Abstract
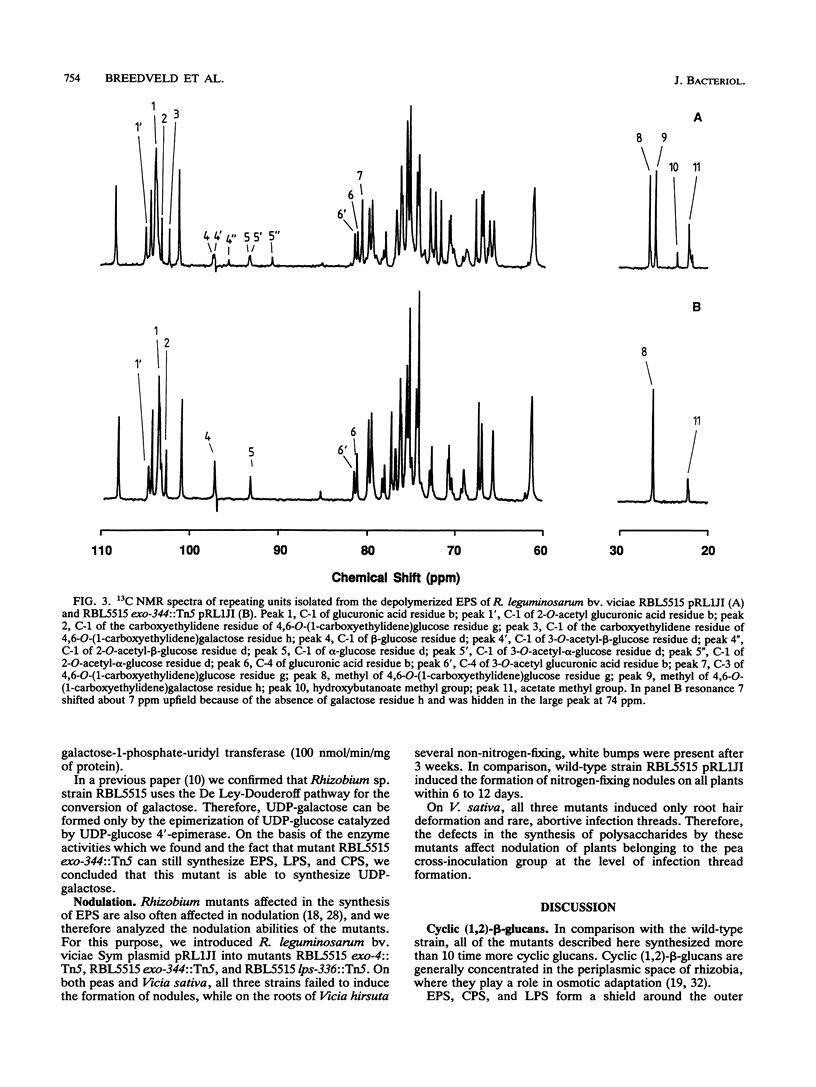
In this study, we characterized four Tn5 mutants derived from Rhizobium leguminosarum RBL5515 with respect to synthesis and secretion of cellulose fibrils, extracellular polysaccharides (EPS), capsular polysaccharides, and cyclic beta-(1,2)-glucans. One mutant, strain RBL5515 exo-344::Tn5, synthesizes residual amounts of EPS, the repeating unit of which lacks the terminal galactose molecule and the substituents attached to it. On basis of the polysaccharide production pattern of strain RBL5515 exo-344::Tn5, the structural features of the polysaccharides synthesized, and the results of an analysis of the enzyme activities involved, we hypothesize that this strain is affected in a galactose transferase involved in the synthesis of EPS only. All four mutants failed to nodulate plants belonging to the pea cross-inoculation group; on Vicia sativa they induced root hair deformation and rare abortive infection threads. All of the mutants appeared to be pleiotropic, since in addition to defects in the synthesis of EPS, lipopolysaccharide, and/or capsular polysaccharides significant increases in the synthesis and secretion of cyclic beta-(1,2)-glucans were observed. We concluded that it is impossible to correlate a defect in the synthesis of a particular polysaccharide with nodulation characteristics.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Borthakur D., Barker R. F., Latchford J. W., Rossen L., Johnston A. W. Analysis of pss genes of Rhizobium leguminosarum required for exopolysaccharide synthesis and nodulation of peas: their primary structure and their interaction with psi and other nodulation genes. Mol Gen Genet. 1988 Jul;213(1):155–162. doi: 10.1007/BF00333413. [DOI] [PubMed] [Google Scholar]
- Breedveld M. W., Zevenhuizen L. P., Zehnder A. J. Excessive excretion of cyclic beta-(1,2)-glucan by Rhizobium trifolii TA-1. Appl Environ Microbiol. 1990 Jul;56(7):2080–2086. doi: 10.1128/aem.56.7.2080-2086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedveld M. W., Zevenhuizen L. P., Zehnder A. J. Synthesis of cyclic beta-(1,2)-glucans by Rhizobium leguminosarum biovar trifolii TA-1: factors influencing excretion. J Bacteriol. 1992 Oct;174(20):6336–6342. doi: 10.1128/jb.174.20.6336-6342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter Cremers H. C., Batley M., Redmond J. W., Eydems L., Breedveld M. W., Zevehuizen L. P., Pees E., Wijffelman C. A., Lugtenberg B. J. Rhizobium leguminosarum exoB mutants are deficient in the synthesis of UDP-glucose 4'-epimerase. J Biol Chem. 1990 Dec 5;265(34):21122–21127. [PubMed] [Google Scholar]
- Canter Cremers H. C., Stevens K., Lugtenberg B. J., Wijffelman C. A., Batley M., Redmond J. W., Breedveld M. W., Zevenhuizen L. P. Unusual structure of the exopolysaccharide of Rhizobium leguminosarum bv. viciae strain 248. Carbohydr Res. 1991 Sep 30;218:185–200. doi: 10.1016/0008-6215(91)84097-x. [DOI] [PubMed] [Google Scholar]
- Canter Cremers H., Spaink H. P., Wijfjes A. H., Pees E., Wijffelman C. A., Okker R. J., Lugtenberg B. J. Additional nodulation genes on the Sym plasmid of Rhizobium leguminosarum biovar viciae. Plant Mol Biol. 1989 Aug;13(2):163–174. doi: 10.1007/BF00016135. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Kalembasa S., Turowski D., Pachori P., Noel K. D. Characterization of the lipopolysaccharide from a Rhizobium phaseoli mutant that is defective in infection thread development. J Bacteriol. 1987 Nov;169(11):4923–4928. doi: 10.1128/jb.169.11.4923-4928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty A. K., Zurkowski W., Shine J., Rolfe B. G. Symbiotic nitrogen fixation: molecular cloning of Rhizobium genes involved in exopolysaccharide synthesis and effective nodulation. J Mol Appl Genet. 1982;1(6):585–596. [PubMed] [Google Scholar]
- Diebold R., Noel K. D. Rhizobium leguminosarum exopolysaccharide mutants: biochemical and genetic analyses and symbiotic behavior on three hosts. J Bacteriol. 1989 Sep;171(9):4821–4830. doi: 10.1128/jb.171.9.4821-4830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic S. P., Chen H., Batley M., Redmond J. W., Rolfe B. G. Nitrogen fixation ability of exopolysaccharide synthesis mutants of Rhizobium sp. strain NGR234 and Rhizobium trifolii is restored by the addition of homologous exopolysaccharides. J Bacteriol. 1987 Jan;169(1):53–60. doi: 10.1128/jb.169.1.53-60.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Helinski D. R., Ditta G. S. Hypoosmotic adaptation in Rhizobium meliloti requires beta-(1----2)-glucan. J Bacteriol. 1990 Mar;172(3):1400–1408. doi: 10.1128/jb.172.3.1400-1408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Nagpal P., Helinski D. R., Ditta G. S. Symbiotic pseudorevertants of Rhizobium meliloti ndv mutants. J Bacteriol. 1990 Mar;172(3):1409–1417. doi: 10.1128/jb.172.3.1409-1417.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R. Immunity in Experimental Salmonellosis I. Protection Induced by Rough Mutants of Salmonella typhimurium. Infect Immun. 1970 Sep;2(3):309–315. doi: 10.1128/iai.2.3.309-315.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J., Walker G. C. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989 Feb 24;56(4):661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Henry R. J., Blakeney A. B., Stone B. A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr Res. 1984 Apr 2;127(1):59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Katsuki H., Yoshida T., Tanegashima C., Tanaka S. Improved direct method for determination of keto acids by 2,4-dinitrophenylhydrazine. Anal Biochem. 1971 Oct;43(2):349–356. doi: 10.1016/0003-2697(71)90263-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leigh J. A., Lee C. C. Characterization of polysaccharides of Rhizobium meliloti exo mutants that form ineffective nodules. J Bacteriol. 1988 Aug;170(8):3327–3332. doi: 10.1128/jb.170.8.3327-3332.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Reed J. W., Hanks J. F., Hirsch A. M., Walker G. C. Rhizobium meliloti mutants that fail to succinylate their calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell. 1987 Nov 20;51(4):579–587. doi: 10.1016/0092-8674(87)90127-9. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton J. D. The relationship between metabolite production and the growth efficiency of the producing organism. FEMS Microbiol Rev. 1990 Mar;6(1):1–18. doi: 10.1111/j.1574-6968.1990.tb04083.x. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Priefer U. B. Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J Bacteriol. 1989 Nov;171(11):6161–6168. doi: 10.1128/jb.171.11.6161-6168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevenhuizen L. P., Bertocchi C., van Neerven A. R. Congo red absorption and cellulose synthesis by Rhizobiaceae. Antonie Van Leeuwenhoek. 1986;52(5):381–386. doi: 10.1007/BF00393465. [DOI] [PubMed] [Google Scholar]
- de Maagd R., van Rossum C., Lugtenberg B. J. Recognition of individual strains of fast-growing rhizobia by using profiles of membrane proteins and lipopolysaccharides. J Bacteriol. 1988 Aug;170(8):3782–3785. doi: 10.1128/jb.170.8.3782-3785.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]