Abstract
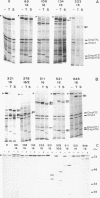
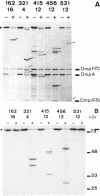
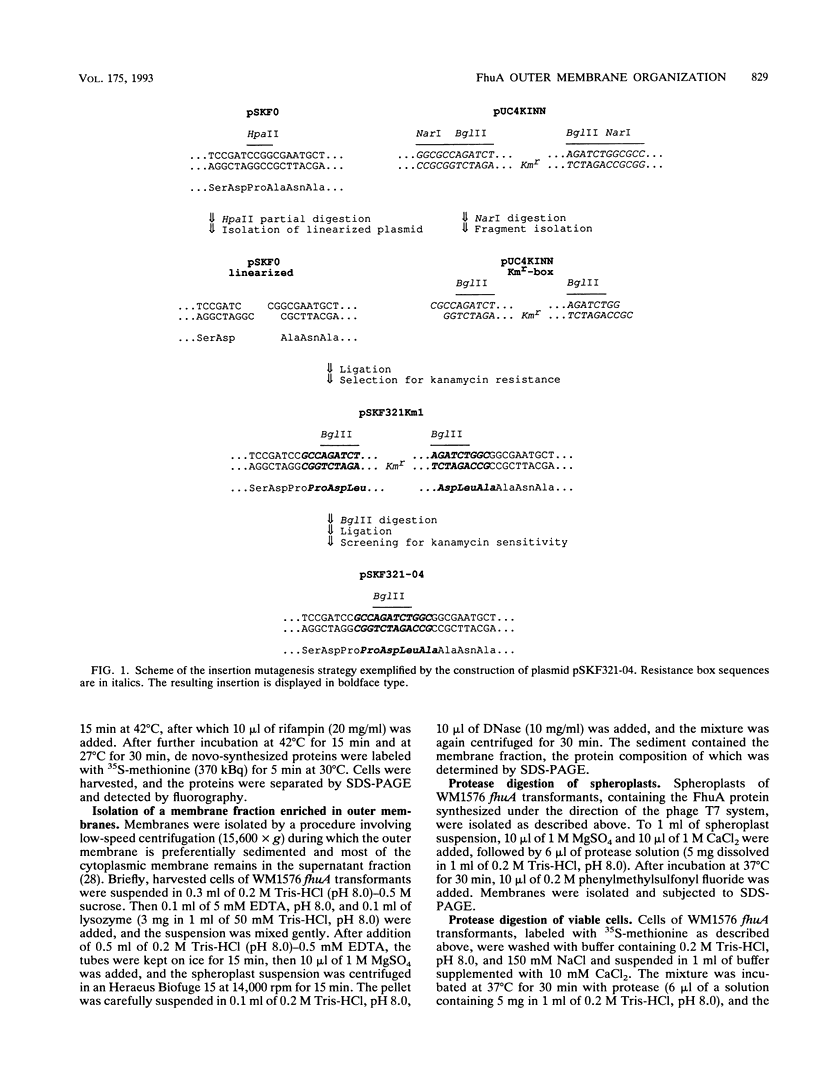
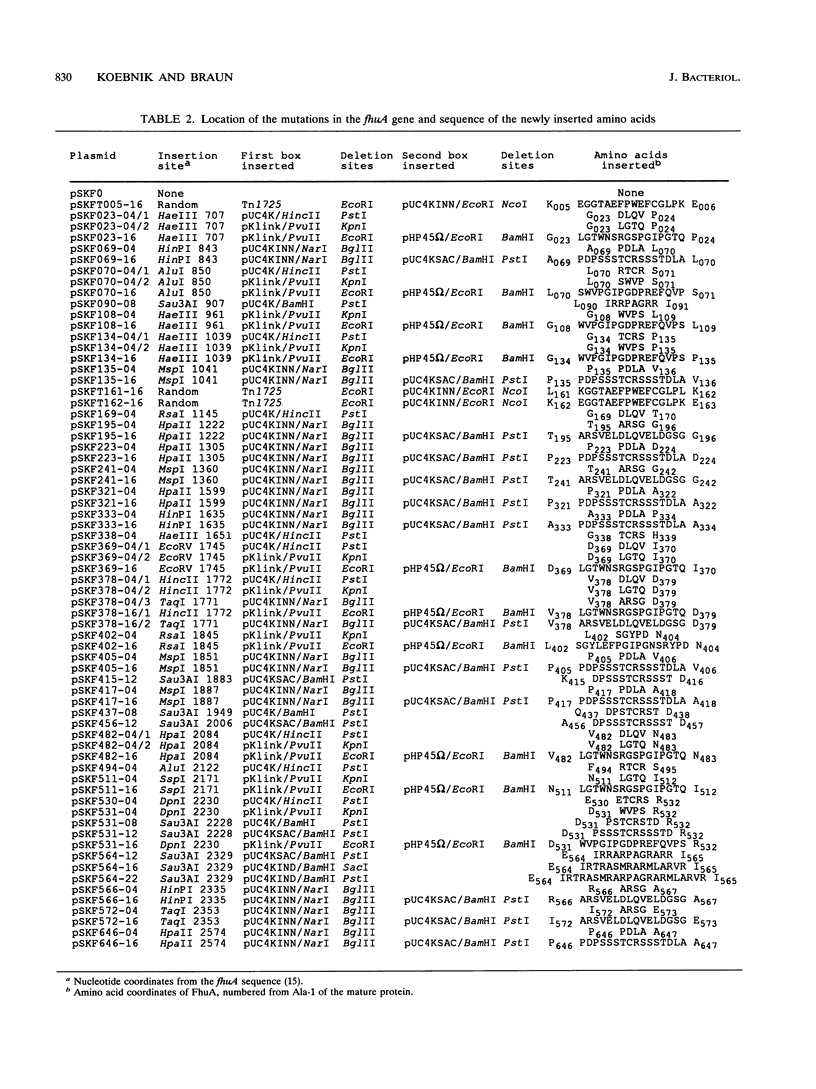
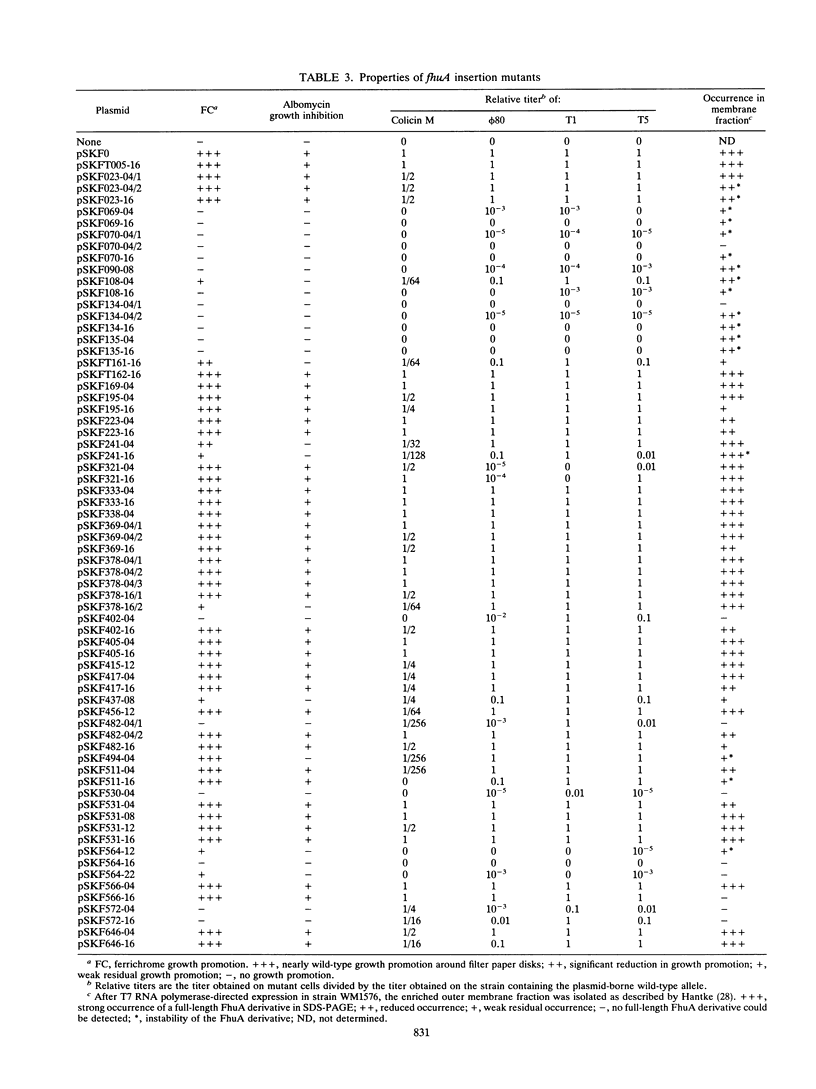
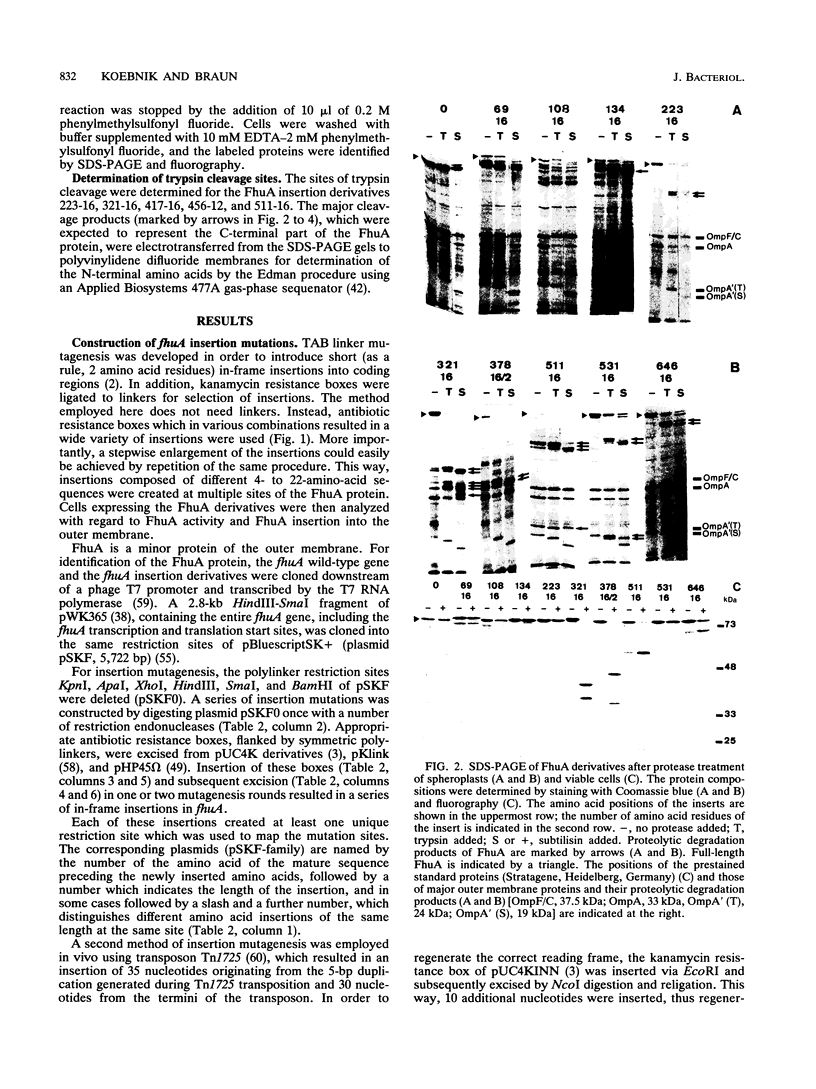
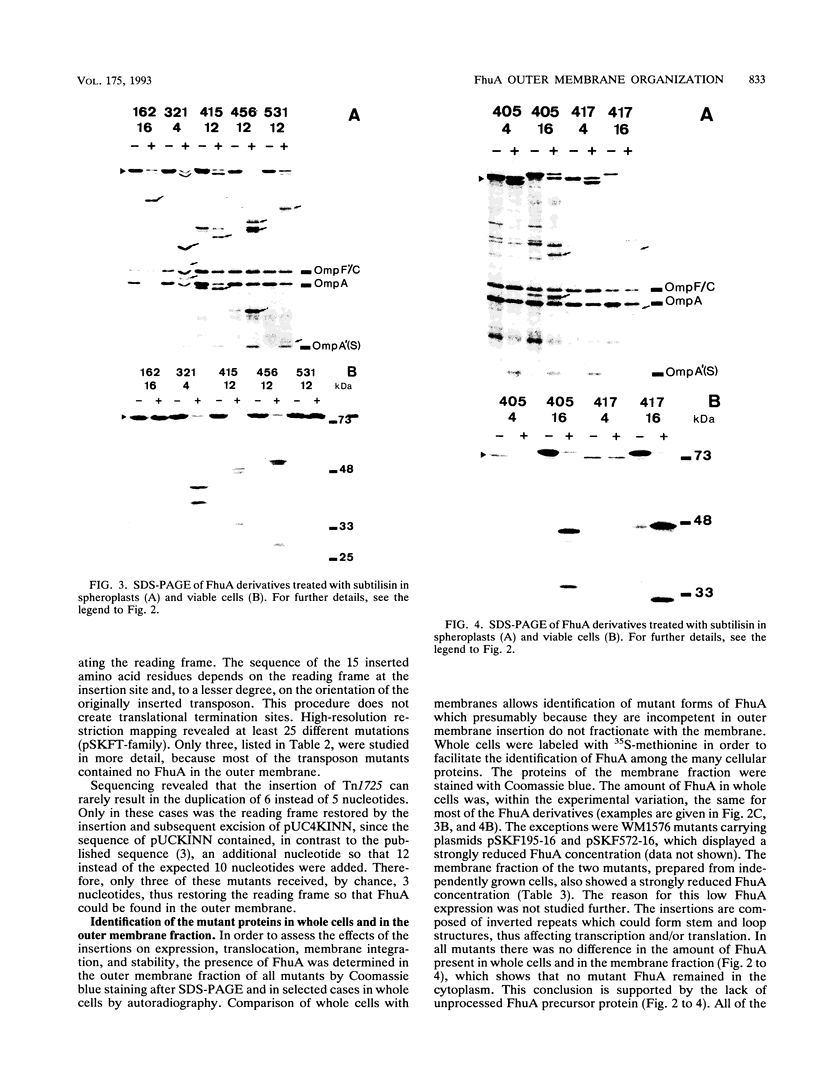
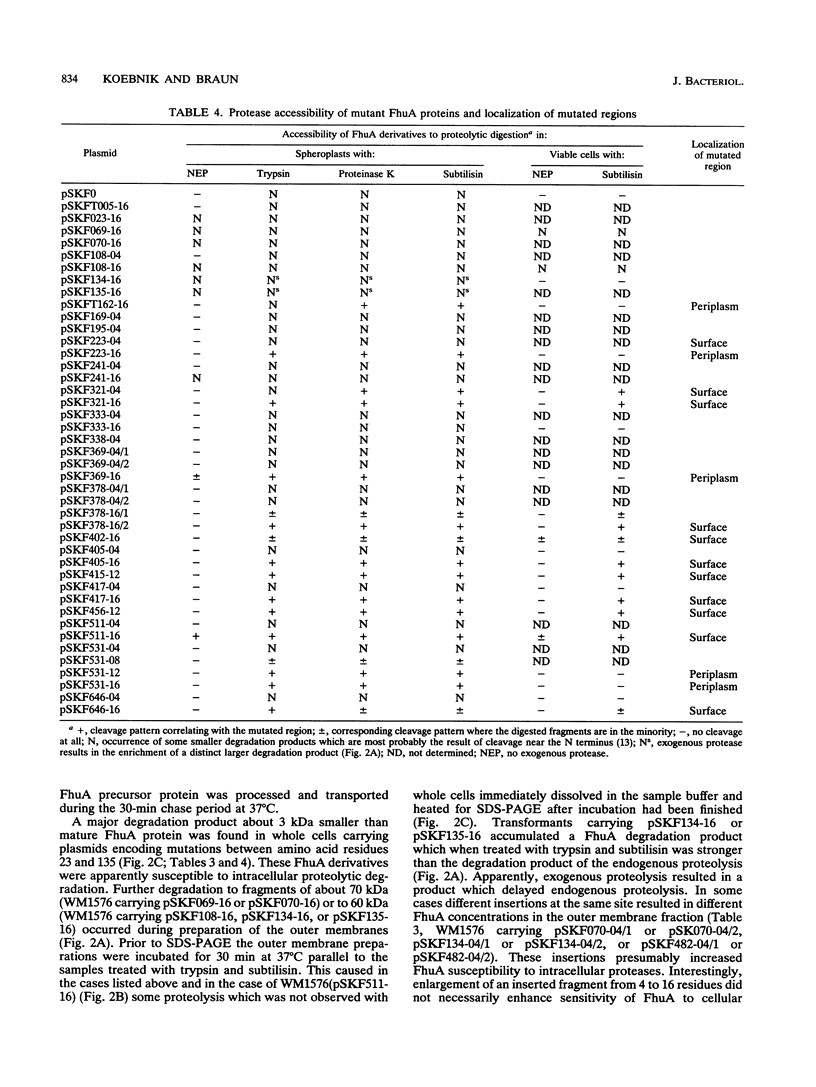
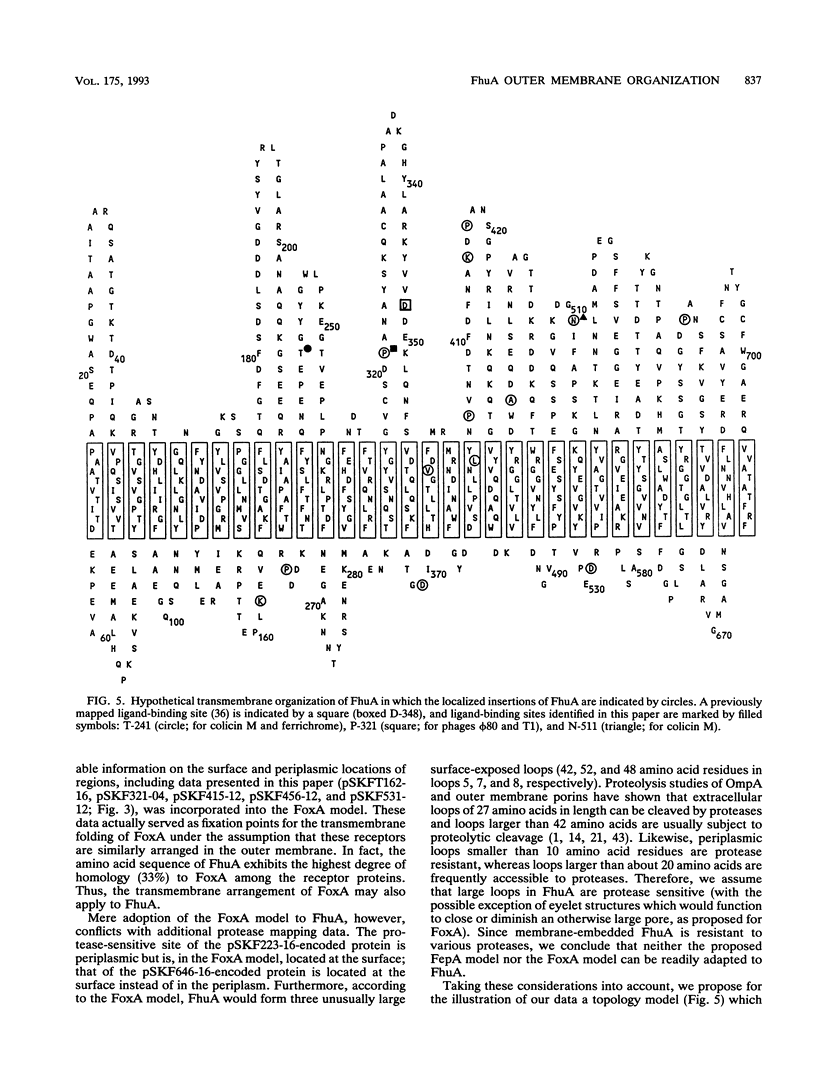
The FhuA receptor in the outer membrane of Escherichia coli K-12 is involved in the uptake of ferrichrome, colicin M, and the antibiotic albomycin and in infection by phages T1, T5, and phi 80. Fragments of up to 16 amino acid residues were inserted into FhuA and used to determine FhuA active sites and FhuA topology in the outer membrane. For this purpose antibiotic resistance boxes flanked by symmetric polylinkers were inserted into fhuA and subsequently partially deleted. Additional in-frame insertions were generated by mutagenesis with transposon Tn1725. The 68 FhuA protein derivatives examined contained segments of 4, 8, 12, 16, and 22 additional amino acid residues at 34 different locations from residues 5 to 646 of the mature protein. Most of the FhuA derivatives were found in normal amounts in the outer membrane fraction. Half of these were fully active toward all ligands, demonstrating proper insertion into the outer membrane. Seven of the 12- and 16-amino-acid-insertion derivatives (at residues 378, 402, 405, 415, 417, 456, and 646) were active toward all of the ligands and could be cleaved by subtilisin in whole cells, suggesting a surface location of the extra loops at sites which did not affect FhuA function. Two mutants were sensitive to subtilisin (insertions at residues 511 and 321) but displayed a strongly reduced sensitivity to colicin M and to phages phi 80 and T1. Four of the insertion derivatives (at residues 162, 223, 369, and 531) were cleaved only in spheroplasts and probably form loops at the periplasmic side of the outer membrane. The number and size of the proteolytic fragments indicate cleavage at or close to the sites of insertion, which has been proved for five insertions by amino acid sequencing. Most mutants with functional defects were affected in their sensitivity to all ligands, yet frequently to different degrees. Some mutants showed a specifically altered sensitivity to a few ligands; for example, mutant 511-04 was partially resistant only to colicin M, mutant 241-04 was reduced in ferrichrome and albomycin uptake and showed a reduced colicin M sensitivity, and mutant 321-04 was fully resistant to phage T1 and partially resistant to phage phi 80. The altered residues define preferential binding sites for these ligands. Insertions of 4 to 16 residues at positions 69, 70, 402, 530, 564, and 572 resulted in strongly reduced amounts of FhuA in the outer membrane fraction, varying in function from fully active to inactive. These results provide the basis for a model of FhuA organization in the outer membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agterberg M., Adriaanse H., van Bruggen A., Karperien M., Tommassen J. Outer-membrane PhoE protein of Escherichia coli K-12 as an exposure vector: possibilities and limitations. Gene. 1990 Mar 30;88(1):37–45. doi: 10.1016/0378-1119(90)90057-x. [DOI] [PubMed] [Google Scholar]
- Barany F. Two-codon insertion mutagenesis of plasmid genes by using single-stranded hexameric oligonucleotides. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4202–4206. doi: 10.1073/pnas.82.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Darveau R. P., Hancock R. E. Outer-membrane protein PhoE from Escherichia coli forms anion-selective pores in lipid-bilayer membranes. Eur J Biochem. 1984 Apr 16;140(2):319–324. doi: 10.1111/j.1432-1033.1984.tb08104.x. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Maier C., Bremer E. Characterization of the nucleoside-binding site inside the Tsx channel of Escherichia coli outer membrane. Reconstitution experiments with lipid bilayer membranes. Eur J Biochem. 1988 Oct 1;176(3):699–705. doi: 10.1111/j.1432-1033.1988.tb14333.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Frenz J., Hantke K., Schaller K. Penetration of colicin M into cells of Escherichia coli. J Bacteriol. 1980 Apr;142(1):162–168. doi: 10.1128/jb.142.1.162-168.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Günter K., Hantke K. Transport of iron across the outer membrane. Biol Met. 1991;4(1):14–22. doi: 10.1007/BF01135552. [DOI] [PubMed] [Google Scholar]
- Bremer E., Middendorf A., Martinussen J., Valentin-Hansen P. Analysis of the tsx gene, which encodes a nucleoside-specific channel-forming protein (Tsx) in the outer membrane of Escherichia coli. Gene. 1990 Nov 30;96(1):59–65. doi: 10.1016/0378-1119(90)90341-n. [DOI] [PubMed] [Google Scholar]
- Brewer S., Tolley M., Trayer I. P., Barr G. C., Dorman C. J., Hannavy K., Higgins C. F., Evans J. S., Levine B. A., Wormald M. R. Structure and function of X-Pro dipeptide repeats in the TonB proteins of Salmonella typhimurium and Escherichia coli. J Mol Biol. 1990 Dec 20;216(4):883–895. doi: 10.1016/S0022-2836(99)80008-4. [DOI] [PubMed] [Google Scholar]
- Bäumler A. J., Hantke K. Ferrioxamine uptake in Yersinia enterocolitica: characterization of the receptor protein FoxA. Mol Microbiol. 1992 May;6(10):1309–1321. doi: 10.1111/j.1365-2958.1992.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Carmel G., Coulton J. W. Internal deletions in the FhuA receptor of Escherichia coli K-12 define domains of ligand interactions. J Bacteriol. 1991 Jul;173(14):4394–4403. doi: 10.1128/jb.173.14.4394-4403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel G., Hellstern D., Henning D., Coulton J. W. Insertion mutagenesis of the gene encoding the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1990 Apr;172(4):1861–1869. doi: 10.1128/jb.172.4.1861-1869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Ronco J., Michel V., Werts C., Hofnung M. Permissive sites and topology of an outer membrane protein with a reporter epitope. J Bacteriol. 1991 Jan;173(1):262–275. doi: 10.1128/jb.173.1.262-275.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Cameron D. R., Carmel G., Jean R., Rode H. N. Protein fusions of beta-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1986 Jan;165(1):181–192. doi: 10.1128/jb.165.1.181-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick-Helmerich K., Braun V. Import of biopolymers into Escherichia coli: nucleotide sequences of the exbB and exbD genes are homologous to those of the tolQ and tolR genes, respectively. J Bacteriol. 1989 Sep;171(9):5117–5126. doi: 10.1128/jb.171.9.5117-5126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick-Helmerich K., Hantke K., Braun V. Cloning and expression of the exbB gene of Escherichia coli K-12. Mol Gen Genet. 1987 Feb;206(2):246–251. doi: 10.1007/BF00333580. [DOI] [PubMed] [Google Scholar]
- Fischer E., Günter K., Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989 Sep;171(9):5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiss E. H., Stanley-Samuelson P., Neilands J. B. Properties and proteolysis of ferric enterobactin outer membrane receptor in Escherichia coli K12. Biochemistry. 1982 Aug 31;21(18):4517–4522. doi: 10.1021/bi00261a050. [DOI] [PubMed] [Google Scholar]
- Freudl R. Insertion of peptides into cell-surface-exposed areas of the Escherichia coli OmpA protein does not interfere with export and membrane assembly. Gene. 1989 Oct 30;82(2):229–236. doi: 10.1016/0378-1119(89)90048-6. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Günter K., Braun V. In vivo evidence for FhuA outer membrane receptor interaction with the TonB inner membrane protein of Escherichia coli. FEBS Lett. 1990 Nov 12;274(1-2):85–88. doi: 10.1016/0014-5793(90)81335-l. [DOI] [PubMed] [Google Scholar]
- Günter K., Braun V. Probing FhuA'-'PhoA fusion proteins for the study of FhuA export into the cell envelope of Escherichia coli K12. Mol Gen Genet. 1988 Dec;215(1):69–75. doi: 10.1007/BF00331305. [DOI] [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Phage T6--colicin K receptor and nucleoside transport in Escherichia coli. FEBS Lett. 1976 Nov;70(1):109–112. doi: 10.1016/0014-5793(76)80737-5. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hoffmann H., Fischer E., Kraut H., Braun V. Preparation of the FhuA (TonA) receptor protein from cell envelopes of an overproducing strain of Escherichia coli K-12. J Bacteriol. 1986 May;166(2):404–411. doi: 10.1128/jb.166.2.404-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H., Fischer E., Schwarz H., Braun V. Overproduction of the proFhuA outer membrane receptor protein of Escherichia coli K-12: isolation, properties, and immunocytochemical localization at the inner side of the cytoplasmic membrane. Arch Microbiol. 1986 Sep;145(4):334–341. doi: 10.1007/BF00470867. [DOI] [PubMed] [Google Scholar]
- Jeanteur D., Lakey J. H., Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991 Sep;5(9):2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- Kadner R. J. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol Microbiol. 1990 Dec;4(12):2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Killmann H., Braun V. An aspartate deletion mutation defines a binding site of the multifunctional FhuA outer membrane receptor of Escherichia coli K-12. J Bacteriol. 1992 Jun;174(11):3479–3486. doi: 10.1128/jb.174.11.3479-3486.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster W., Braun V. Iron-hydroxamate transport into Escherichia coli K12: localization of FhuD in the periplasm and of FhuB in the cytoplasmic membrane. Mol Gen Genet. 1989 Jun;217(2-3):233–239. doi: 10.1007/BF02464886. [DOI] [PubMed] [Google Scholar]
- Köster W., Gudmundsdottir A., Lundrigan M. D., Seiffert A., Kadner R. J. Deletions or duplications in the BtuB protein affect its level in the outer membrane of Escherichia coli. J Bacteriol. 1991 Sep;173(18):5639–5647. doi: 10.1128/jb.173.18.5639-5647.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Morona R., Tommassen J., Henning U. Demonstration of a bacteriophage receptor site on the Escherichia coli K12 outer-membrane protein OmpC by the use of a protease. Eur J Biochem. 1985 Jul 1;150(1):161–169. doi: 10.1111/j.1432-1033.1985.tb09002.x. [DOI] [PubMed] [Google Scholar]
- Murphy C. K., Kalve V. I., Klebba P. E. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J Bacteriol. 1990 May;172(5):2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau C. D., Konisky J. Evolutionary relationship between the TonB-dependent outer membrane transport proteins: nucleotide and amino acid sequences of the Escherichia coli colicin I receptor gene. J Bacteriol. 1989 Feb;171(2):1041–1047. doi: 10.1128/jb.171.2.1041-1047.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C., Rosenbusch J. P. Folding patterns of porin and bacteriorhodopsin. EMBO J. 1985 Jun;4(6):1593–1597. doi: 10.1002/j.1460-2075.1985.tb03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauptit R. A., Schirmer T., Jansonius J. N., Rosenbusch J. P., Parker M. W., Tucker A. D., Tsernoglou D., Weiss M. S., Schultz G. E. A common channel-forming motif in evolutionarily distant porins. J Struct Biol. 1991 Oct;107(2):136–145. doi: 10.1016/1047-8477(91)90017-q. [DOI] [PubMed] [Google Scholar]
- Postle K. TonB and the gram-negative dilemma. Mol Microbiol. 1990 Dec;4(12):2019–2025. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Ronco J., Charbit A., Hofnung M. Creation of targets for proteolytic cleavage in the LamB protein of E coli K12 by genetic insertion of foreign sequences: implications for topological studies. Biochimie. 1990 Feb-Mar;72(2-3):183–189. doi: 10.1016/0300-9084(90)90144-6. [DOI] [PubMed] [Google Scholar]
- Schmid K., Ebner R., Jahreis K., Lengeler J. W., Titgemeyer F. A sugar-specific porin, ScrY, is involved in sucrose uptake in enteric bacteria. Mol Microbiol. 1991 Apr;5(4):941–950. doi: 10.1111/j.1365-2958.1991.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Schöffler H., Braun V. Transport across the outer membrane of Escherichia coli K12 via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol Gen Genet. 1989 Jun;217(2-3):378–383. doi: 10.1007/BF02464907. [DOI] [PubMed] [Google Scholar]
- Schülein K., Schmid K., Benzl R. The sugar-specific outer membrane channel ScrY contains functional characteristics of general diffusion pores and substrate-specific porins. Mol Microbiol. 1991 Sep;5(9):2233–2241. doi: 10.1111/j.1365-2958.1991.tb02153.x. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare J. T., Postle K. Evidence for a TonB-dependent energy transduction complex in Escherichia coli. Mol Microbiol. 1991 Dec;5(12):2883–2890. doi: 10.1111/j.1365-2958.1991.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Crouse G. F. Construction of linker-scanning mutations using a kanamycin-resistance cassette with multiple symmetric restriction sites. Gene. 1989 Dec 7;84(1):159–164. doi: 10.1016/0378-1119(89)90150-9. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubben D., Schmitt R. Tn1721 derivatives for transposon mutagenesis, restriction mapping and nucleotide sequence analysis. Gene. 1986;41(2-3):145–152. doi: 10.1016/0378-1119(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Abele U., Weckesser J., Welte W., Schiltz E., Schulz G. E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991 Dec 13;254(5038):1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- van der Ley P., Struyvé M., Tommassen J. Topology of outer membrane pore protein PhoE of Escherichia coli. Identification of cell surface-exposed amino acids with the aid of monoclonal antibodies. J Biol Chem. 1986 Sep 15;261(26):12222–12225. [PubMed] [Google Scholar]
- von Heijne G., Manoil C. Membrane proteins: from sequence to structure. Protein Eng. 1990 Dec;4(2):109–112. doi: 10.1093/protein/4.2.109. [DOI] [PubMed] [Google Scholar]