Abstract
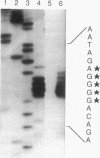
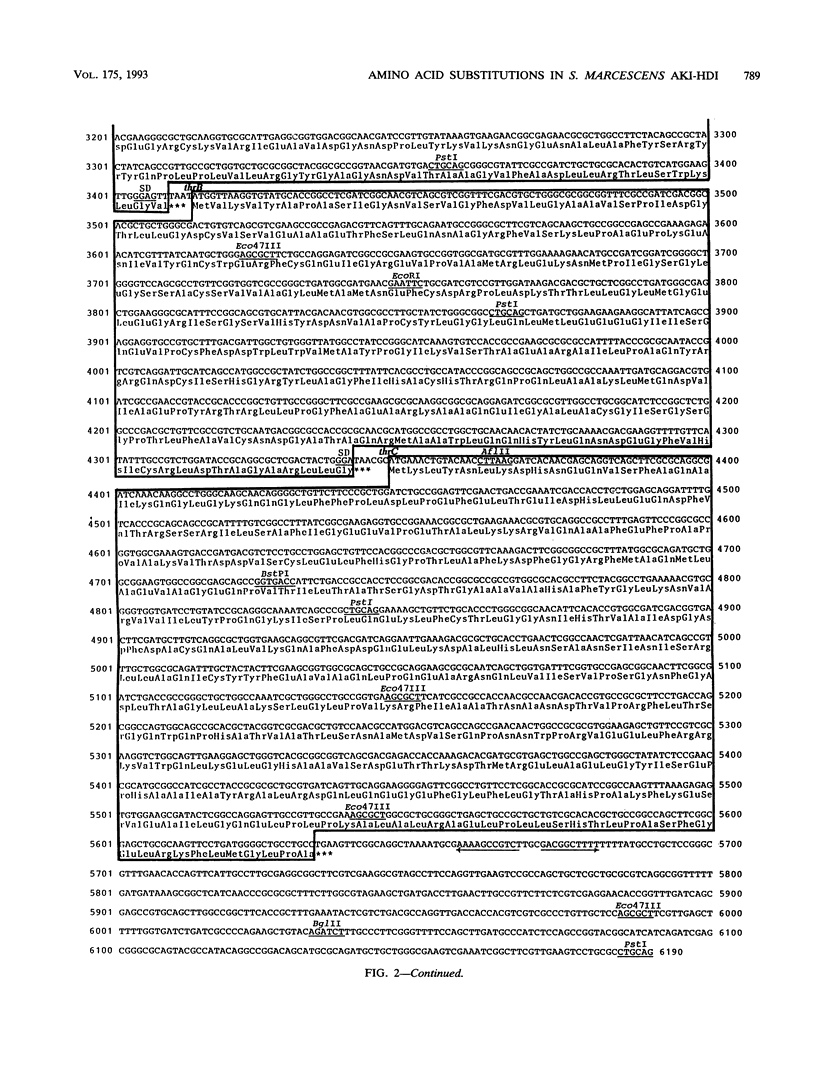
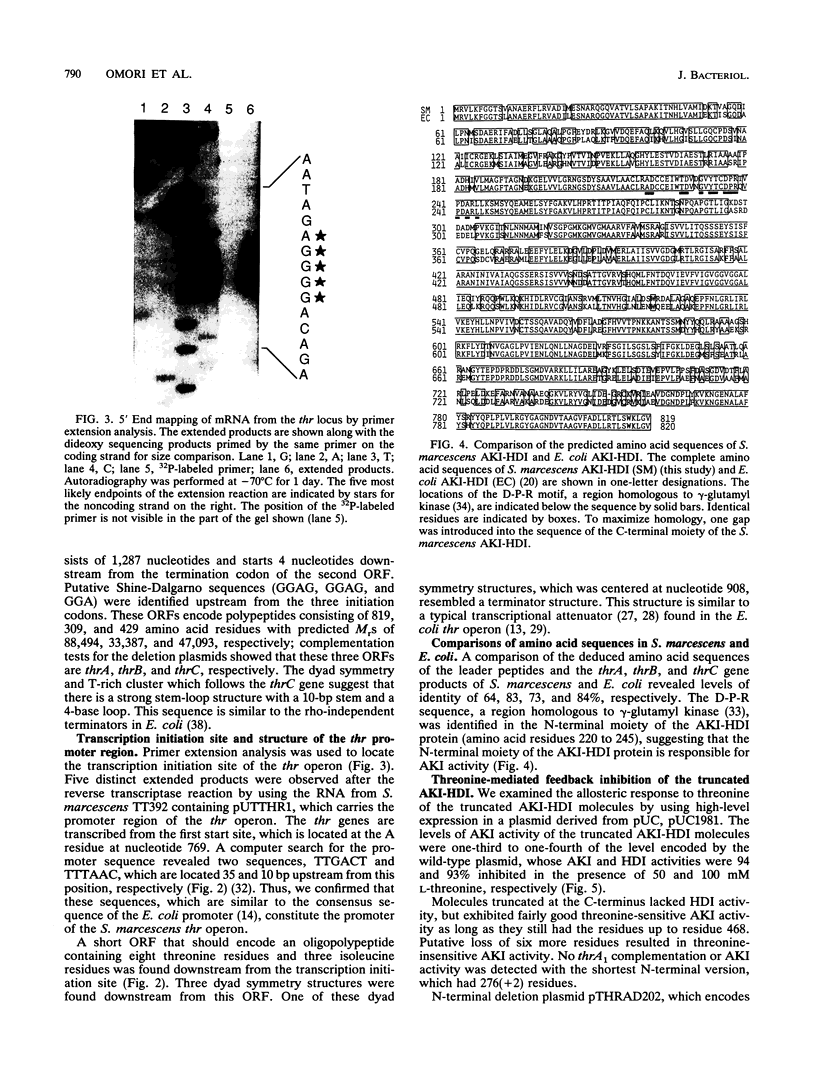
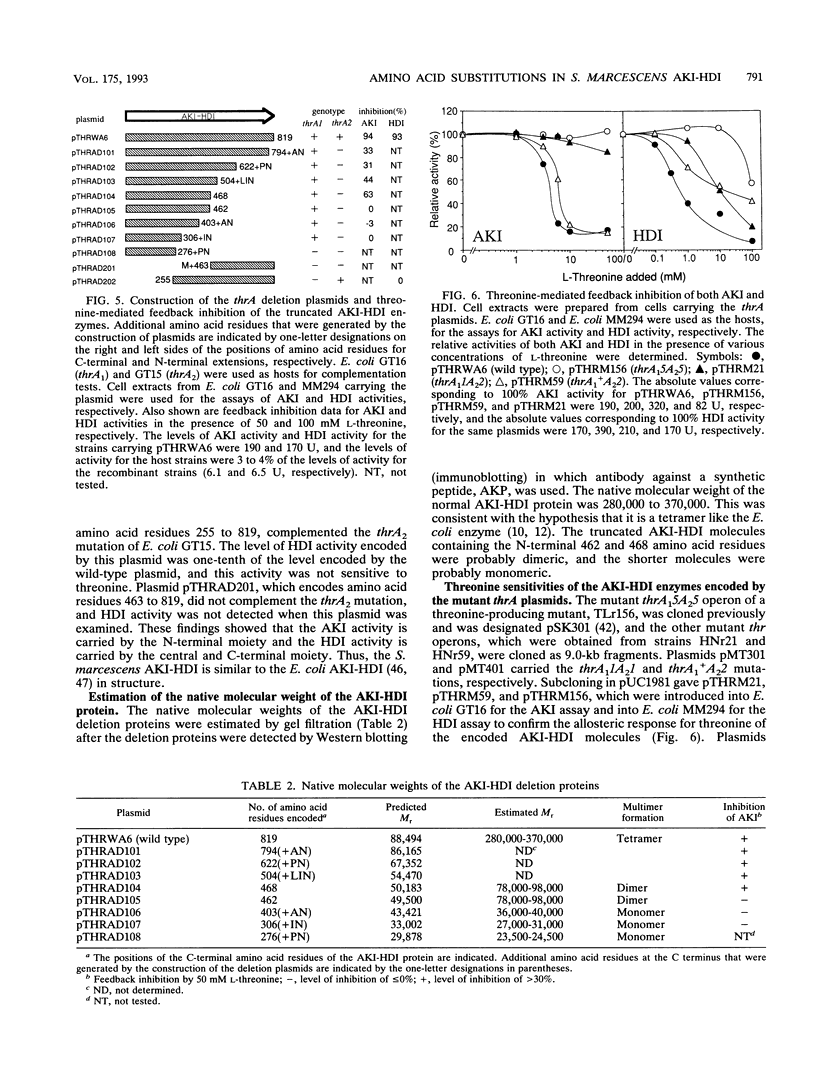
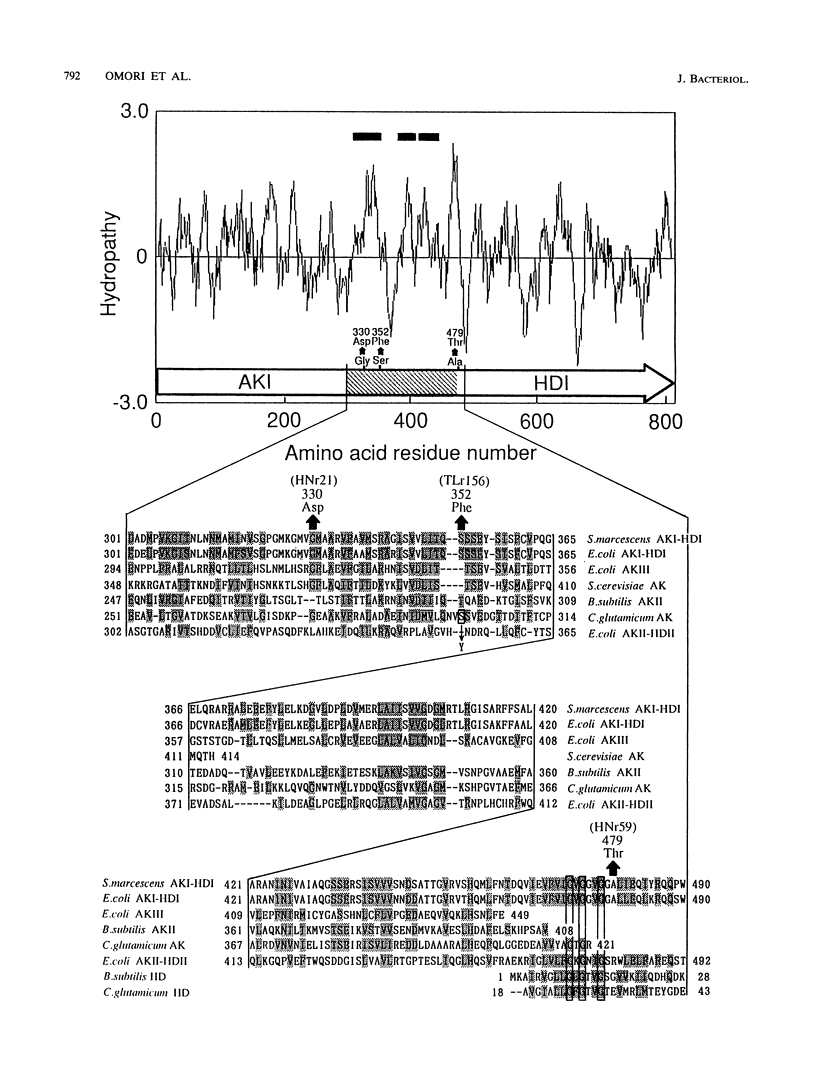
The nucleotide sequence of the Serratia marcescens threonine operon (thrA1A2BC) was determined. Three long open reading frames were identified; these open reading frames code for aspartokinase I (AKI)-homoserine dehydrogenase I (HDI), homoserine kinase, and threonine synthase, in that order. The predicted amino acid sequences of these enzymes were similar to the amino acid sequences of the corresponding enzymes in Escherichia coli. The AKI-HDI protein is apparently a tetramer composed of monomer polypeptides that are 819 amino acids long. A deletion analysis revealed that the central and C-terminal region was responsible for threonine-resistant HDI activity, a monomeric fragment extending from the N terminus to residue 306 was responsible for threonine-resistant AKI activity, and an N-terminal portion containing 468 residues was responsible for threonine-sensitive AKI activity. The thrA(1)1A(2)1 and thrA(1)5A(2)5 mutations of threonine-excreting strains HNr21 and TLr156, which result in the loss of threonine-mediated feedback inhibition of both AKI activity and HDI activity, cause single amino acid substitutions (Gly to Asp at position 330 and Ser to Phe at position 352, respectively) in the central region of the AKI-HDI protein. The thrA1+A(2)2 mutation of strain HNr59, which results in a threonine-sensitive AKI and a threonine-resistant HDI, also causes a single amino acid substitution (Ala to Thr at position 479).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brosius J., Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassan M., Parsot C., Cohen G. N., Patte J. C. Nucleotide sequence of lysC gene encoding the lysine-sensitive aspartokinase III of Escherichia coli K12. Evolutionary pathway leading to three isofunctional enzymes. J Biol Chem. 1986 Jan 25;261(3):1052–1057. [PubMed] [Google Scholar]
- Chen N. Y., Hu F. M., Paulus H. Nucleotide sequence of the overlapping genes for the subunits of Bacillus subtilis aspartokinase II and their control regions. J Biol Chem. 1987 Jun 25;262(18):8787–8798. [PubMed] [Google Scholar]
- Cossart P., Katinka M., Yaniv M. Nucleotide sequence of the thrB gene of E. coli, and its two adjacent regions; the thrAB and thrBC junctions. Nucleic Acids Res. 1981 Jan 24;9(2):339–347. doi: 10.1093/nar/9.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcoz-Kelly F., Janin J., Saari J. C., Véron M., Truffa-Bachi P., Cohen G. N. Revised structure of aspartokinase I-homoserine dehydrogenase I of Escherichia coli K12. Evidence for four identical subunits. Eur J Biochem. 1972 Aug 4;28(4):507–519. doi: 10.1111/j.1432-1033.1972.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Fazel A., Guillou Y., Cohen G. N. A hybrid proteolytic fragment of Escherichia coli aspartokinase I-homoserine dehydrogenase I. Structure, inhibition pattern, dissociation properties, and generation of two homodimers. J Biol Chem. 1983 Nov 25;258(22):13570–13574. [PubMed] [Google Scholar]
- Fazel A., Müller K., Le Bras G., Garel J. R., Véron M., Cohen G. N. A triglobular model for the polypeptide chain of aspartokinase I-homoserine dehydrogenase I of Escherichia coli. Biochemistry. 1983 Jan 4;22(1):158–165. doi: 10.1021/bi00270a023. [DOI] [PubMed] [Google Scholar]
- Gardner J. F. Regulation of the threonine operon: tandem threonine and isoleucine codons in the control region and translational control of transcription termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1706–1710. doi: 10.1073/pnas.76.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G., Fink G. R. Repeated DNA sequences upstream from HIS1 also occur at several other co-regulated genes in Saccharomyces cerevisiae. J Biol Chem. 1983 Apr 25;258(8):5238–5247. [PubMed] [Google Scholar]
- Kalinowski J., Cremer J., Bachmann B., Eggeling L., Sahm H., Pühler A. Genetic and biochemical analysis of the aspartokinase from Corynebacterium glutamicum. Mol Microbiol. 1991 May;5(5):1197–1204. doi: 10.1111/j.1365-2958.1991.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Katinka M., Cossart P., Sibilli L., Saint-Girons I., Chalvignac M. A., Le Bras G., Cohen G. N., Yaniv M. Nucleotide sequence of the thrA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5730–5733. doi: 10.1073/pnas.77.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Enhancement of isoleucine hydroxamate-mediated growth inhibition and improvement of isoleucine-producing strains of Serratia marcescens. Appl Environ Microbiol. 1977 Dec;34(6):647–653. doi: 10.1128/aem.34.6.647-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Kisumi M., Chibata I. Transductional construction of a threonine-producing strain of Serratia marcescens. Appl Environ Microbiol. 1979 Dec;38(6):1045–1051. doi: 10.1128/aem.38.6.1045-1051.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Kisumi M., Murata K., Chibata I. Threonine production by regulatory mutants of Serratia marcescens. Appl Environ Microbiol. 1978 May;35(5):834–840. doi: 10.1128/aem.35.5.834-840.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn S. P., Burton W. S., Donohue T. J., Gould R. M., Gumport R. I., Gardner J. F. Specificity of the attenuation response of the threonine operon of Escherichia coli is determined by the threonine and isoleucine codons in the leader transcript. J Mol Biol. 1987 Mar 5;194(1):59–69. doi: 10.1016/0022-2836(87)90715-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Hosogaya S., Suzuki K., Tazaki T. Arginine gene cluster of Serratia marcescens. Jpn J Microbiol. 1975 Feb;19(1):35–44. doi: 10.1111/j.1348-0421.1975.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori K., Suzuki S., Imai Y., Komatsubara S. Analysis of the Serratia marcescens proBA operon and feedback control of proline biosynthesis. J Gen Microbiol. 1991 Mar;137(3):509–517. doi: 10.1099/00221287-137-3-509. [DOI] [PubMed] [Google Scholar]
- Parsot C., Cohen G. N. Cloning and nucleotide sequence of the Bacillus subtilis hom gene coding for homoserine dehydrogenase. Structural and evolutionary relationships with Escherichia coli aspartokinases-homoserine dehydrogenases I and II. J Biol Chem. 1988 Oct 15;263(29):14654–14660. [PubMed] [Google Scholar]
- Parsot C., Cossart P., Saint-Girons I., Cohen G. N. Nucleotide sequence of thrC and of the transcription termination region of the threonine operon in Escherichia coli K12. Nucleic Acids Res. 1983 Nov 11;11(21):7331–7345. doi: 10.1093/nar/11.21.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples O. P., Liebl W., Bodis M., Maeng P. J., Follettie M. T., Archer J. A., Sinskey A. J. Nucleotide sequence and fine structural analysis of the Corynebacterium glutamicum hom-thrB operon. Mol Microbiol. 1988 Jan;2(1):63–72. doi: 10.1111/j.1365-2958.1988.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Rafalski J. A., Falco S. C. Structure of the yeast HOM3 gene which encodes aspartokinase. J Biol Chem. 1988 Feb 15;263(5):2146–2151. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Margarita D. Fine structure analysis of the threonine operon in Escherichia coli K-12. Mol Gen Genet. 1978 Jun 1;162(1):101–107. doi: 10.1007/BF00333856. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Sugita T., Komatsubara S., Kisumi M. Cloning and characterization of the mutated threonine operon (thrA(1)5A(2)5BC) of Serratia marcescens. Gene. 1987;57(2-3):151–158. doi: 10.1016/0378-1119(87)90118-1. [DOI] [PubMed] [Google Scholar]
- Takagi T., Kisumi M. Isolation of a versatile Serratia marcescens mutant as a host and molecular cloning of the aspartase gene. J Bacteriol. 1985 Jan;161(1):1–6. doi: 10.1128/jb.161.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Margarita D., Cohen G. N., Borne F., Patte J. C. Mapping of the structural genes of the three aspartokinases and of the two homoserine dehydrogenases of Escherichia coli K-12. J Bacteriol. 1974 Jan;117(1):133–143. doi: 10.1128/jb.117.1.133-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Saint-Girons I. Threonine locus of Escherichia coli K-12: genetic structure and evidence for an operon. J Bacteriol. 1974 Jun;118(3):990–998. doi: 10.1128/jb.118.3.990-998.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron M., Guillou Y., Cohen G. N. Isolation of the aspartokinase domain of bifunctional aspartokinase I-homoserine dehydrogenase I from E.coli K12. FEBS Lett. 1985 Feb 25;181(2):381–384. doi: 10.1016/0014-5793(85)80297-0. [DOI] [PubMed] [Google Scholar]
- Véron M., Falcoz-Kelly F., Cohen G. N. The threonine-sensitive homoserine dehydrogenase and aspartokinase activities of Escherichia coli K12. The two catalytic activities are carried by two independent regions of the polypeptide chain. Eur J Biochem. 1972 Aug 4;28(4):520–527. doi: 10.1111/j.1432-1033.1972.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zakin M. M., Duchange N., Ferrara P., Cohen G. N. Nucleotide sequence of the metL gene of Escherichia coli. Its product, the bifunctional aspartokinase ii-homoserine dehydrogenase II, and the bifunctional product of the thrA gene, aspartokinase I-homoserine dehydrogenase I, derive from a common ancestor. J Biol Chem. 1983 Mar 10;258(5):3028–3031. [PubMed] [Google Scholar]
- de la Peña P., Zasloff M. Enhancement of mRNA nuclear transport by promoter elements. Cell. 1987 Aug 14;50(4):613–619. doi: 10.1016/0092-8674(87)90034-1. [DOI] [PubMed] [Google Scholar]