Abstract
The origin recognition complex (ORC) marks chromosomal positions as replication origins and is essential for replication initiation. At a few loci, the ORC functions in heterochromatin formation. We show that the ORC's two roles at the heterochromatic HMRa locus in Saccharomyces cerevisiae were regulated by differences in the ORC's interaction with its target site. At HMRa, a strong ORC-DNA interaction inhibited and delayed replication initiation but promoted heterochromatin formation, whereas a weak ORC-DNA interaction allowed for increased and earlier replication initiation but reduced heterochromatin formation. Therefore, the ORC's interaction with its target site could modulate ORC activity within a heterochromatin domain in vivo.
Keywords: Heterochromatin, silencers, origins, ORC, yeast
The origin recognition complex (ORC) is essential for genome replication in eukaryotes and functions by binding to specific chromosomal positions and recruiting additional proteins essential for origin firing (Bell 2002). Because each chromosome requires the activity of many replication origins for its duplication, ORCs function at hundreds of positions distributed throughout the genome. Although ORCs function at all origins, individual origins vary in activity. Some initiate replication efficiently and early during S phase, whereas others initiate in only a small fraction of cell cycles and later during S phase (Friedman et al. 1997; Yamashita et al. 1997; Polomienko et al. 2001). In budding yeast, a small subset of origins, termed silencers, is associated with the formation of a specialized chromatin structure that represses transcription (Loo and Rine 1995). Significantly, a role for the ORC in repressive chromatin is conserved in metazoans (Pak et al. 1997).
Silencing of HMRa in Saccharomyces cerevisiae is a form of transcription repression requiring a specialized chromatin structure called silent chromatin that is similar to heterochromatin (Pillus and Grunstein 1995; Rusche et al. 2003). Like heterochromatin, silent chromatin causes gene-independent, position-dependent transcription repression. Both heterochromatin and silent chromatin contain relatively hypoacetylated nucleosomes as well as specialized nonhistone chromatin-binding proteins that help assemble a repressive chromatin domain encompassing many kilobase pairs of DNA. In addition, silent chromatin, like many forms of heterochromatin, is replicated late during S phase (Raghuraman et al. 2001).
DNA elements bound by sequence-specific DNA-binding proteins target specialized chromatin structures to specific chromosomal domains. At HMRa, the critical DNA element is the 150-bp HMR-E silencer that contains a binding site for the ORC (A-element) as well as a single binding site for each of the abundant nuclear proteins, Rap1p and Abf1p (Loo and Rine 1995). Together, silencer-binding proteins nucleate the assembly of silenced chromatin by direct physical interactions with nonhistone chromatin-binding proteins called Sirs, which, in turn, recruit additional Sir proteins that modify and bind nucleosomes, forming silent chromatin (Gasser and Cockell 2001).
In addition to its silencer function, the activity of HMR-E differs from that of a typical nonsilencer origin such as ARS1 in other ways. HMR-E is an inefficient origin, initiating replication in <10% of cell cycles (DeBeer and Fox 1999; Polomienko et al. 2001) compared with many nonsilencer origins that initiate replication in a majority of cell cycles (Friedman et al. 1997; Polomienko et al. 2001). Here, we show that the ORC bound the HMR-E silencer tightly and that this ORC/silencer interaction enhanced silencer activity but inhibited origin activity of HMR-E independently of the chromatin state at HMRa.
Results and Discussion
To compare the relative strengths of the ORC-DNA interaction at HMR-E and nonsilencer origins, we performed electrophoretic mobility shift assays (EMSAs) with DNA fragments from several identified origins (ARS elements) within the yeast genome (Fig. 1; Supplementary Table 1). We used ARS1, a well-characterized origin in terms of ORC binding in vitro and origin activity in vivo to guide these experiments (Fig. 1A). ARS1 consists of a conserved 11-bp A-element found in virtually all yeast origins and a less conserved series of B-elements 5′ to the A-rich strand of the A-element (Marahrens and Stillman 1992). The A-element is essential for replication initiation in vivo and ORC binding in vitro (Bell and Stillman 1992; Marahrens and Stillman 1992). The B-elements contribute to but are not essential for replication initiation. Mutations in the B1-element reduce ORC binding in vitro, and footprinting and cross-linking experiments indicate that the ORC contacts a bipartite element in ARS1 consisting of the B1- and A-elements (B1/A-element; Rao and Stillman 1995; Lee and Bell 1997).
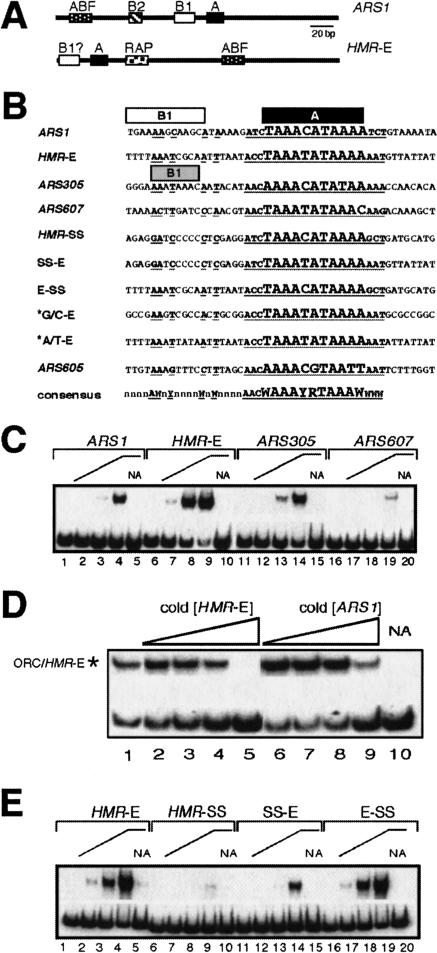
Figure 1.
ORC binding to the B1/A-elements of various origins. (A) Structural organization of ARS1 as defined by linker-scanning mutagenesis (Marahrens and Stillman 1992) and the HMR-E silencer as defined by deletion analysis (for review, see Loo and Rine 1995). A B1-element (?) for HMR-E was functionally characterized in this study. (B) Sequences of B1/A-elements for a variety of origins examined for ORC binding in vitro. B1-elements as defined by mutagenesis are shown for ARS1 and ARS305 with boxes above the element (Marahrens and Stillman 1992; Huang and Kowalski 1996). The “expanded” ORC consensus site is shown at the bottom (Theis and Newlon 1997). The conserved A-element is shown in larger, bold font and underlined. The conserved nucleotides in the more variable B1-element are shown as bold and underlined. The * marks B1/A-elements in which every nucleotide in the fragment other than the conserved nucleotides of the expanded ORC consensus site has been changed to G or C (GC-E) or Aand T (A/T-E). (C) Equilibrium binding of purified ORC to B1/A-elements of indicated origins as measured by EMSAs (Bell et al. 1995; Rao and Stillman 1995). Binding reactions used 0.1 nM radiolabeled DNA and 0 nM (lanes 1,6,11,16), 0.1 nM (lanes 2,7,12,17), 1 nM (lanes 3,8,13,18), and 10 nM (lanes 4,5,9,10,14,15,19,20) ORC. All binding reactions contained 2 mM ATP except those in lanes marked NA (No ATP; lanes 5,10,15,20). (D) Competition binding experiments to measure relative affinities of ORC for HMR-E and ARS1. An EMSA was performed with 0.02 nM radiolabeled HMR-E DNA (as in B) and ORC to obtain ∼50% binding of DNA substrate (lane 1). The ORC/HME-E complex is indicated by an *. Increasing concentrations of cold HMR-E substrate DNA (lanes 2-5) or ARS1 substrate DNA (as in B; lanes 6-9) were added to identical reactions to determine the concentrations needed to compete the binding of ORC to radiolabeled HMR-E. (Lane 10) Ano-ATP control experiment. The concentrations of cold DNA substrate were 0.6 nM (lanes 2,6), 1 nM (lanes 3,7), 2 nM (lanes 4,8), and 20 nM (lanes 5,9). (E) ORC binding as described in C to the natural (HMR-E) and synthetic silencer's (HMR-SS) putative B1/A-elements and B1/Ahybrid DNA templates as shown in B.
Other origins that have been examined contain A- and B-elements including a B1-element in similar relative positions as those in ARS1 (Fig. 1B, see ARS305; Huang and Kowalski 1996). Also, a sequence comparison of multiple origins revealed an expanded ORC consensus-binding site (Fig. 1B) that includes both the A-element and sequences expected to contain a B1-element (Theis and Newlon 1997). Although a B1-element has not been defined for HMR-E or several nonsilencer origins, we used these observations to design DNA probes that contained the best matches for both the B1- and A-elements from natural and engineered origins (Fig. 1B) for EMSAs with purified recombinant ORC (Bell et al. 1995).
Interestingly, ORC bound the putative B1/A-element from HMR-E with a 10-fold higher affinity than the analogous region of ARS1 as measured by both direct EMSAs of radioactive substrates and competition experiments with appropriate unlabeled DNA substrates (Fig. 1C,D). Additional experiments indicated that ORC bound the B1/A-element of HMR-E more tightly than it bound the analogous regions of several other origins known to initiate replication much more efficiently than HMR-E, including ARS305 and ARS607 (Fig. 1B; Supplementary Table 1).
To test whether HMR-E's putative B1-element contributed to high-affinity binding by ORC, we used an engineered version of HMR-E called the synthetic silencer (HMR-SS) whose replication and silencing activities have been well defined (McNally and Rine 1991; Fox et al. 1995). HMR-SS contains an A-element and binding sites for Rap1p and Abf1p positioned with the same spacing and order as in HMR-E. However, the binding sites as well as the sequences between them differ between HMR-E and HMR-SS. We noted that HMR-SS contains a poor match to the expanded ORC consensus site, particularly in the region that should contain a B1-element (Fig. 1B). Therefore, we tested whether the putative B1/A-element of HMR-SS would bind ORC with a lower affinity compared with HMR-E. Significantly, HMR-SS bound ORC with >100-fold lower affinity than HMR-E (Fig. 1E), even though HMR-SS initiates replication more efficiently than HMR-E on the chromosome (Fox et al. 1995; DeBeer and Fox 1999).
To test whether the B1-element contributed to high-affinity ORC binding at HMR-E, we engineered hybrid B1/A-elements from HMR-SS and HMR-E and tested them in EMSAs (Fig. 1B,E). Ahybrid DNA element containing the B1-element from HMR-SS and the A-element from HMR-E (SS-E) bound ORC only slightly better than the B1/A-element from HMR-SS. However, the hybrid element containing the B1-element of HMR-E and the A-element of HMR-SS (E-SS) bound ORC as tightly as the B1/A-element of HMR-E (Fig. 1E). Therefore, HMR-E contains a B1-element important for high-affinity binding by ORC.
We next tested whether a high-affinity ORC-DNA interaction within a silencer could contribute to the silencer's origin or silencing activities at HMRa. As described above, HMR-E is an extremely weak replication origin but a strong silencer. In contrast, HMR-SS is a stronger origin but a weaker silencer, and both of its activities are reduced by the orc2-1 mutation (Fox et al. 1995; DeBeer and Fox 1999). Therefore, we engineered two strains that differed only in terms of the silencer that controlled their HMRa locus (Fig. 2A). One strain contained HMR-SS as the only silencer at HMRa (low-affinity silencer). The other strain contained a hybrid version of HMR-E and HMR-SS in which the low-affinity ORC-binding site (B1/A-element) of HMR-SS was replaced with the high-affinity ORC-binding site of HMR-E (high-affinity silencer; Fig. 2A). These strains were examined for replication (Figs. 2B, 3) and silencing (Fig. 4) of HMRa.
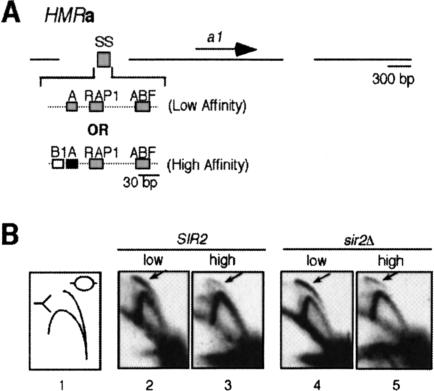
Figure 2.
A high-affinity ORC-binding site inhibits replication initiation by the HMR-SS silencer. (A) Structure of the two versions of HMRa analyzed for origin function in vivo. The HMR-I silencer and the cryptic origins flanking the defined HMR-E silencer were deleted (shown as gaps; DeBeer and Fox 1999). In one strain, the HMR-SS containing a low-affinity ORC-binding site was the only silencer at HMRa (low affinity). In a second strain, an HMR-SS engineered to contain the high-affinity ORC-binding site of natural HMR-E was the only silencer at HMRa (high affinity). (B) 2D origin analysis of the HMR-SS origins described in A in both SIR2 and sir2Δ strains. 2D origin mapping experiments allow the analysis of chromosomal replication intermediates formed in vivo by allowing the separation of origin bubbles from replication forks (Friedman and Brewer 1995). Arrows indicate origin bubbles. Except for differences noted, the strains were MATα and isogenic to W303.
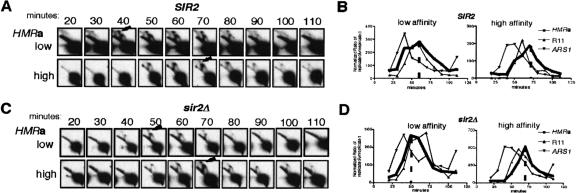
Figure 3.
A high-affinity ORC-binding site in HMR-SS delayed the replication time of HMRa. (A) Two strains, differing only in terms of their silencers at HMRa (Fig. 2A) were examined by 2D origin analysis at 10-min intervals throughout S phase after release from synchronized G1 arrest. Arrows indicate the first detectable origin bubbles. (B) A plot of quantified data obtained from analysis of replication intermediates for HMR, R11, and ARS1 for the two strains examined in A. (C) Replication times of HMRa controlled by the two different versions of HMR-SS were not affected by SIR genotype. The same experiments as in A were performed in sir2Δ strains. Arrows indicate the peaks of replication. (D) A plot of quantified data obtained from analysis of replication intermediates for HMR, R11, and ARS1 for strains examined in C. For B and D, replication data for HMR, R11, and ARS1 were graphed as ratios of replication intermediates to unreplicated linear DNA (Y-axis) versus time after release from G1 arrest (X-axis). Data were quantified using a PhosphorImager. The peak replication time for HMRa in each strain is indicated with a broken vertical line.
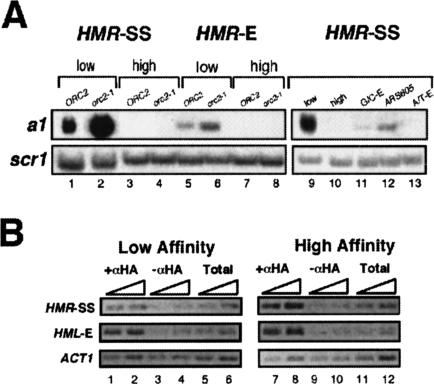
Figure 4.
A high-affinity ORC-binding site contributes to silencing of and efficient Sir1p binding to HMRa. (A) RNA blot hybridization of a1 mRNA expressed from HMRa in strains containing the HMR-SS (lanes 1-4) or natural HMR-E (lanes 5-8) silencers with the indicated B1/A-element ORC-binding site and either ORC2 (lanes 1,3,5,7) or the orc2-1 allele (lanes 2,4,6,8). Hybrid versions of HMR-SS containing high-affinity ORC-binding sites different from the B1/A-element of HMR-E (see Fig. 1B) also silenced HMRa (lanes 9-13). scr1 RNA was used as an RNA loading control. (B) ChIP of Sir1p-3xHA in yeast strains with either the low-affinity or high-affinity silencers was performed as described previously (Gardner and Fox 2001) except that HML-E was also examined as an internal control with HML-specific primers.
Significantly, at HMRa, the low-affinity silencer initiated replication more efficiently than the high-affinity silencer as determined by 2D origin mapping gels (Fig. 2B; SIR2), indicating that the high-affinity ORC-binding site could inhibit origin activity at HMRa.
In some contexts, SIR-dependent silent chromatin can inhibit replication initiation and cause early origins to fire later in S phase (Stevenson and Gottschling 1999; Zappulla et al. 2002). However, HMRa is replicated late in S phase even in strains containing SIR gene mutations that eliminate SIR-dependent silent chromatin (Dubey et al. 1991). In addition, the low efficiency of replication initiation at HMRa is not affected dramatically by deletions in SIR genes (Rivier and Rine 1992; Fox et al. 1995; DeBeer and Fox 1999). Thus, some feature other than the heterochromatic state inhibits replication initiation in this region. Therefore, we tested whether a high-affinity ORC/DNA interaction within a silencer could inhibit replication initiation by HMR-E in cells containing a deletion of the SIR2 gene (Fig. 2B). Significantly, the high-affinity silencer still initiated replication less efficiently than the low-affinity silencer as determined by the reduced number of bubble intermediates observed in 2D origin mapping experiments (Fig. 2B; sir2Δ). Thus, the SIR-dependent chromatin state could not explain the inhibited origin firing at HMRa.
Silent chromatin, like many forms of heterochromatin, is one of the last regions of the genome to be replicated. To test whether inhibiting replication initiation at HMRa via a strong ORC-binding site contributed to the late replication time of this locus, we compared replication timing of HMRa and two other well-characterized chromosomal regions, ARS1 and R11, during S phase in strains that differed only in terms of their silencers (Fig. 2A).
2D origin mapping performed on samples harvested during S phase in cell cycle arrest and release experiments revealed that a strong silencer ORC-binding site caused a later replication timing profile of HMRa (Fig. 3). HMRa containing the low-affinity silencer showed measurable replication intermediates, including origin bubbles as early as 40 min after release into S phase (Fig. 3A). In contrast, HMRa containing the high-affinity silencer did not show replication intermediates until 50 min, and origin bubbles were first and barely detectable at 70 min (Fig. 3A). The replication-timing profiles of the late (R11) and mid-early (ARS1) controls were similar in the two strains (Fig. 3B). Thus, the low-affinity silencer led to substantial replication of HMRa prior to the peak replication time of R11 (40-60 min for HMRa vs. 50-60 min for R11), whereas the high-affinity silencer led to a replication peak for HMRa that occurred after the peak replication time for R11 (70 min for HMRa vs. 60 min for R11). To the best of our knowledge, this is the first example of any mutation affecting the replication time of HMRa.
Because SIR-dependent silent chromatin can affect the time during S phase at which an origin fires (Stevenson and Gottschling 1999; Zappulla et al. 2002), we tested whether it had an effect on the relative replication-timing profiles of the low- and high-affinity silencers at HMRa (Fig. 3C,D). Significantly, the SIR2 genotype had a negligible effect on the absolute or the relative replication timing profiles of HMRa compared with R11 and ARS1. Thus, a silencer that can bind ORC tightly inhibits replication origin activity at HMRa and contributes to the SIR-independent late replication time of this locus.
We also tested whether a strong ORC-binding site was important for silencing of HMRa. We examined relevant yeast strains with silencers as described above (Fig. 2A) for their ability to silence HMRa (Fig. 4A). HMR-SS (low-affinity silencer) is a weak silencer that provides for only partial silencing of HMRa (Fox et al. 1995). This reduced ability to form silent chromatin can be measured by the appearance of a1 mRNA transcribed from HMRa in an RNA blot hybridization experiment (Fig. 4A, lane 1). The orc2-1 mutation completely abolished the low-affinity silencer's ability to silence (Fig. 4A, lane 2). In contrast, the high-affinity silencer provided for full silencing of HMRa (Fig. 4A, lanes 3,10) that was unaffected by the orc2-1 mutation (Fig. 4A, lane 4). Thus, the high-affinity ORC-binding site, owing in large part to the B1-element at HMR-E (Fig. 1D), was required for ORC-dependent silent chromatin formation.
These data indicated that a high-affinity ORC-binding site was sufficient to convert the weak HMR-SS silencer into a strong silencer whose ability to silence HMRa was insensitive to the orc2-1 mutation. Because the synthetic silencer is an engineered version of HMR-E that contains differences in addition to the A/B1-element, we also tested the effect of a low-affinity ORC-binding site on natural HMR-E (Fig. 4A, lanes 5-8). A low-affinity ORC-binding site reduced silencing by HMR-E and made it more sensitive to orc2-1 (Fig. 4A, lanes 5,6). The silencer activity of HMR-E was not as sensitive to a low-affinity ORC-binding site as HMR-SS, consistent with other differences between the two elements having roles in silencer strength. Nevertheless, these data indicate that a strong ORC-binding site was necessary for full silencing of HMRa.
Conceivably, some unique sequence in the B1-element of HMR-E could promote the ORC's function in silencing rather than high-affinity binding per se. To test this, we engineered silencers containing high-affinity B1/A-elements that were relatively “unrelated” to the B1/A-element of HMR-E (Fig. 1B). These hybrid silencers, including one that contained the B1/A-element from the exceptional nonsilencer origin ARS605 that bound ORC with a particularly high affinity in vitro (Supplementary Table 1) silenced HMRa more effectively than the low-affinity silencer, suggesting that a high-affinity binding site rather than some specific sequence was important for silencer function at HMRa (Fig. 4A, lanes 9-13).
One mechanism to reduce silencing at HMRa is to reduce the recruitment of Sir proteins; however, Sir recruitment alone is not sufficient for silencing HMRa (Lau et al. 2002). Because the primary role for ORC in silencing HMRa is to recruit Sir1p (for review, see Gasser and Cockell 2001), we tested whether the high-affinity ORC-binding site was necessary for efficient Sir1p binding in vivo by chromatin immunoprecipitation (ChIP; Fig. 4B). Significantly, Sir1p-3xHA bound more efficiently to HMRa in the strain controlled by the silencer with a high-affinity ORC-binding site, indicating that this site contributes to Sir1p's association with HMRa.
A major conclusion of this study is that stable binding of ORC to its target site in HMRa favors ORC's role in silencing over its role in replication initiation at this locus. We propose that the low origin activity at HMRa is not necessarily a prerequisite for silent chromatin formation, but rather a consequence of a stable ORC-chromosome interaction that is optimal for formation of silent chromatin. Tight binding by ORC would enhance the probability of a Sir complex remaining stably associated with HMR-E because ORC binds Sir1p (Triolo and Sternglanz 1996), and this, in turn, would stabilize silent chromatin at HMRa. Alternatively, the tight binding may reflect a conformation of the ORC at the silencer optimal for recruitment of Sir1p. The late replication time of HMRa during S phase may therefore be a consequence of an ORC-DNA interaction optimized for Sir1p recruitment rather than replication.
This study also provides an explanation for why defects in ORC caused by mutations in ORC genes fail to cause either silencing or replication defects at wild-type HMRa and why compromised silencers were useful in identifying the first mutant alleles of ORC genes (Foss et al. 1993). For example, the orc2-1 mutant allele lowers the concentration of ORC in vivo (Shimada et al. 2002) and reduces replication initiation by many origins, including the HMR-SS silencer origin (Fox et al. 1995; Liang et al. 1995). However, because the HMR-E silencer has a high affinity for ORC, it is resistant to reductions in ORC concentration and may have a competitive advantage over other lower-affinity chromosomal origins in an orc2-1 mutant strain.
The observation that the high-affinity ORC-binding site did not promote enhanced replication initiation at HMRa compared with the low-affinity silencer may seem nonintuitive at one level because such a site should increase the probability that the silencer origin is bound by ORC in any given cell, and ORC binding is necessary for origin activity. However, it is clear that there is enough ORC present in yeast to occupy origins with measurably lower affinities sufficiently enough that they initiate in a majority of cell cycles (Supplementary Table 1). Perhaps the silencer origin used here is revealing something about ORC function in replication initiation that is not possible to observe at nonsilencer origins optimized for origin firing. If true and if the high-affinity binding by ORC to silencer DNA observed in this study reflects a slow off-rate, then perhaps a step in replication initiation requires the ORC's dissociation from double-stranded origin DNA. Such a mechanism would be akin to Escherichia coli RNA polymerase promoter clearance (for review, see Hsu 2002). Alternatively, other features of the silencer origin may collaborate with some as yet unknown feature of the HMR-E A/B1-element that influences both ORC binding affinity and conformation and Sir binding, resulting in low origin efficiency and late replication of HMR-E.
Interestingly, genome-wide binding studies of ORC and MCM proteins reveal that the heterochromatic loci HMRa and HMLα are the only genomic regions that exhibit inefficient origin activity yet bind both ORC and MCM in vivo quite efficiently as measured by ChIP experiments (Wyrick et al. 2001). These data support the idea that origin inefficiency associated with a stable ORC-chromosome interaction may reflect the role for ORC in silencing. Our data indicate that at HMRa, a significant contributor to a stable ORC-chromosome interaction may be the ORC DNA-binding site (B1/A-element) itself. But it is probable that other features of silencers and heterochromatic regions in yeast and metazoans could contribute substantially to a stable interaction between the ORC and its chromosomal target site.
Silencers use several of the same proteins necessary for both replication initiation and transcription activation at other loci, and a central question is how these proteins can perform different functions depending on their genome position. We propose that highly stable binding of these factors to silencers may be critical for their roles in forming a stable silent chromatin structure but less essential, and possibly even detrimental, for their other roles at other positions. Further biochemical analysis of silencer-protein complexes from yeast and other organisms should address this idea and help define the mechanisms that govern the duplication and expression of the eukaryotic genome.
Materials and methods
Yeast strains
All experiments were carried out with yeast strains isogenic to W303-1A. Chromosomal gene deletions and mutations were generated using standard recombinant DNA technology and yeast molecular biology.
In vitro ORC-binding assays
The DNA probes for EMSAs were prepared by annealing complementary 55-bpair oligonucleotides containing the core 11-bp ORC-binding site (A-element) and best match to the 5′ putative B1-element from relevant ARSs (Fig. 1B). The annealed fragments were subcloned into pSTBlue1 (Novagen) and then used as templates to generate 260-bp radiolabeled probes (32P-dCTP) by PCR with a set of common primers complementary to pSTBlue1. Because all A/B1-elements contained the same flanking sequences, the specific activity of probes was virtually the same, and DNA concentrations of different probes varied by at most 1.2-fold. The binding reactions were done under equilibrium conditions in which ORC was in excess over probe DNA such that the concentration of ORC necessary to bind 50% of the probe DNA was approximately equal to the apparent Kd. Binding reactions contained 20 μg/mL dI-dC as nonspecific competitor (Amersham Pharmacia). The concentrations of ORC and DNA used in reactions are indicated in the figure legends.
2D origin analysis and replication timing experiments
2D origin analysis was performed as described (Friedman and Brewer 1995; Kim and Huberman 2001). For replication-timing experiments, the strains were as described in Figure 2 except they were additionally engineered to contain MATa and bar1Δ::HIS3 for arrest and release experiments. To examine the effect that silent chromatin had on replication-timing profiles of HMRa, the strains were additionally modified to contain sir2Δ::URA3 and hmlΔ::LEU2. First, 4 L of yeast cells was grown in YPD to 0.7 O.D. and concentrated for arrest in G1 with α-factor at 23°C. Arrested cells were harvested, washed, and released into S phase in YPD plus 0.025 mg/mL Pronase (CalBiochem). Every 10 min, 400 mL of cells was harvested and genomic DNA was isolated for each sample. The DNA was split into three samples and analyzed for relevant replication intermediates directly after restriction digest. To examine replication intermediates, genomic DNA was digested with HindIII (for HMRa), with NcoI (for ARS1), and with EcoRI (for R11). Hybridization probes were generated by PCR with appropriate primers.
Acknowledgments
We thank Bruce Stillman for the ORC baculoviruses and purification protocol; Michael Weinreich for additional protocols; Jim Dahlberg for critical reading of the manuscript; and Paul Kaufman, Ann Kirchmaier, and Michael Sheets for productive discussions. We also thank J.A. Huberman and M.K. Raghuraman for technical advice on replication timing experiments. This work was supported by predoctoral fellowships from the NSF and Ford Foundation (to M.A.P.D.), and by a grant from the NIH (GM056890 to C.A.F).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Corresponding author.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1096703.
Footnotes
Supplemental material is available at http://www.genesdev.org.
References
- Bell S.P. 2002. The origin recognition complex: From simple origins to complex functions. Genes & Dev. 16: 659-672. [DOI] [PubMed] [Google Scholar]
- Bell S.P. and Stillman, B. 1992. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 357: 128-134. [DOI] [PubMed] [Google Scholar]
- Bell S.P., Mitchell, J., Leber, J., Kobayashi, R., and Stillman, B. 1995. The multidomain structure of Orc1p reveals similarity to regulators of DNA replication and transcriptional silencing. Cell 83: 563-568. [DOI] [PubMed] [Google Scholar]
- DeBeer M.A.P. and Fox, C.A. 1999. A role for a replicator dominance mechanism in silencing. EMBO J. 18: 3808-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey D.D., Davis, L.R., Greenfeder, S.A., Ong, L.Y., Zhu, J.G., Broach, J.R., Newlon, C.S., and Huberman, J.A. 1991. Evidence suggesting that the ARS elements associated with silencers of the yeast mating-type locus HML do not function as chromosomal DNA replication origins. Mol. Cell. Biol. 11: 5346-5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss M., McNally, F.J., Laurenson, P., and Rine, J. 1993. Origin recognition complex (ORC) in transcriptional silencing and DNA replication in S. cerevisiae. Science 262: 1838-1844. [DOI] [PubMed] [Google Scholar]
- Fox C.A., Loo, S., Dillin, A., and Rine, J. 1995. The origin recognition complex has essential functions in transcriptional silencing and chromosomal replication. Genes & Dev. 9: 911-924. [DOI] [PubMed] [Google Scholar]
- Friedman K.L. and Brewer, B.J. 1995. Analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Methods Enzymol. 262: 613-627. [DOI] [PubMed] [Google Scholar]
- Friedman K.L., Brewer, B.J., and Fangman, W.L. 1997. Replication profile of Saccharomyces cerevisiae chromosome VI. Genes to Cells 2: 667-678. [DOI] [PubMed] [Google Scholar]
- Gardner K.A. and Fox, C.A. 2001. The Sir1 protein's association with a silenced chromosome domain. Genes & Dev. 15: 147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S.M. and Cockell, M.M. 2001. The molecular biology of the SIR proteins. Gene 279: 1-16. [DOI] [PubMed] [Google Scholar]
- Hsu L.M. 2002. Promoter clearance and escape in prokaryotes. Biochim. Biophys. Acta 1577: 191-207. [DOI] [PubMed] [Google Scholar]
- Huang R.Y. and Kowalski, D. 1996. Multiple DNA elements in ARS305 determine replication origin activity in a yeast chromosome. Nucleic Acids Res. 24: 816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.M. and Huberman, J.A. 2001. Regulation of replication timing in fission yeast. EMBO J. 20: 6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A., Blitzblau, H., and Bell, S.P. 2002. Cell cycle control of the establishment of mating-type silencing in S. cerevisiae. Genes & Dev. 16: 2935-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.G. and Bell, S.P. 1997. Architecture of the yeast origin recognition complex bound to origins of DNA replication. Mol. Cell. Biol. 17: 7159-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Weinreich, M., and Stillman, B. 1995. ORC and Cdc6p interact and determine the frequency of initiation of DNA replication in the genome. Cell 81: 667-676. [DOI] [PubMed] [Google Scholar]
- Loo S. and Rine, J. 1995. Silencing and domains of heritable gene expression. Annu. Rev. Cell Dev. Biol. 11: 519-548. [DOI] [PubMed] [Google Scholar]
- Marahrens Y. and Stillman, B. 1992. Ayeast chromosomal origin of DNA replication defined by multiple functional elements. Science 255: 817-823. [DOI] [PubMed] [Google Scholar]
- McNally F.J. and Rine, J. 1991. Asynthetic silencer mediates SIR-dependent functions in Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 5648-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak D.T., Pflumm, M., Chesnokov, I., Huang, D.W., Kellum, R., Marr, J., Romanowski, P., and Botchan, M.R. 1997. Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell 91: 311-323. [DOI] [PubMed] [Google Scholar]
- Pillus L. and Grunstein, M. 1995. Chromatin structure in epigenetic regulation in yeast. In Chromatin structure and gene expression (ed. S.C.R. Elgin), pp. 123-141. Oxford University Press, New York.
- Polomienko A., Dershowitz, A., De, J., and Newlon, C.S. 2001. Completion of replication map of Saccharomyces cerevisiae chromosome III. Mol. Cell. Biol. 12: 3317-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman M.K., Winzeler, E.A., Collingwood, D., Hunt, S., Wodicka, L., Conway, A., Lockhart, D.J., Davis, R.W., Brewer, B.J., and Fangman W.L. 2001. Replication dynamics of the yeast genome. Science 294: 115-121. [DOI] [PubMed] [Google Scholar]
- Rao H. and Stillman, B. 1995. The origin recognition complex interacts with a bipartite DNA binding site within yeast replicators. Proc. Natl. Acad. Sci. 92: 2224-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier D.H. and Rine, J. 1992. An origin of DNA replication and a transcriptional silencer require a common element. Science 256: 659-663. [DOI] [PubMed] [Google Scholar]
- Rusche L.N., Kirchmaier, A.L., and Rine, J. 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72: 481-516. [DOI] [PubMed] [Google Scholar]
- Shimada K., Pasero, P., and Gasser, S.M. 2002. ORC and the intra-S-phase checkpoint: Athreshold regulates Rad53p activation in S phase. Genes & Dev. 16: 3236-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J.B. and Gottschling, D.E. 1999. Telomeric chromatin modulates replication timing near chromosome ends. Genes & Dev. 13: 146-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis J.F. and Newlon, C.S. 1997. The ARS309 chromosomal replicator of Saccharomyces cerevisiae depends on an exceptional ARS consensus sequence. Proc. Natl. Acad. Sci. 94: 10786-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triolo T. and Sternglanz, R. 1996. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature 381: 251-253. [DOI] [PubMed] [Google Scholar]
- Wyrick J.J., Aparicio, J.G., Chen, T., Barnett, J.D., Jennings, E.G., Young, R.A., Bell, S.P., and Aparicio, O.M. 2001. Genome-wide distribution of ORC and MCM proteins in S. cerevisiae: High-resolution mapping of replication origins. Science 14: 2357-2360. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Hori, Y., Shinomiya, T., Obuse, C., Tsurimoto, T., Yoshikawa, H., and Shirahige, K. 1997. The efficiency and timing of initiation of replication of multiple replicons of Saccharomyces cerevisiae chromosome VI. Genes to Cells 2: 655-665. [DOI] [PubMed] [Google Scholar]
- Zappulla D.C., Sternglanz, R., and Leatherwood, J. 2002. Control of replication timing by a transcriptional silencer. Curr. Biol. 12: 869-875. [DOI] [PubMed] [Google Scholar]