorectal tumors by antagonist RC-3095, was associated with a major decrease in EGF receptor levels in tumor membranes (17–27). Conversely, the absence of, or a major decrease in, BN/GRP receptors abolishes the inhibitory effect of BN/GRP antagonist on tumor growth and prevents the concomitant down-regulation of EGF receptors (22).
The molecular mechanism of action of BN and GRP antagonists is incompletely understood. After binding to the receptors, the peptides of the BN/GRP family initiate a complex cascade of intracellular signals mediated by second messengers. The activation of protein kinase C is one of the early cellular events that follow the interaction of BN-like peptides with the receptors (38, 39). Zachary et al. (38) reported that in 3T3 cells the activation of protein kinase C, caused by BN-like peptides, leads to a rapid transmodulation of EGF receptors with a decrease in EGF binding. These effects were selectively blocked by early BN antagonists such as analogs of substance P (SP) (38). Thus, SP analog (D-Arg1, D-Pro2, D-Trp7,9, Leu11)SP markedly inhibited both [125I-Tyr15]GRP binding and BN- and GRP-induced mitogenesis in 3T3 cells (38). Studies in various SCLC lines have demonstrated that some early events such as stimulation by GRP of mobilization of intracellular Ca2+ and inositol phosphate turnover are similar to those in murine 3T3 cells. Broad spectrum SP antagonists (38, 40) could block the effects of Ca2+-mobilizing neuropeptides (38, 40). Tallett et al. (41) showed that BN and other neuropeptides stimulated protein tyrosine phosphorylation and protein tyrosine kinase activity in intact SCLC H-345 cells. The broad spectrum neuropeptide receptor antagonist [D-Arg1, D-Phe5, D-Trp7,9, Leu11]SP inhibited all neuropeptide-mediated signals including protein tyrosine kinase activation and H-345 SCLC cell growth in vivo and in vitro.
Studies by other groups indicate that additional distinct mechanisms could be involved in the stimulatory action of BN/GRP and its blockade by the BN antagonists. In the HT-29 colon carcinoma cell line, BN stimulated cell proliferation, but it did not induce an increase in intracellular Ca2+ (42). Antagonist RC-3095 powerfully inhibited BN-stimulated proliferation of HT-29 cells (42). Liebow et al. (43) showed that in various cancers and cancer cell lines, BN enhanced the phosphorylation of the substrates phosphorylated by EGF receptors or amplified the tyrosine kinase response of the EGF receptor. BN/GRP antagonist RC-3095 inhibited the phosphorylation responses. Their results also suggest that BN/GRP peptides may function by up-regulating EGF receptors and that antagonist RC-3095 blocks this action and causes a down-regulation of EGF binding sites (43).
The EGF receptor, whose cytoplasmic domain bears an intrinsic tyrosine-specific protein kinase activity, has been implicated in the regulation of cellular proliferation and in the progression of a wide variety of human tumors (43–45). The binding of EGF to the extracellular domain of its receptor leads to autophosphorylation as well as increased phosphorylation of intracellular substrates (45) and stimulates the tyrosine kinase activity. The activation of tyrosine kinase of the EGF receptor produces mitogenic signals that result in cellular proliferation (44, 45).
Additional investigations are required to elucidate the mechanism of antitumor action of BN antagonists. However, the inhibitory action of BN/GRP antagonist RC-3095 on growth of H-128 SCLC tumors and other cancers (9, 17–27) was invariably associated with a down-regulation of EGF receptors. Consequently, it would not be unreasonable to hypothesize that the antitumor effect may be related to the reduction in EGF receptor concentration produced by the BN antagonist, because it has been previously shown that the decrease in EGF receptor autophosphorylation appears to be due to a fall in the EGF receptor number (46).
Acknowledgments
Some work described in this paper was supported by the Medical Research Service of the Veterans Affairs Department (to A.V.S.). We acknowledge the participation of Dr. Jacek Pinski in the oncological part of this work. We thank Dr. Charles Liebow for advice in the preparation of the manuscript and Dora Rigo and Katalin Halmos for excellent technical assistance.
Footnotes
Abbreviations: SCLC, small-cell lung cancer; EGF, epidermal growth factor; BN, bombesin; GRP, gastrin-releasing peptide; Bmax, maximal binding capacity; Kd, dissociation constant; SP, substance P; NMB, neuromedin B.
References
- 1.Smyth J F, Fowlie S M, Gregor A, Crompton G K, Busutill A, Leonard R C F, Grant I W B. Quart J Med. 1986;61:969–976. [PubMed] [Google Scholar]
- 2.Cuttitta F, Carney D N, Mulshine J, Moody T W, Fedorko J, Fischler A, Minna J D. Nature (London) 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 3.Moody T W, Cuttitta F. Life Sci. 1993;53:1161–1173. doi: 10.1016/0024-3205(93)90098-n. [DOI] [PubMed] [Google Scholar]
- 4.Veale S, Ashcroft T, March C, Gibson G J, Harris A L. Br J Cancer. 1987;55:513–516. doi: 10.1038/bjc.1987.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minuto F, Del Monte P, Baarraeca A, Alama A, Cariola G, Giordano G. Cancer Res. 1988;48:3716–3719. [PubMed] [Google Scholar]
- 6.Macaulay V M, Evereard M J, Teale D, Troutt P A, van Wyk J J, Smith I E, Millar J L. Cancer Res. 1990;50:2511–2517. [PubMed] [Google Scholar]
- 7.Damstrup L, Rygaarad K, Spang-Thomsen M, Poulson H S. Cancer Res. 1992;52:3089–3093. [PubMed] [Google Scholar]
- 8.Reeve J G, Morgan J, Schwander J, Bleehen N M. Cancer Res. 1993;53:4680–4685. [PubMed] [Google Scholar]
- 9.Pinski J, Schally A V, Halmos G, Szepeshazi K, Groot K, O’Byrne K, Cai R-Z. Br J Cancer. 1994;70:886–892. doi: 10.1038/bjc.1994.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moody T W, Carney D N, Cuttitta F, Quattrocchi K, Minna J D. Life Sci. 1985;37:105–113. doi: 10.1016/0024-3205(85)90413-8. [DOI] [PubMed] [Google Scholar]
- 11.Spindel E R, Giladi E, Segerson T P, Nagalla S. Recent Prog Horm Res. 1993;48:365–391. doi: 10.1016/b978-0-12-571148-7.50017-8. [DOI] [PubMed] [Google Scholar]
- 12.Sunday M E, Kaplan L M, Motoyama E, Chin W W, Spindel E R. Lab Invest. 1988;59:5–23. [PubMed] [Google Scholar]
- 13.Von Schrenck T, Heinz-Erian P, Moran T, Mantey S A, Gardner J D, Jenson R T. Am J Physiol. 1989;256:747–758. doi: 10.1152/ajpgi.1989.256.4.G747. [DOI] [PubMed] [Google Scholar]
- 14.Fathi Z, Corjay M H, Shapira J, Wada E, Benya R, Jensen R, Viallet J, Sausville E A, Battey J F. J Biol Chem. 1993;268:5979–5984. [PubMed] [Google Scholar]
- 15.Nagalla S R, Barry B J, Creswick K C, Eden P, Taylor J T, Spindel E R. Proc Natl Acad Sci USA. 1995;92:6205–6209. doi: 10.1073/pnas.92.13.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radulovic S, Cai R-Z, Serfozo P, Groot K, Redding T W, Pinski J, Schally A V. Int J Pept Protein Res. 1991;38:593–600. doi: 10.1111/j.1399-3011.1991.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 17.Radulovic S, Miller G, Schally A V. Cancer Res. 1991;51:6006–6009. [PubMed] [Google Scholar]
- 18.Szepeshazi K, Schally A V, Halmos G, Groot K, Radulovic S. J Natl Cancer Inst. 1992;84:1915–1922. doi: 10.1093/jnci/84.24.1915. [DOI] [PubMed] [Google Scholar]
- 19.Szepeshazi K, Schally A V, Groot K, Halmos G. Int J Cancer. 1993;54:282–289. doi: 10.1002/ijc.2910540220. [DOI] [PubMed] [Google Scholar]
- 20.Pinski J, Halmos G, Schally A V. Cancer Lett (Shannon, Irel) 1993;71:189–196. doi: 10.1016/0304-3835(93)90115-p. [DOI] [PubMed] [Google Scholar]
- 21.Pinski J, Halmos G, Yano T, Szepeshazi K, Qin Y, Ertl T, Schally A V. Int J Cancer. 1994;57:574–580. doi: 10.1002/ijc.2910570422. [DOI] [PubMed] [Google Scholar]
- 22.Pinski J, Reile H, Halmos G, Groot K, Schally A V. Cancer Res. 1994;54:169–174. [PubMed] [Google Scholar]
- 23.Pinski J, Schally A V, Halmos G, Szepeshazi K, Groot K. Cancer Res. 1994;54:5895–5901. [PubMed] [Google Scholar]
- 24.Qin Y, Ertl T, Cai R-Z, Halmos G, Schally A V. Cancer Res. 1994;54:1035–1041. [PubMed] [Google Scholar]
- 25.Qin Y, Halmos G, Cai R-Z, Szoke B, Ertl T, Schally A V. Cancer Res Clin Oncol. 1994;120:519–528. doi: 10.1007/BF01221028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radulovic S, Schally A V, Reile H, Halmos G, Szepeshazi K, Groot K, Milovanovic S, Miller G, Yano T. Acta Oncol. 1994;33:693–701. doi: 10.3109/02841869409121784. [DOI] [PubMed] [Google Scholar]
- 27.Shirahige Y, Cai R-Z, Szepeshazi K, Halmos G, Pinski J, Groot K, Schally A V. Biomed Pharmacother. 1994;48:465–472. doi: 10.1016/0753-3322(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 28.Halmos G, Pinski J, Szoke B, Schally A V. Cancer Lett (Shannon, Irel) 1994;85:111–118. doi: 10.1016/0304-3835(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 29.Halmos G, Wittliff J L, Schally A V. Cancer Res. 1995;55:280–287. [PubMed] [Google Scholar]
- 30.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Munson P J, Rodbard D A. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 32.McPherson G A. J Pharmacol Methods. 1985;14:213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- 33.Scatchard B. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- 34.Steel R G D, Torrie J. Principles and Procedures of Statistics. New York: McGraw–Hill; 1976. p. 113. [Google Scholar]
- 35.Mahmoud D, Staley Y, Taylor J, Bodgen A, Moreau J P, Coy D, Avis I, Cuttitta F, Mulshine J L, Moody T W. Cancer Res. 1991;51:1798–1802. [PubMed] [Google Scholar]
- 36.Thomas F, Arvelo F, Antoine E, Jacrot M, Poupon M R. Cancer Res. 1992;52:4872–4877. [PubMed] [Google Scholar]
- 37.Moody T W, Lee M, Kris R M, Bellot F, Bepler G, Oie H, Gazdar A. J Cell Biochem. 1990;43:139–147. doi: 10.1002/jcb.240430205. [DOI] [PubMed] [Google Scholar]
- 38.Zachary I, Sinnett-Smith J W, Rozengurt E. J Cell Biol. 1986;102:2111–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zachary I, Rozengurt E. Cancer Surv. 1985;4:729–765. [PubMed] [Google Scholar]
- 40.Sethi T, Langdon S, Smyth J, Rozengurt E. Cancer Res. 1992;52:2737s–2742s. [PubMed] [Google Scholar]
- 41.Tallett A, Chilvers E R, Hannah S, Dransfield I, Lawson M F, Haslett C, Sethi T. Cancer Res. 1996;56:4255–4263. [PubMed] [Google Scholar]
- 42.Casanueva F, Perez F R, Casabiell X, Camiña J, Cai R-Z, Schally A V. Proc Natl Acad Sci USA. 1996;93:1406–1411. doi: 10.1073/pnas.93.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liebow C, Crean D H, Lee M T, Kamer A R, Mang T S, Schally A V. Proc Natl Acad Sci USA. 1994;91:3804–3808. doi: 10.1073/pnas.91.9.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carpenter G, Cohen S. J Biol Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- 45.Gill G N, Bertics P J, Santon J B. Mol Cel Endocrinol. 1987;51:169–186. doi: 10.1016/0303-7207(87)90027-x. [DOI] [PubMed] [Google Scholar]
- 46.King I, Sartorelli A C. Cancer Res. 1989;49:5677–5681. [PubMed] [Google Scholar]